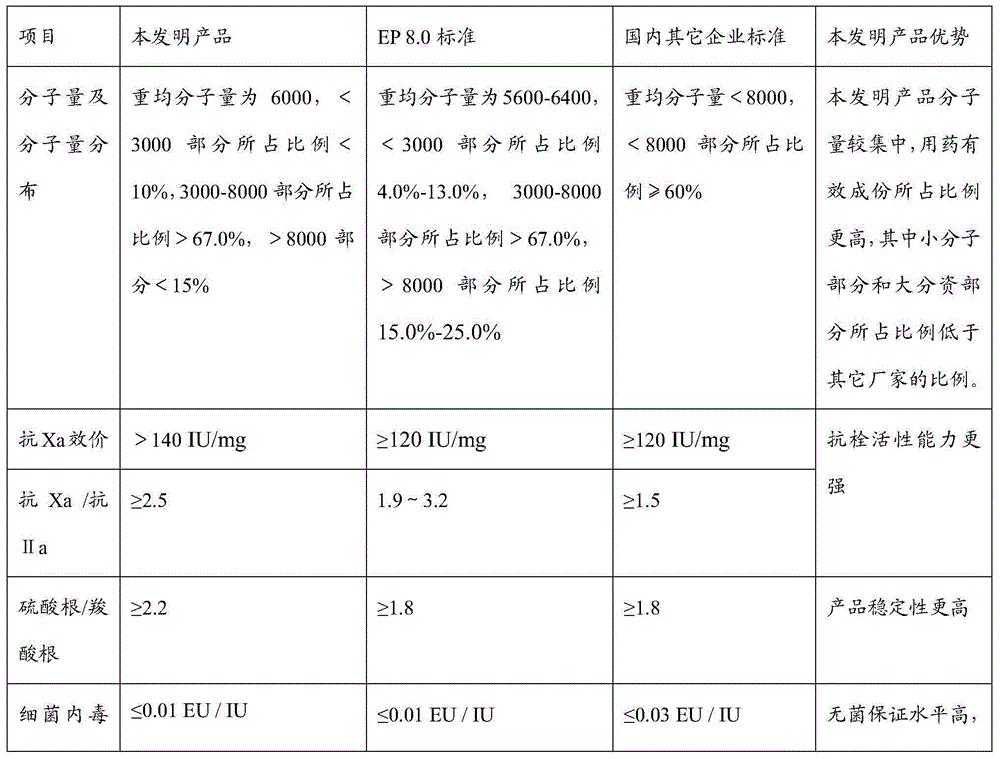
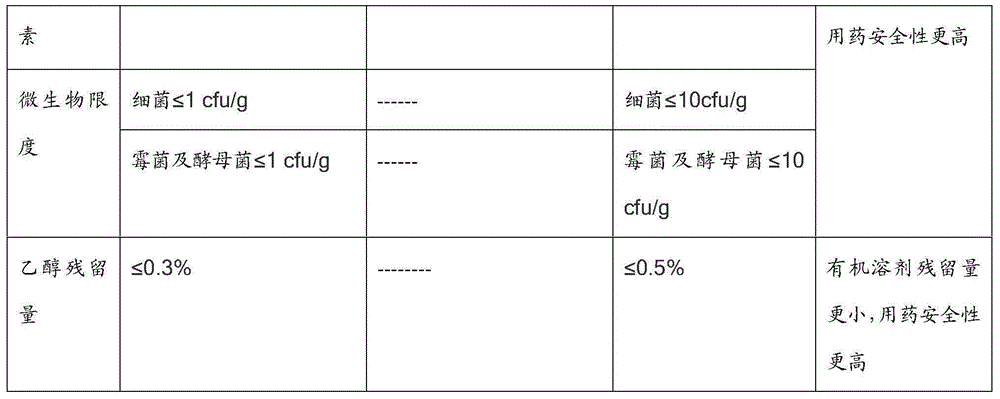
Production method of dalteparin sodium fine product
A technology of dalteparin sodium and its production method, which is applied in the field of preparation and purification of high-quality dalteparin sodium, can solve the problems of raw material acquisition limitation, increase of process production cost, and high cost, achieve wide application range, save time and labor costs , the effect of simplifying the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 90L of water and 10kg of crude heparin sodium to the reaction tank, stir to dissolve it completely, adjust the pH of the solution in the tank to 2.7 with 4mol / L hydrochloric acid, weigh 0.18kg of sodium nitrite and add it to the beaker, and add 1620mL of it to the beaker Purified water, stir to dissolve, add to the reaction tank, maintain the pH of the solution in the tank at 2.6-2.8, stir and react for 20 minutes, test the solution with starch-potassium iodide test paper, observe the color of the test paper until the test paper does not show blue. Use 5mol / L sodium hydroxide solution to adjust the pH of the system to 9.6, add 0.072kg of sodium borohydride to the reaction tank, stir for 16 hours, then adjust the pH of the solution to 3.5 with 4mol / L hydrochloric acid, and stir for 20 minutes. Then use 5mol / L sodium hydroxide solution to adjust the pH value of the system to 7.5, turn on the UV lamp with a wavelength of 254nm, and put it into the reaction tank for 20 m...
Embodiment 2
[0050] Add 85L of water and 10kg of crude heparin sodium to the reaction tank, stir to dissolve it completely, adjust the pH of the solution in the tank to 2.7 with 4mol / L hydrochloric acid, weigh 0.15kg of sodium nitrite and add it to the beaker, and add 1650mL of it to the beaker Purified water, stir to dissolve, add to the reaction tank, maintain the pH value of the solution in the tank at 2.6-2.8, stir and react for 25 minutes, test the solution with starch-potassium iodide test paper, observe the color of the test paper until the test paper does not show blue. Use 5mol / L sodium hydroxide solution to adjust the pH of the system to 9.8, add 0.045kg of sodium borohydride to the reaction tank, stir for 18 hours, then adjust the pH of the solution to 3.5 with 4mol / L hydrochloric acid, and stir for 23 minutes. Then use 5mol / L sodium hydroxide solution to adjust the pH value of the system to 7.3, turn on the ultraviolet lamp with a wavelength of 254nm, put it into the reaction ta...
Embodiment 3
[0057] Add 100L of water and 10kg of crude heparin sodium into the reaction tank, stir to dissolve it completely, adjust the pH value of the solution in the tank to 2.7 with 4mol / L hydrochloric acid, weigh 0.25kg of sodium nitrite and add it to the beaker, add 1550mL of purified Water, stir to dissolve, add to the reaction tank, maintain the pH value of the solution in the tank at 2.6-2.8, stir the reaction for 25 minutes, test the solution with starch-potassium iodide test paper, observe the color of the test paper until the test paper does not show blue. Use 5mol / L sodium hydroxide solution to adjust the pH of the system to 10.0, add 0.125kg of sodium borohydride to the reaction tank, stir for 16 hours, then adjust the pH of the solution to 3.6 with 4mol / L hydrochloric acid, and stir for 25 minutes. Then use 5mol / L sodium hydroxide solution to adjust the pH value of the system to 7.5, turn on the ultraviolet lamp with a wavelength of 254nm, put it into the reaction tank and i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com