Mesenchymal stem cell injection as well as preparation method and application thereof
A technology of stem cells and injections, applied in the field of stem cells, can solve problems such as pain of patients, and achieve the effect of being convenient to use, convenient to obtain materials, and treating Parkinson's disease.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1 Preparation of placental mesenchymal stem cells
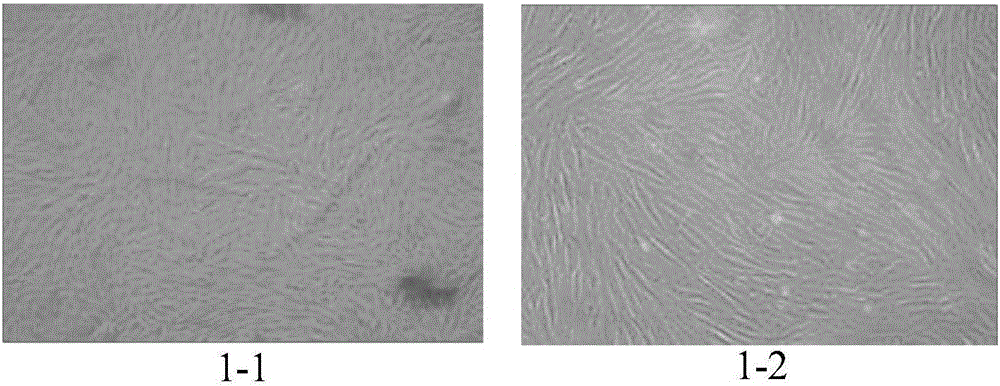
[0071] 1. Primary isolation of placental mesenchymal stem cells
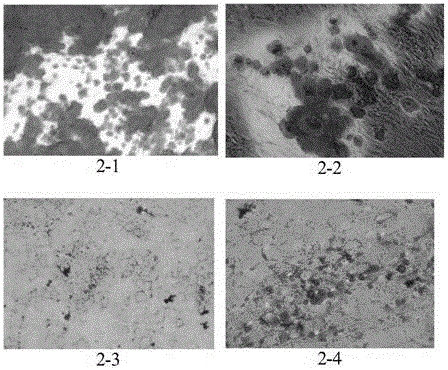
[0072] Under sterile conditions, take the fresh placenta discarded after delivery, peel off the placenta, wash it with PBS buffer solution, and cut the placenta tissue into 5×5cm 2 , add 3-5 times the volume of 0.25% trypsin to digest, place on a 200r / min 37°C constant temperature shaker, digest for 30min, and shake vigorously several times every 10min to remove epithelial cells.
[0073] After the digestion is complete, transfer it to a storage bottle, add 150 mL of PBS buffer solution, shake vigorously, and repeat this operation 2 times. Transfer placental tissue to a small beaker and chop to 1mm 3 . Then transfer to a storage bottle, add 20 mL of 0.5% type I collagenase and complete medium (high glucose DMEM+10% FBS), so that the final concentration of collagenase is 0.1-0.2%. Place in a constant temperature shaker at 37°C, 250r / min, un...
Embodiment 2
[0087] Preparation of embodiment 2 placental mesenchymal stem cell preparation
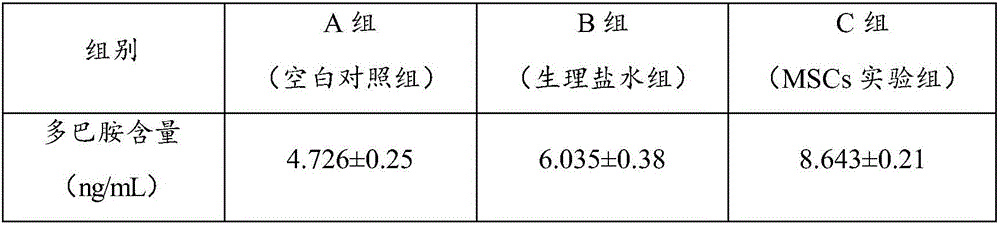
[0088] The method for preparing 100mL stem cell injection: 30mL of human serum albumin stock solution with an albumin concentration of 10% (the final concentration of the mass volume ratio is 3%), 0.5mL of low molecular weight heparin calcium (the final concentration of the mass volume ratio is 0.5%), Vitamin C 0.5g (mass volume ratio final concentration is 0.5%), lentinan 0.4g (mass volume ratio final concentration is 0.4%) and 0.9% sodium chloride injection 61.6mL are mixed, slowly add DMSO 7mL (volume percentage Content is 7%), put into 4 ℃ of refrigerators and pre-cool, placental mesenchymal stem cells (cell final concentration 1×10 6 cells / mL) were resuspended in the mixed solution, and the prepared placental mesenchymal stem cell injection was dispensed in The cells are stored in the cryopreservation bag, and the preparation is complete.
Embodiment 3
[0089] Preparation of embodiment 3 placental mesenchymal stem cell preparation
[0090] The method for preparing 100mL stem cell injection: 5mL of human serum albumin stock solution with an albumin concentration of 20% (the final mass-volume concentration is 1%), 0.5mL low-molecular-weight heparin calcium (0.5% mass-volume final concentration), Vitamin C 0.3g (mass volume ratio final concentration is 0.3%), lentinan 0.2g (mass volume ratio final concentration is 0.2%) and 0.9% sodium chloride injection 88.8mL are mixed, slowly add DMSO 5mL (volume percentage Content is 5%), put into 4 ℃ of refrigerators and pre-cool, placental mesenchymal stem cells (cell final concentration 1×10 6 cells / mL) were resuspended in the mixed solution, and the prepared placental mesenchymal stem cell injection was dispensed in The cells are stored in the cryopreservation bag, and the preparation is complete.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com