Method of producing heparin calcium from crude heparin sodium
A technology of heparin sodium and heparin calcium, which is applied in the field of biomedicine, can solve the problems of cumbersome operation, high production cost, and high requirements for resin activation, and achieve the effect of high activity quality, low production cost, and easy industrialized large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
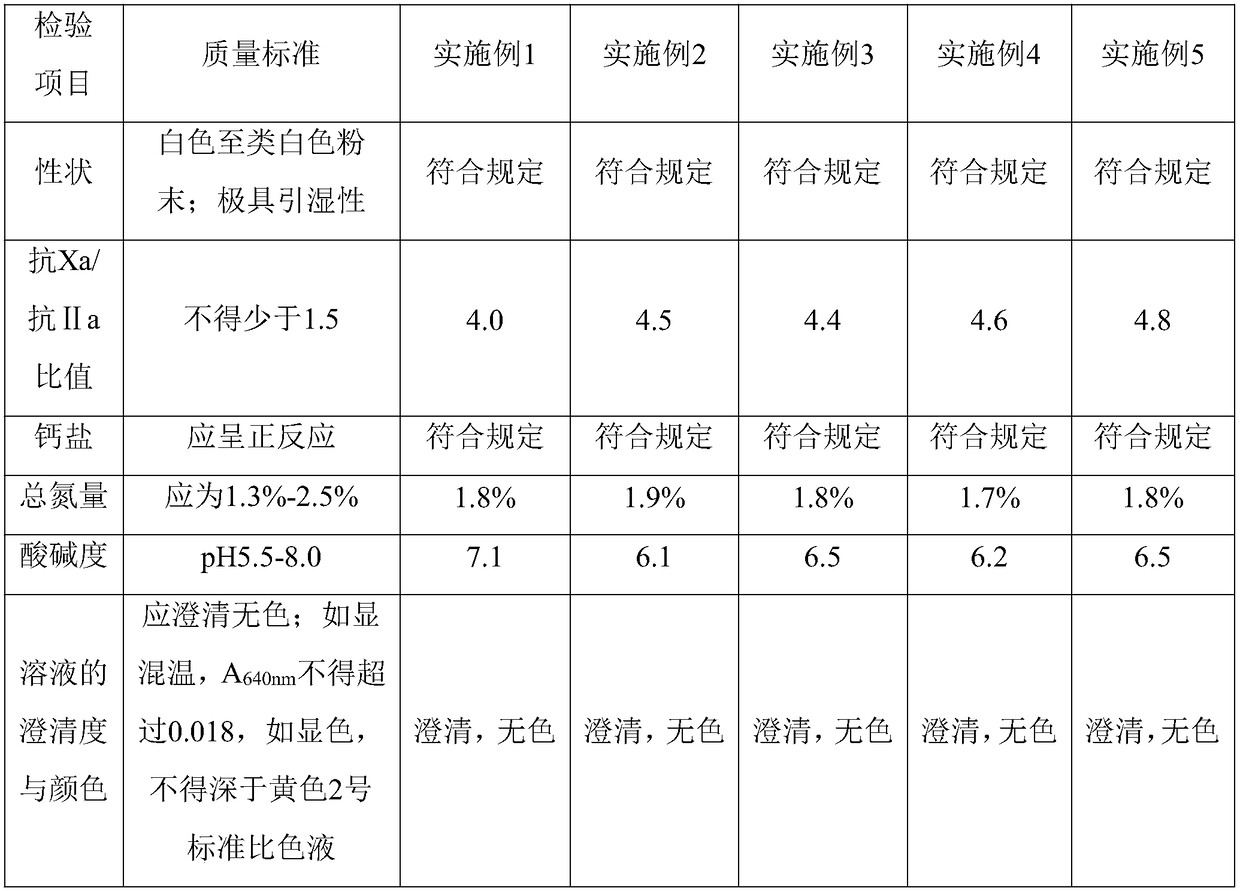
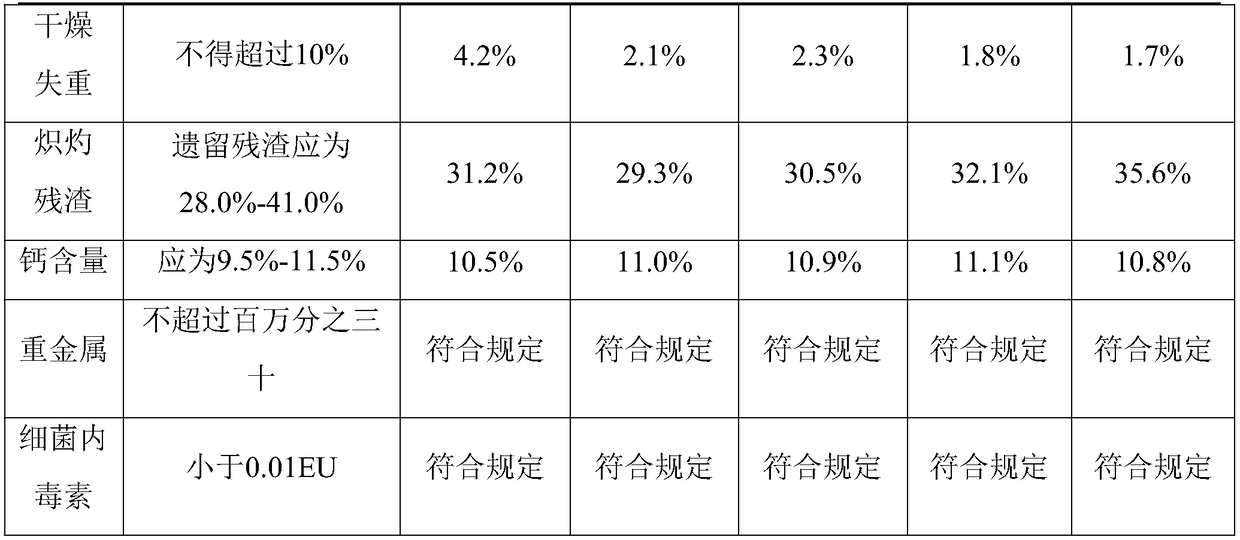
Embodiment 1
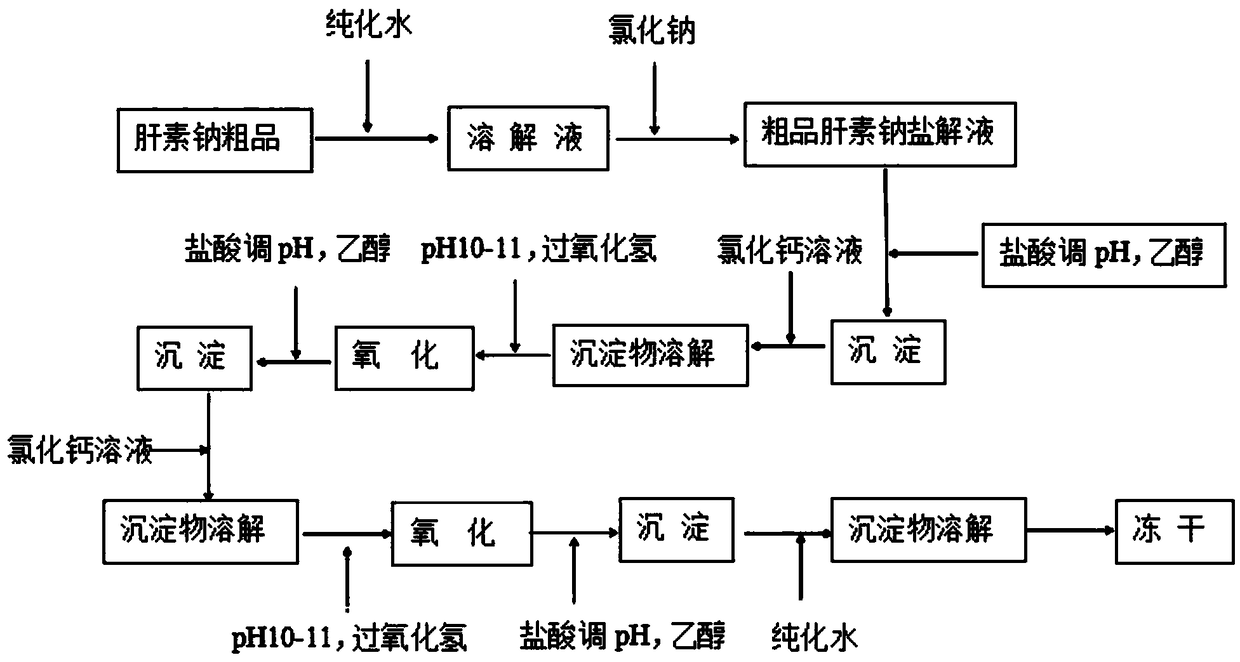
[0031] A method for producing heparin calcium from crude heparin sodium is provided in this embodiment, the method comprising the steps of:
[0032] 1. Weigh 10kg of crude heparin sodium and add 10 times of purified water, stir at 50°C for 2.0 hours and then dissolve; then add 2kg of sodium chloride to the aqueous solution, adjust the pH to 8.0 with sodium hydroxide, then heat up to 43 ℃, stirring and reacting for 3 hours, adding 2kg of calcium chloride, raising the temperature to 85 ℃, keeping warm for 30 minutes, cooling to 60 ℃, filtering to obtain crude heparin sodium salt solution.
