Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Implantation failure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
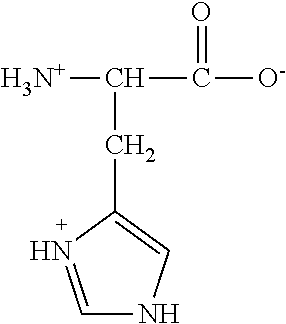
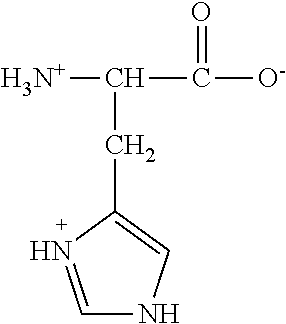
Failure. Implantation failure is considered to be caused by inadequate uterine receptivity in two-thirds of cases, and by problems with the embryo itself in the other third. Inadequate uterine receptivity may be caused by abnormal cytokine and hormonal signaling as well as epigenetic alterations.
Oral formulations of pyrrolidine derivatives
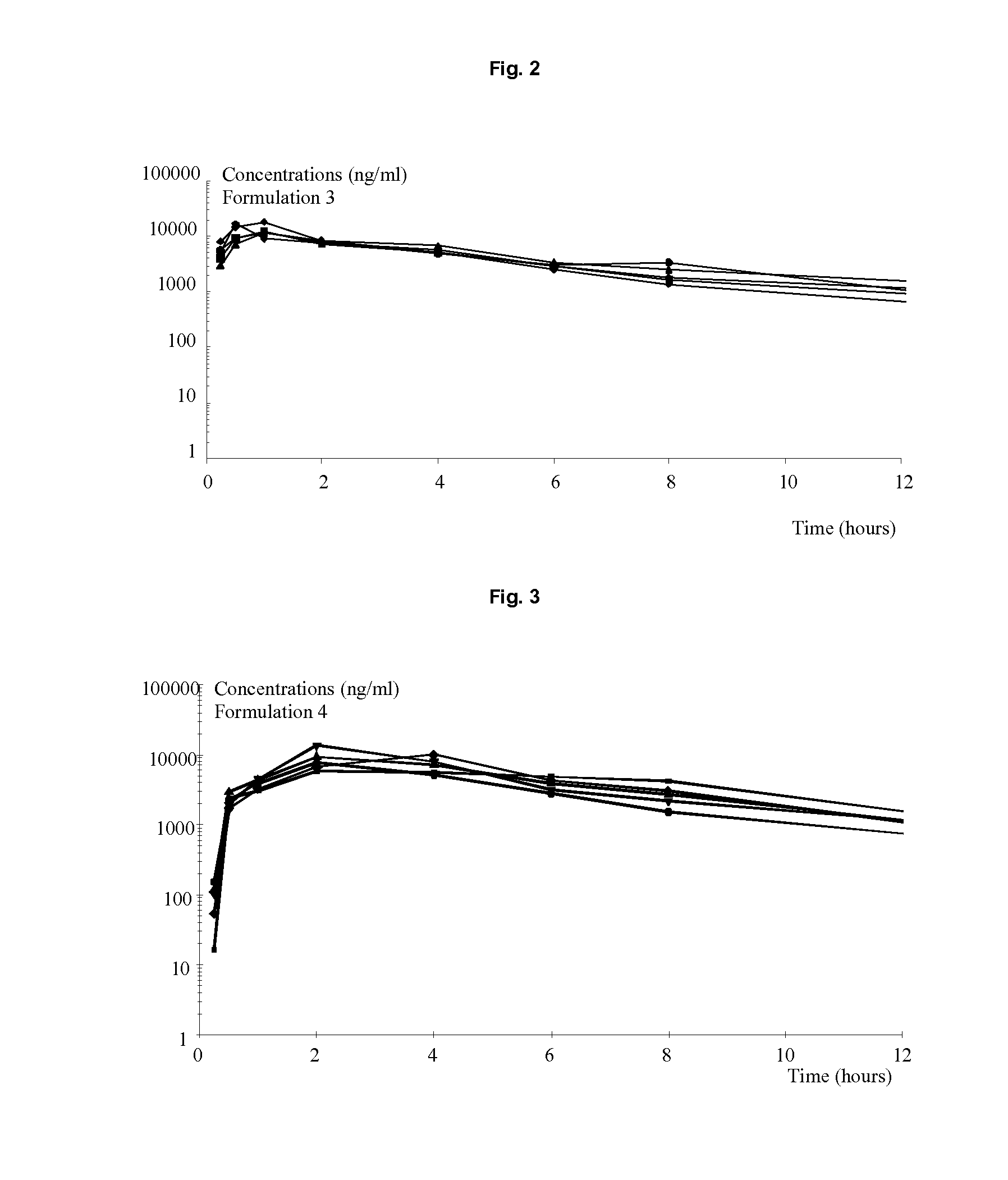
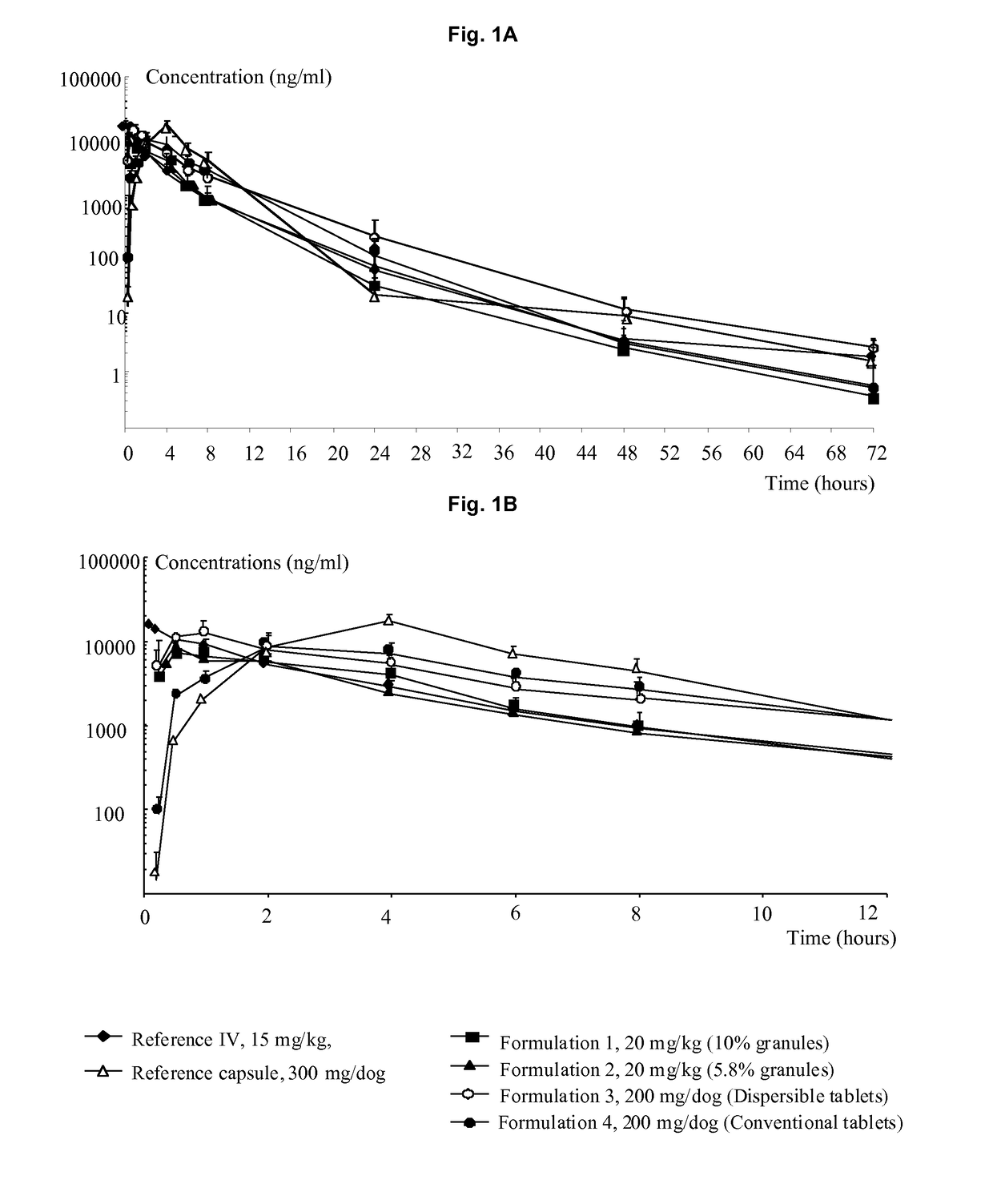
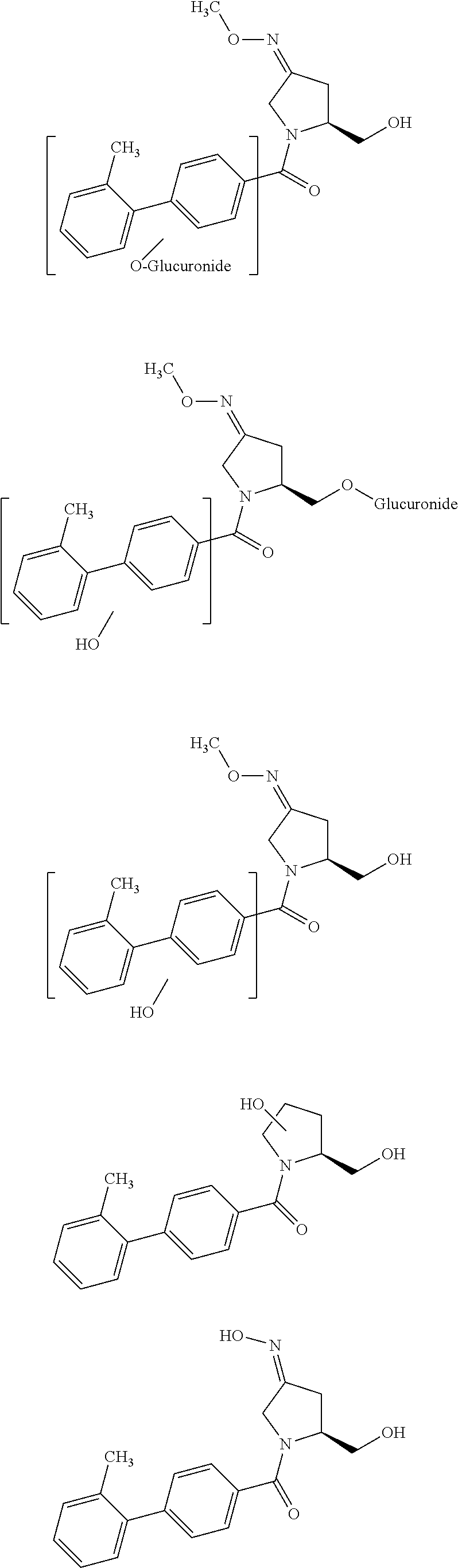
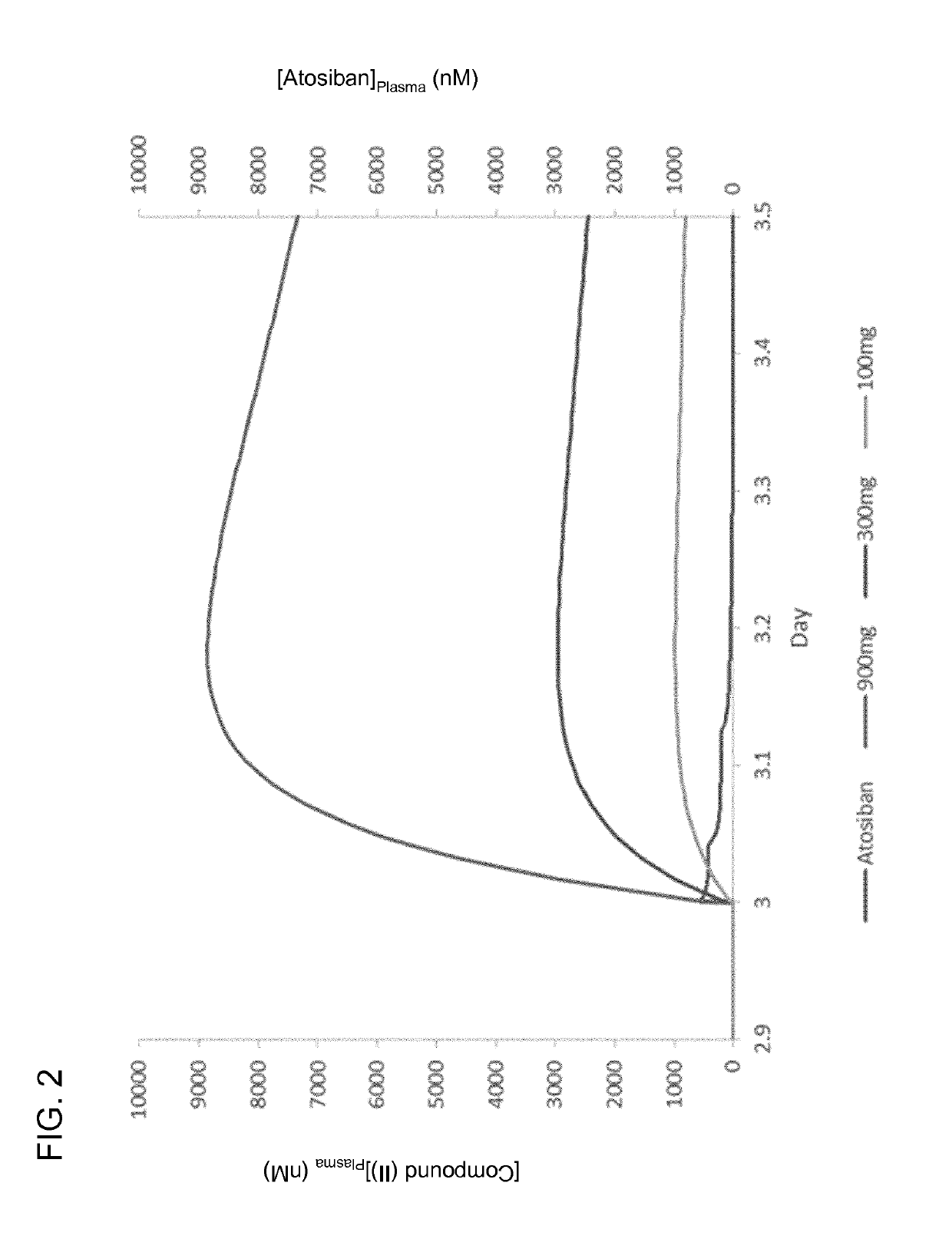
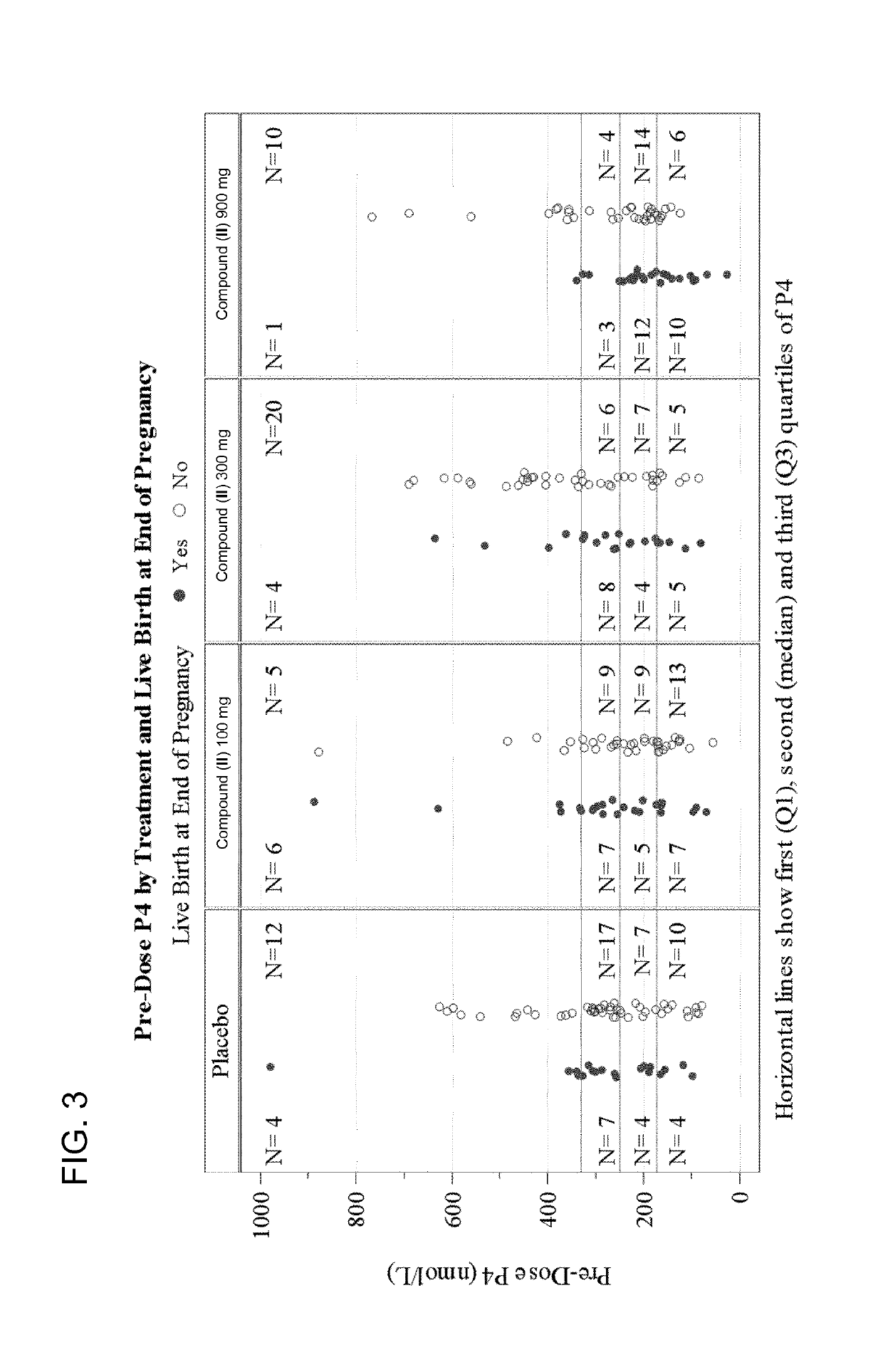
The present invention relates to solid oral formulations comprising a compound of formula (3Z,5S)-5-(hydroxymethyl)-1-[(2′-methyl-1,1′-biphenyl-4-yl)carbonyl]pyrrolidin-3-one-O-methyloxime, and / or an active metabolite thereof, and the use of