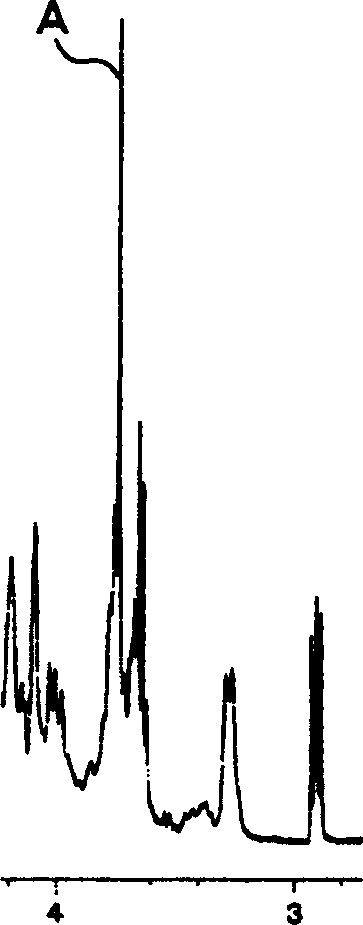Low molecule heparin and salt thereof, pharmaceutical composition comprising same and producing method thereof
A low-molecular-weight heparin and its manufacturing method technology, applied in the field of low-molecular-weight heparin or its salt, can solve the problems of cost and difficult implementation of equipment, and achieve the effects of simple and efficient refining, economical production, and reduced impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
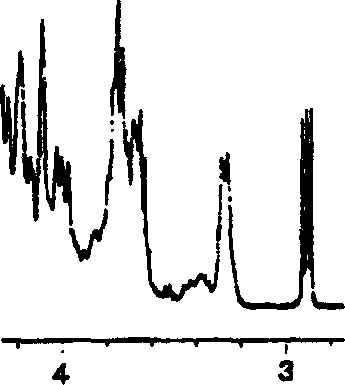
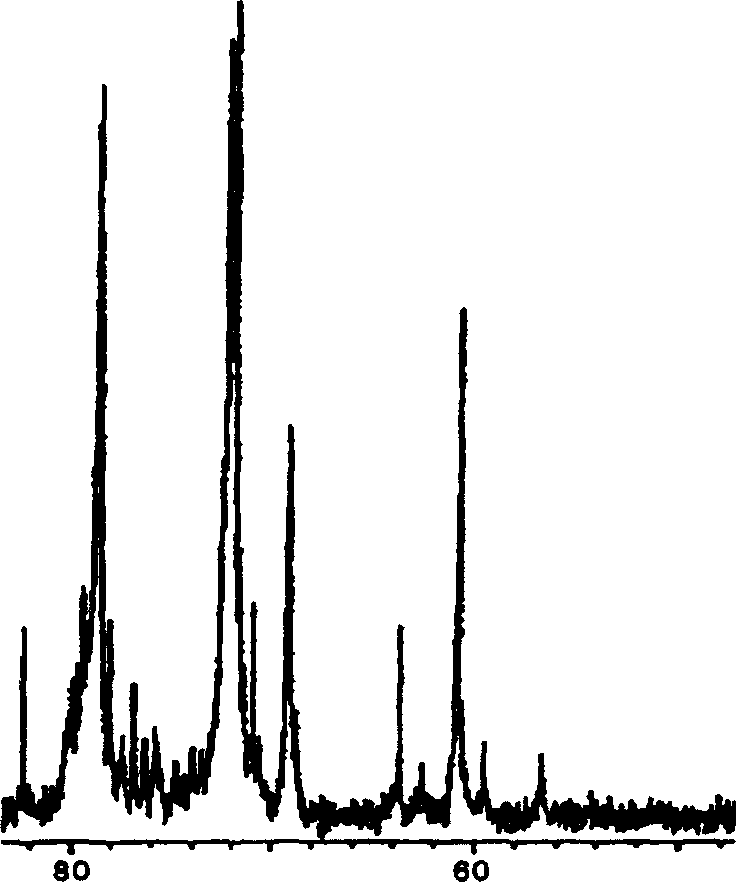
Image
Examples
Embodiment 1
[0116] (a) 15.0 g of heparin sodium specified in the Japanese Pharmacopoeia was dissolved in 250 mL of solvent (ionized water) to prepare a reaction solution, and then the pH of the reaction solution was adjusted to 2.5 with 4 mol / L hydrochloric acid aqueous solution.
[0117] (b) Next, 10 mL of 5% sodium nitrite was added to the reaction liquid, and a low molecular weight reaction (depolymerization reaction) was performed at room temperature for 1 hour. During the reaction, the pH of the reaction solution was maintained at pH 2.5 with 4 mol / L hydrochloric acid aqueous solution.
[0118] (c) Next, the pH of the reaction solution was adjusted to 7.0 with 4 mol / L sodium hydroxide aqueous solution to stop the reaction.
[0119] (d) Next, the reaction solution was heated to 40° C., 1.0 g of a reducing agent (sodium borohydride) was added, and a reduction reaction was performed for 2 hours.
[0120] (e) Next, adjust the pH of the reaction solution to 4.0 with 4 mol / L hydrochloric ...
Embodiment 2
[0131] Low-molecular-weight heparin sodium (dalteparin sodium) was obtained in the same manner as in Example 1, except that the pH of the solution in step (i) in Example 1 was adjusted to 3.8±0.3.
Embodiment 3
[0133] Except that the ethanol solvent in the steps (g), (k) and (m) in Example 1 is replaced with a mixture of methanol-acetone (1:1), use the same method as in Example 1 to obtain Low molecular weight heparin sodium (dalteparin sodium).
[0134] 4.5. Embodiment 4
[0135] Except that after the step (g) in Example 1, the chromatographic column purification of the step (l) is carried out, and then the treatment of the steps (h) to (k) is carried out, the method is the same as that of the example 1 to obtain low molecular weight heparin sodium (dalteparin sodium).
[0136] 4.6. Comparative example 1
[0137] Except that steps (h) to (k) in Example 1 are deleted (that is, step (l) is performed after step (g)), the same method as in Example 1 is used to obtain low-molecular-weight heparin sodium (dalteparin sodium ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com