Pharmaceutical composition for treating rhinitis
A composition and drug technology, applied in the field of medicine, can solve problems such as ineffective treatment and capillary atrophy, and achieve the effect of facilitating drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Naphthalene Naphthalene Nasal Spray
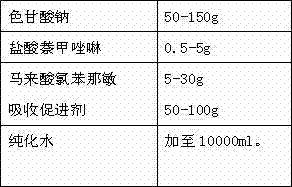
[0024] Prescription: 1000 sticks
[0025]
[0026] Preparation process: Take the prescribed amount of sodium cromoglycate, naphazoline hydrochloride, chlorpheniramine maleate, polyethylene glycol (PEG) lauryl hydroxystearate in an appropriate amount of container, add purified water, and place at room temperature Stir under low pressure, after the above materials are dissolved, add purified water to the full amount, filter through a 0.22 μm microporous membrane to obtain a chromagannaphthylamine solution with a pH value of 6.4, and distribute it in a qualified container under the condition of avoiding bacteria. In a spray bottle, inspected, packaged.
[0027]
Embodiment 2
[0028] Embodiment 2 Naphthalene Naphthalene Nasal Spray
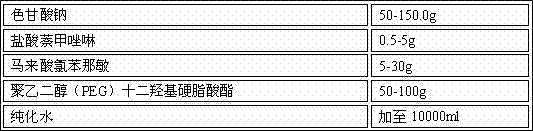
[0029] Prescription: 1000 sticks
[0030] sodium cromolyn 150.0g Naphazoline Hydrochloride 5g Chlorpheniramine maleate 30g Polyethylene glycol (PEG) lauryl hydroxystearate 100g purified water Add to 10000ml
[0031] Preparation process: Take the prescribed amount of sodium cromoglycate, naphazoline hydrochloride, chlorpheniramine maleate, polyethylene glycol (PEG) lauryl hydroxystearate in an appropriate amount of container, add purified water, and place at room temperature Stir under low pressure, after the above materials are dissolved, finally add purified water to the full amount, filter through a 0.22 μm microporous membrane to obtain a chromagannaphthylamine solution with a pH value of 5.7, and pack it in a qualified container under the condition of avoiding bacteria. spray bottle, inspection, packaging.
[0032]
Embodiment 3
[0033] Example 3 Naphthalene Naphthalene Nasal Spray
[0034] Prescription: 1000 sticks
[0035] sodium cromolyn 100.0g Naphazoline Hydrochloride 2.5g Chlorpheniramine maleate 25.0g Polyethylene glycol (PEG) lauryl hydroxystearate 55g purified water Add to 10000ml
[0036] Preparation process: Take the prescribed amount of sodium cromoglycate, naphazoline hydrochloride, chlorpheniramine maleate, polyethylene glycol (PEG) lauryl hydroxystearate in an appropriate amount of container, add purified water, and place at room temperature Stir under low pressure, after the above-mentioned materials are dissolved, add purified water to the full amount, filter with a 0.22 μm microporous membrane to obtain a chromagannaphthylamine solution with a pH value of 5.6, and distribute it in a qualified container under the condition of avoiding bacteria. In a spray bottle, inspected, packaged.
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com