said formulations in the treatment and / or prevention of preterm labor, premature birth, dysmenorrhea and embryo implantation failure due to uterine contractions. The present invention is furthermore related to processes for their preparation.
Owner:OBSEVA
Oral formulations of pyrrolidine derivatives
The present invention relates to solid oral formulations comprising a compound of formula (3Z,5S)-5-(hydroxymethyl)-1-[(2′-methyl-1,1′-biphenyl-4-yl)carbonyl]pyrrolidin-3-one-O-methyloxime, and / or an active metabolite thereof, and the use of said formulations in the treatment and / or prevention of preterm labor, premature birth, dysmenorrhea and embryo implantation failure due to uterine contractions. The present invention is furthermore related to processes for their preparation.
Owner:OBSEVA
Preparation method for micro-arc oxidation-dopamine coupling carried traditional Chinese medicine coating of pure titanium oral implant
InactiveCN106521601APromote growth and developmentPromote substance metabolismSurface reaction electrolytic coatingTissue regenerationMicro arc oxidationBiocompatibility Testing
The invention discloses a preparation method for a micro-arc oxidation-dopamine coupling carried traditional Chinese medicine coating of a pure titanium oral implant and relates to a preparation method for a pure titanium oral implant. The preparation method disclosed by the invention aims to solve the technical problem of implantation failure as an existing titanium oral implant is slowly healed in the early implanting period and bacteria invade after implantation to lead to early infection after an implant surgery. The preparation method provided by the invention comprises the steps of: I, pre-treatment of the surface of a pure titanium test sample; II, preparation of a micro-arc oxidation electrolyte; III, ultrasonic micro-arc oxidation treatment; and IV, post-treatment. After micro-arc oxidation on the surface of a pure titanium implanting material, the material is modified by taking dopamine as a coupling agent carrying traditional Chinese medicines, so that growth and development of skeleton are promoted, material metabolism of human cells is enhanced, aging of muscles and skeleton is prevented, and the broad-spectrum antibacterial action is improved, and therefore, a novel bone implant which is better in biocompatibility and faster to heal is obtained.
Owner:JIAMUSI UNIVERSITY
Compositions and methods for healthy pregnancy
InactiveUS20070071716A1Avoid laborReduce frequencyOrganic active ingredientsPeptide/protein ingredientsPreterm laborImplantation failure
Compositions, kits and methods for the prevention of, for example, spontaneous abortion, preeclampsia, preterm labor or implantation failure during assisted reproduction are provided. The compositions, kits and methods provide an effective amount of granulocyte colony stimulating factor to prevent, for example, spontaneous abortion, preeclampsia, preterm labor or implantation failure of an embryo.
