Preparation method of novel source of low molecular weight heparin from nitrous acid degradation
A technology of sodium nitrite and heparin sodium, applied in the field of preparation of new sources of nitrous acid to degrade low molecular weight heparin, can solve the problem of single source of species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
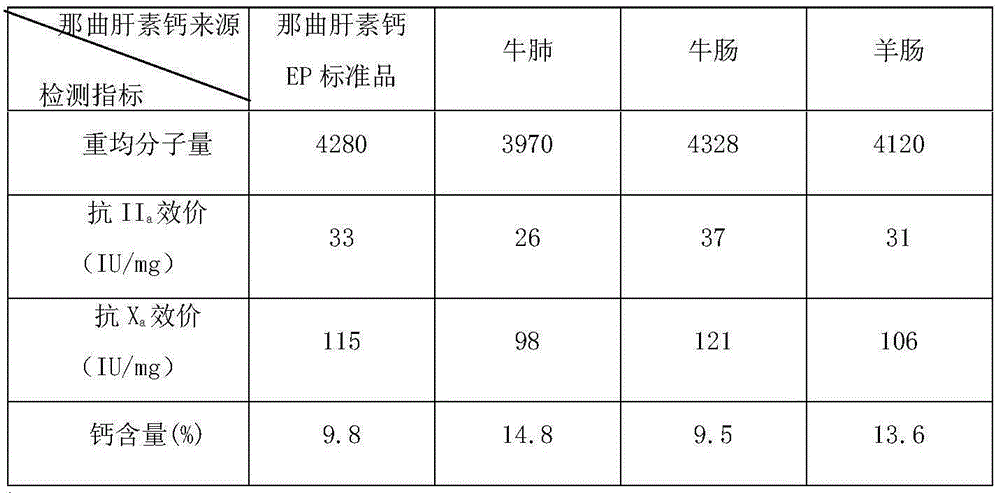
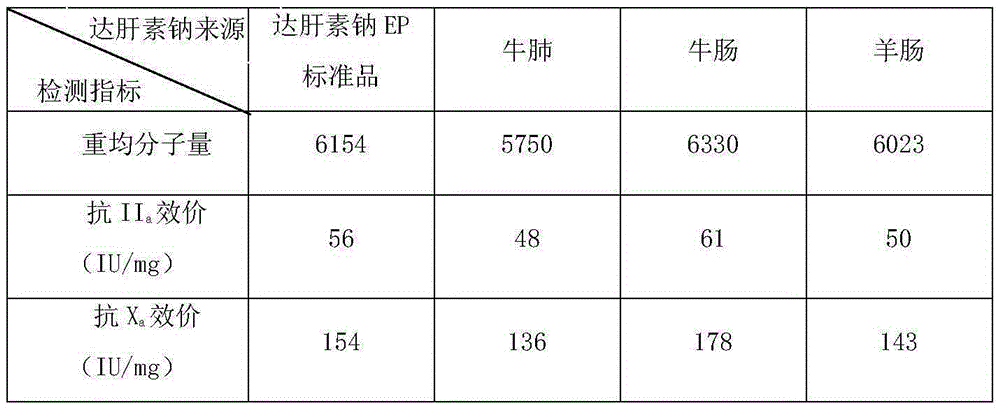
[0007] The preparation method of nadroparin calcium, the steps are as follows:
[0008] (i) Completely dissolve the heparin sodium prepared from the raw material bovine lung, bovine intestine and / or sheep intestine in water, add sodium nitrite, the heparin sodium is 10 to 30 times the mass of sodium nitrite, adjust the pH to 2 to 5, and react 80 to 120 minutes, then adjust the pH to 9 to 12 to prepare the reaction solution A;
[0009] (ii) Add sodium borohydride to the reaction liquid A prepared in step (i), the sodium borohydride is 1-3% of the mass of heparin sodium, react for 4-8 hours, adjust the pH to 2-5 with hydrochloric acid, mix well , and then adjusted to neutrality with sodium hydroxide to prepare reaction solution B;
[0010] (iii) In the reaction solution B prepared in step (ii), add 3 to 5 times the volume of alcohol, separate the solid and liquid, and dry to obtain solid A, then dissolve solid A in water, the mass of solid A and water The volume ratio is 1: (5...
specific Embodiment approach
[0031] The technical solutions of the present invention will be further described below in conjunction with the examples, but the protection scope of the present invention is not limited thereto.
[0032] The raw material bovine lung described in the embodiment and comparative example, bovine intestine, the heparin sodium prepared by sheep intestine is purified and prepared according to the prior art, can refer to Wang Qingyuan's " heparin sodium process research " (" China Pharmaceutical Industry Journal " 1991 05 phases ) to describe the method to obtain heparin sodium.
Embodiment 1
[0034] Bovine lung derived heparin sodium to prepare nadroparin calcium samples, the steps are as follows:
[0035] (i) Completely dissolve sodium heparin derived from bovine lung in water, add sodium nitrite, the sodium heparin is 30 times the mass of sodium nitrite, adjust the pH to 2.5, react for 80 minutes, and then adjust the pH to 10 to prepare the reaction solution A ;
[0036] (ii) Add sodium borohydride to the reaction solution A prepared in step (i), the mass percentage of sodium borohydride and heparin sodium is 1%, react for 6 hours, adjust the pH to 3 with hydrochloric acid, mix well, and then use hydrogen Sodium oxide was adjusted to neutrality to prepare reaction solution B;
[0037] (iii) In the reaction solution B prepared in step (ii), add 4 times the volume of ethanol, separate the solid from the liquid, and dry to obtain solid A, then dissolve solid A in water, the mass volume ratio of solid A to water The ratio is 1:10, the unit is g / mL, and the reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com