Preparation and purification process for nadroparin calcium
A technology of heparin calcium and process, applied in the field of preparation and purification process of nadroparin calcium, can solve the problems of difficulty in separating fragments and failing to be separated, and achieve the effect of optimizing conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
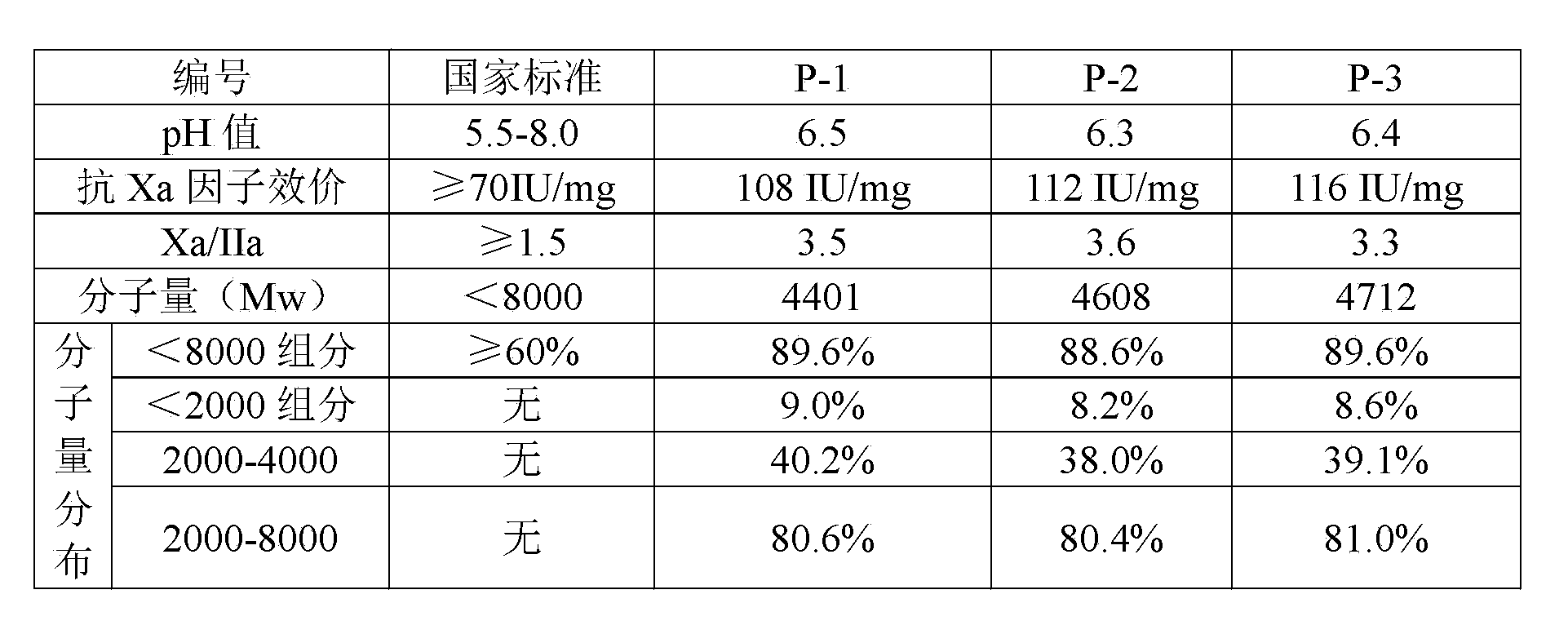
Embodiment 1)
[0026] Weigh 1 kg of undegraded heparin sodium, and the specific steps include:
[0027] a. Prepare 1 kg of undegraded heparin sodium with 5% acetic acid solution into a 5% (w / v) aqueous solution, add 40 g of sodium nitrite solution dropwise for depolymerization reaction, and adjust the pH value to 2.8 in the presence of acetic acid. The temperature was controlled at 20°C, the depolymerization reaction was carried out for 150 minutes, and then the pH value of the reaction solution was adjusted to 6.5 with sodium hydroxide solution to terminate the reaction;
[0028] b. Add 100 g of sodium borohydride to the feed solution whose depolymerization reaction has been terminated in the previous step. The amount of sodium borohydride added is 10% of the weight of undepolymerized heparin sodium. When the temperature of the feed liquid is lowered to 15°C, slowly add hydrochloric acid dropwise, adjust the pH of the reaction feed liquid to 2.0, and stir for 10 minutes; then continue to dr...
Embodiment 2)
[0035] Weigh 1 kg of undegraded heparin sodium, and the specific steps include:
[0036] a. Prepare 1 kg of undegraded heparin sodium with 6% acetic acid solution into a 5% (w / v) aqueous solution, add 36 g of sodium nitrite solution dropwise for depolymerization reaction, and adjust the pH value to 3.0 in the presence of acetic acid, and react The temperature was controlled at 22°C, the depolymerization reaction was carried out for 180 minutes, and then the pH value of the reaction solution was adjusted to 7.0 with sodium hydroxide solution to terminate the reaction;
[0037] b. Add 80 g of sodium borohydride to the feed solution whose depolymerization reaction has been terminated in the previous step. The amount of sodium borohydride added is 8% of the weight of undepolymerized heparin sodium. When the temperature of the feed liquid is lowered to 10°C, slowly add hydrochloric acid dropwise, adjust the pH of the reaction feed liquid to 2.2, and stir for 12 minutes; then contin...
Embodiment 3)
[0044] Weigh 1 kg of undegraded heparin sodium, and the specific steps include:
[0045] a. Prepare 1 kg of undegraded heparin sodium with 8% acetic acid solution into a 5% (w / v) aqueous solution, add 30 g of sodium nitrite solution dropwise for depolymerization reaction, and adjust the pH value to 3.4 in the presence of acetic acid. The temperature was controlled at 25°C, the depolymerization reaction was carried out for 210 minutes, and then the pH value of the reaction solution was adjusted to 7.5 with sodium hydroxide solution to terminate the reaction;
[0046] b. Add 60 g of sodium borohydride to the feed solution whose depolymerization reaction has been terminated in the previous step. The amount of sodium borohydride added is 6% of the weight of undepolymerized heparin sodium. When the temperature of the feed liquid is lowered to 12°C, slowly add hydrochloric acid dropwise, adjust the pH of the reaction feed liquid to 2.5, and stir for 15 minutes; then continue to drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com