Owner:NORA THERAPEUTICS
Method of reducing the likelihood of implantation failure during an assisted reproduction in a female subject in a need thereof
InactiveUS7470662B2Reduce frequencyPrevent spontaneous abortionOrganic active ingredientsPeptide/protein ingredientsGynecologyAbortion
Compositions, kits and methods for the prevention of, for example, spontaneous abortion, preeclampsia, preterm labor or implantation failure during assisted reproduction are provided. The compositions, kits and methods provide an effective amount of granulocyte colony stimulating factor to prevent, for example, spontaneous abortion, preeclampsia, preterm labor or implantation failure of an embryo.
Owner:NORA THERAPEUTICS
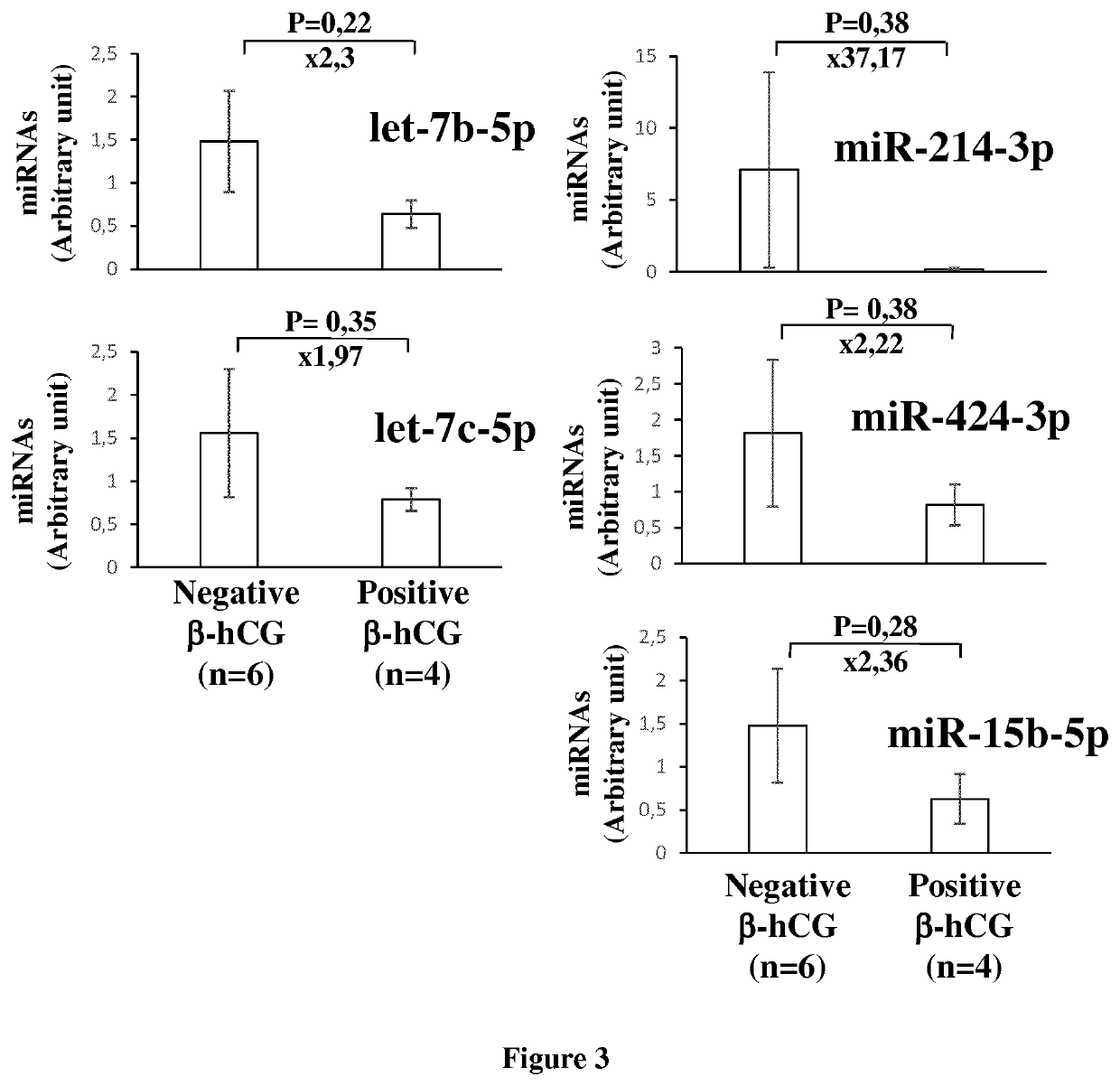
Methods for assessing pregnancy outcome
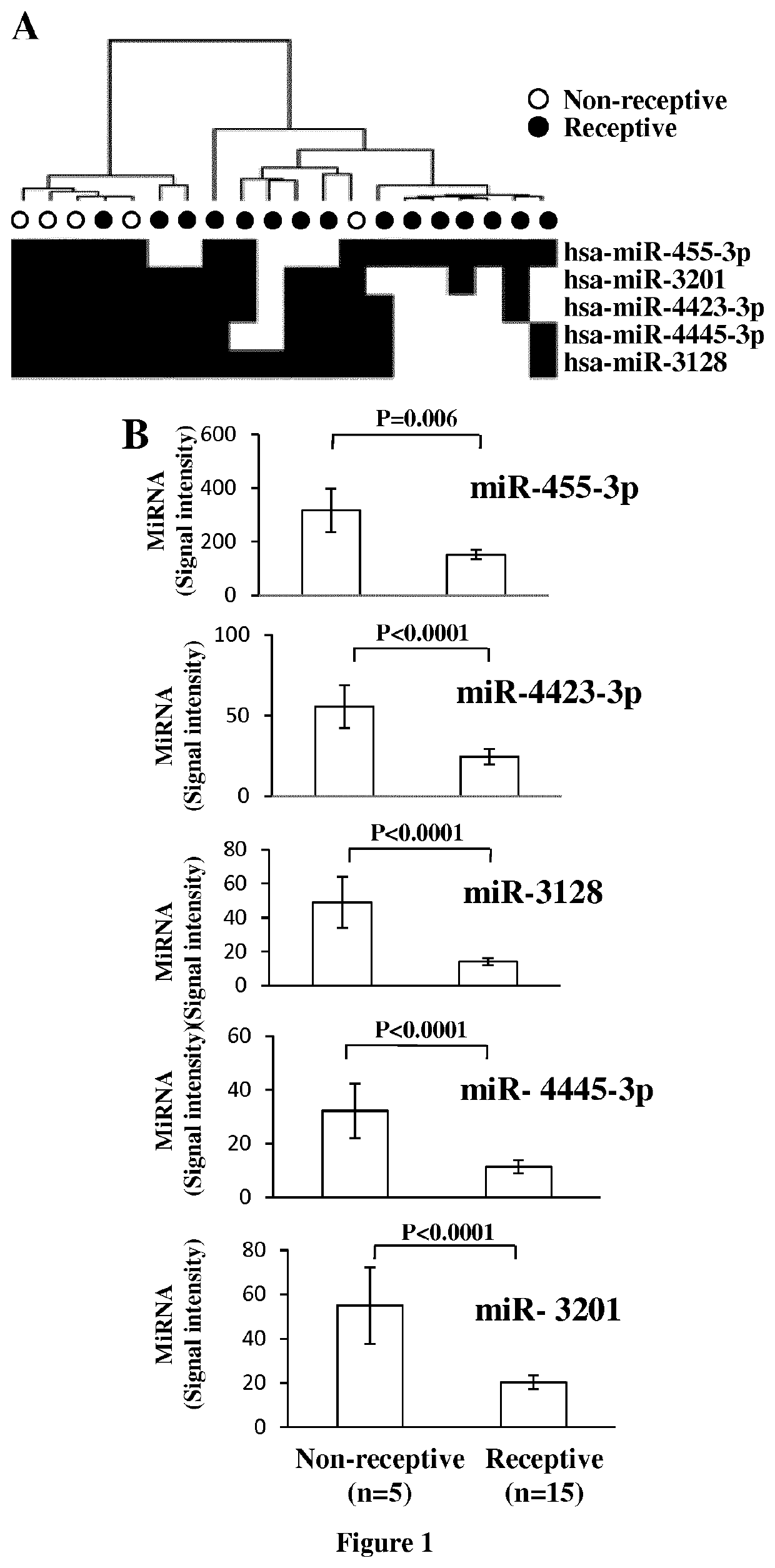
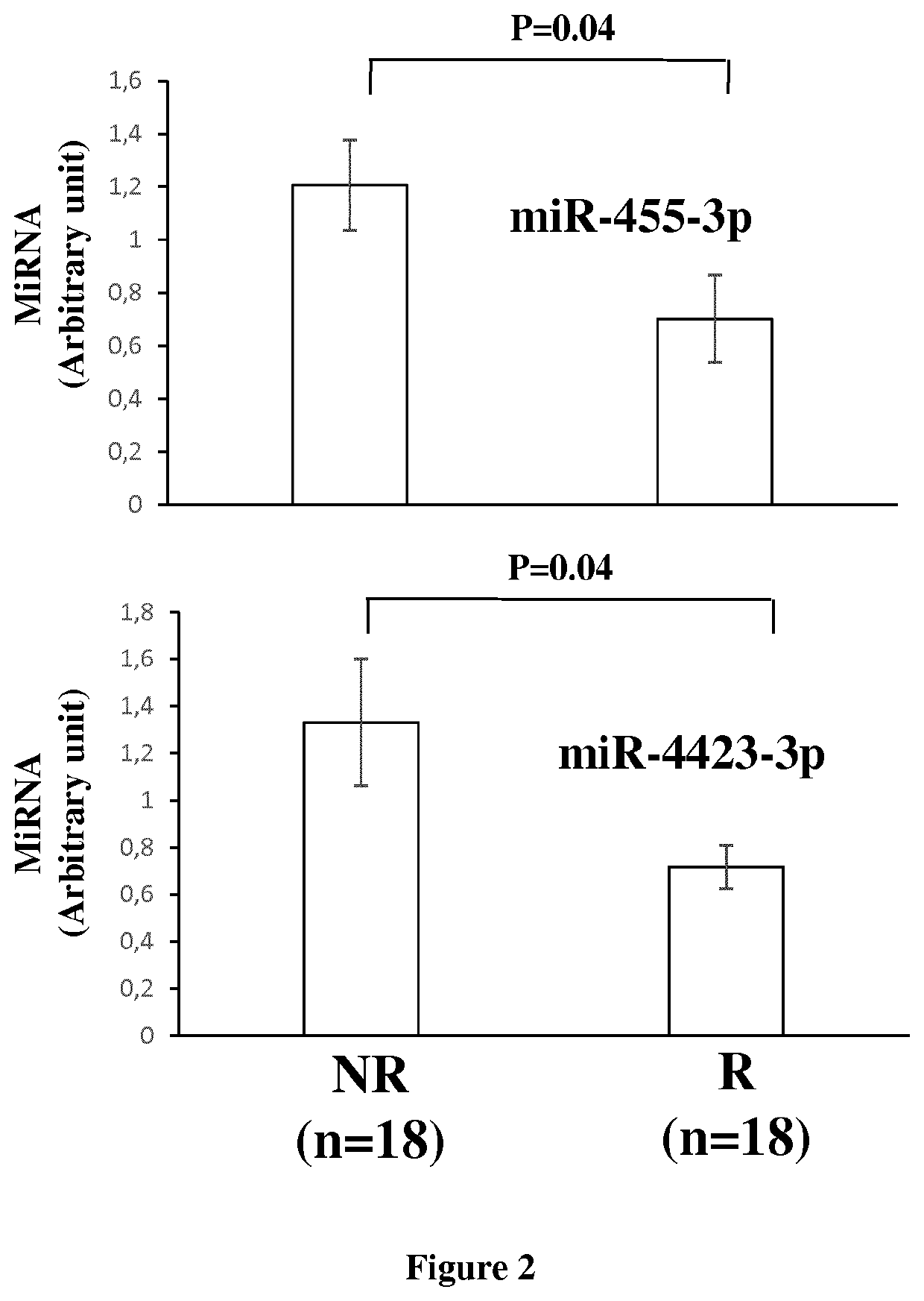
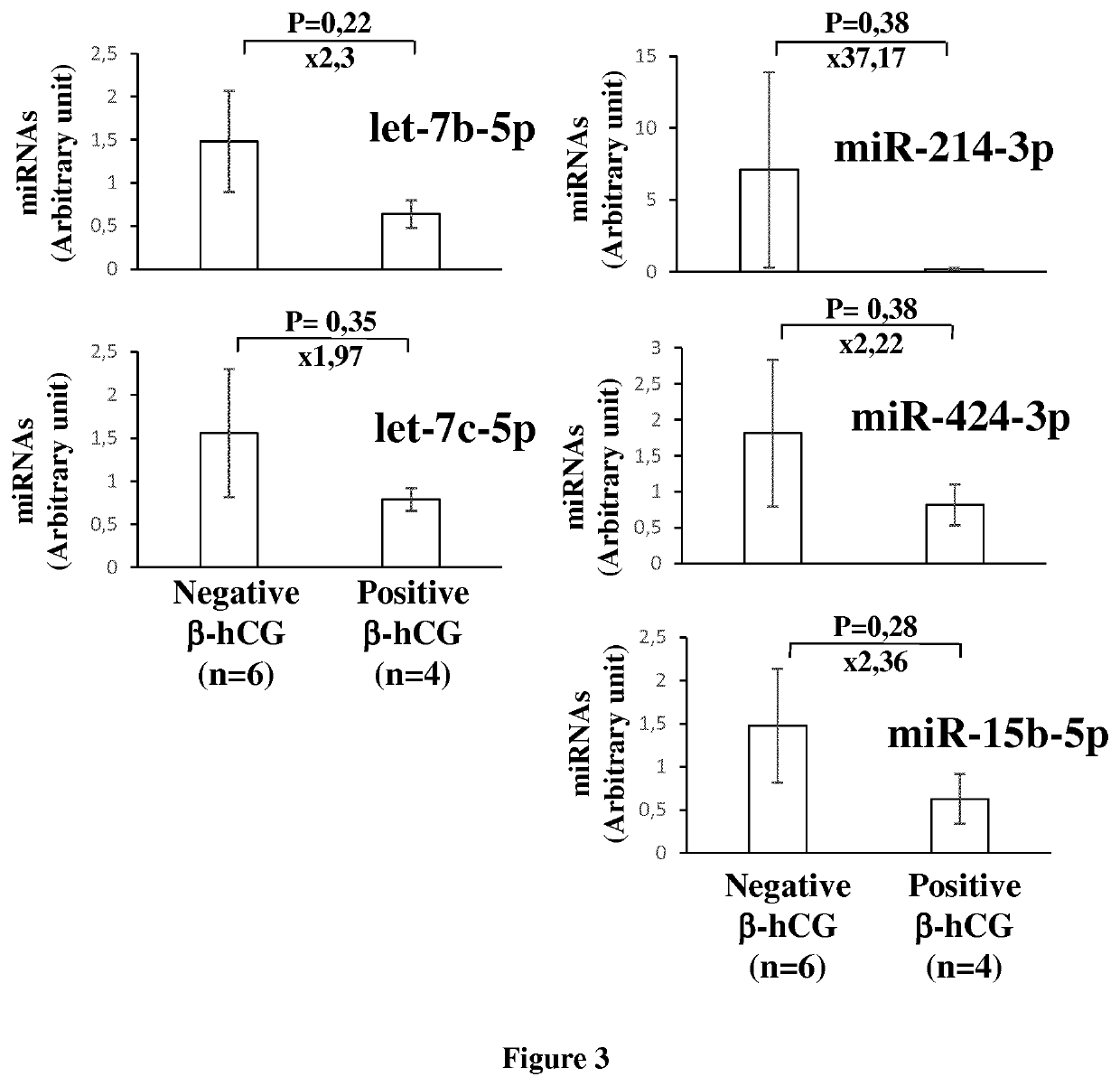
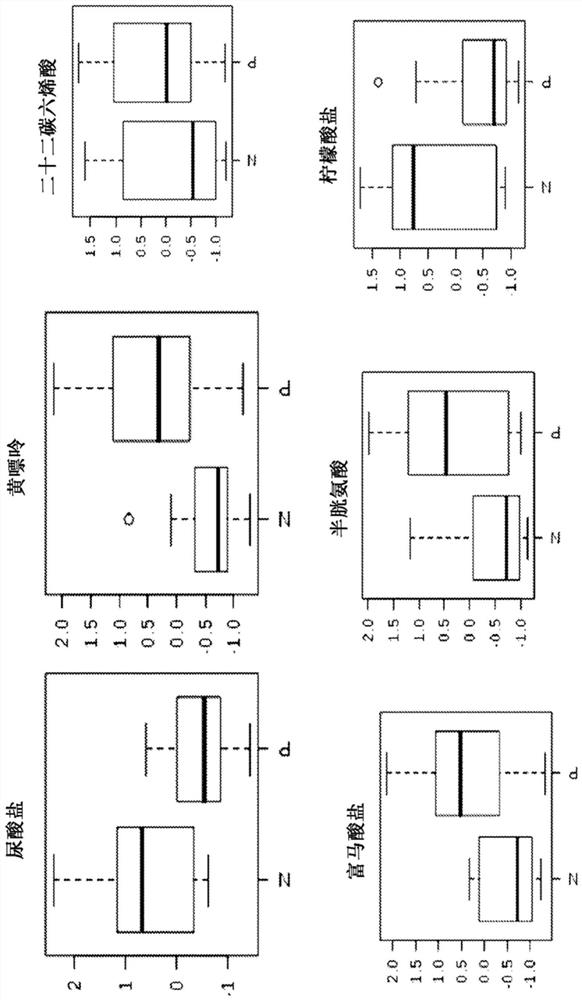