[0033] 2. Adjust the crude heparin sodium salt solution in step 1 with hydrochloric acid to pH = 7.0, add 0.8 times of ethanol according to the volume of the feed solution, and separate the mixture after standing for 10 hours;
[0034] 3. Discard the supernatant, and add 180 kg of 10% calcium chloride solution to dissolve the lower sediment;
[0035] 4. Adjust the solution in step 3 with sat...
Embodiment 2
[0042] A method for producing heparin calcium from crude heparin sodium is provided in this embodiment, the method comprising the steps of:
[0043] 1. Weigh 10kg of crude heparin sodium, add 10 times of purified water, and stir at 50°C for 2.0 hours to dissolve; then add 2kg of sodium chloride to the aqueous solution, adjust the pH to 8.2 with sodium hydroxide, and stir to react 3 hour, add 2.0kg of calcium chloride, heat up to 83°C, keep warm for 30 minutes, drop to 60°C, filter to obtain crude heparin sodium salt solution;
[0044] 2. Adjust the crude heparin sodium salt solution in step 1 with hydrochloric acid to pH = 7.0, add 0.8 times of ethanol according to the volume of the feed solution, and separate the mixture after standing for 10 hours;
[0045]3. Discard the supernatant, and add 18 times of 15% calcium chloride solution to dissolve the lower sediment;
[0046] 4. After adjusting the solution in step 3 with saturated calcium hydroxide solution to make the pH to ...
Embodiment 3
[0053] A method for producing heparin calcium from crude heparin sodium is provided in this embodiment, the method comprising the steps of:
[0054] 1. Weigh 10kg of crude heparin sodium and add 12 times of purified water, and stir at 55°C for 1.5 hours to dissolve; then add 3kg of sodium chloride to the aqueous solution, adjust the pH to 8.5 with sodium hydroxide, and stir to react 2 hour, add 3kg of calcium chloride, heat up to 88°C, stir and react for 30 minutes, drop to 60°C, filter to obtain crude heparin sodium salt solution;
[0055] 2. Adjust the crude heparin sodium salt solution in step 1 with hydrochloric acid to pH = 7.0, add 1.0 times of ethanol according to the volume of the feed solution, and separate the mixture after standing for 10 hours;
[0056] 3. Discard the supernatant, and add 200 kg of 15% calcium chloride solution to dissolve the lower sediment;
[0057] 4. After adjusting the solution in step 3 with saturated calcium hydroxide solution to make the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com