The invention relates to a method of assessing pregnancy outcome. The inventors investigated endometrial miRNAs associated with pregnancy outcome by studying miRNAs associated with endometrial receptivity, implantation failure and embryo miscarriage. They performed a miRNomic study to find miRNAs that are differentially expressed according to the endometrial receptivity status, compared the endometrial miRNome between receptive patients with negative beta-hCG and receptive patients with positive beta-hCG, and compared the endometrial miRNome between receptive patients with a miscarriage between 8-12 weeks of amenorrhoea and receptive patients with a live birth. They demonstrated miRNA differential expression in endometrial samples according to the pregnancy outcome. Thus, the invention relates to a method of assessing pregnancy outcome of a patient, comprising a step of measuring in a biological sample obtained from said patient the expression level of at least one miRNA selected from the group consisting of miR-455-3p, miR-4423-3p, miR-4445-3p, miR-3128, miR-3201, let-7b-5p, let-7c-5p, miR-4534, miR-214-3p, miR-15b-5p, miR-424-3p, miR-181a-5p, miR-574-3p, miR-92a-3p, miR-320c, let-7d-5p, miR-125a-5p, miR-320a, miR-320b and let-7f-5p.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
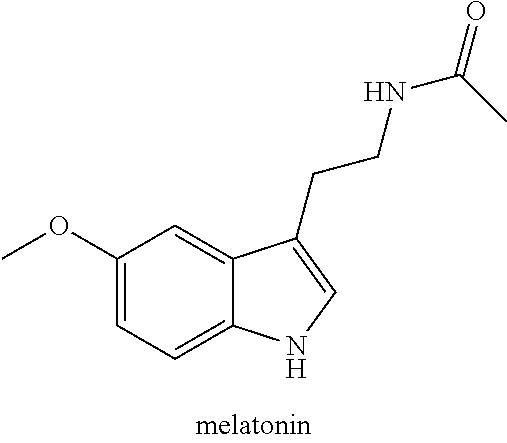
Use of N-acetyl-5-methoxytryptamine or analogues thereof, for promoting the mechanism of implantation of the embryo and related compositions and culture media
InactiveCN104334167AInhibit synthesisSmall shrinkageOrganic active ingredientsPharmaceutical delivery mechanismGynecologyN-Acetyl-5-methoxytryptamine
The present invention refers to the use of N-acetyl-5-methoxytryptamine (melatonin) and / or an analogue thereof, for use in the medical or veterinary field in the assisted reproduction for promoting the mechanism of implantation of the embryo, and in particular for the prevention of implantation failure into the uterus, by topical administration of an effective amount in a mammalian subject female in need of such treatment, and related compositions, culture media and medical devices.
Owner:ARES TRADING SA
Application of cyclosporine A in preparation of medicine for improving embryo implantation rate of patient suffering from repeated embryo implantation failure
ActiveCN113769065AImprove biological behaviorImprove planting rateCyclic peptide ingredientsSexual disorderBiotechnologyEmbryo transplantation
The invention discloses application of cyclosporine A in preparation of a medicine for improving the embryo implantation rate of a patient suffering from repeated embryo implantation failure. In the prior art, the cyclosporine A is used as an immunosuppressor or a medicine for preventing miscarriage and preventing abortion, the application proposes that the cyclosporine A can improve the embryo planting rate for the first time, and particularly discloses improvement of the embryo planting rate of secondary embryo transplantation of the patient suffering from the repeated embryo implantation failure, and the research result is different from that in the prior art. Specifically, according to the technical scheme, clinical application of the cyclosporine A can be effectively guided, and side effects caused by improper medication are avoided.
Owner:SUZHOU MUNICIPAL HOSPITAL
Biomarkers and agents for recurrent implant failure
PendingCN114107480AOrganic active ingredientsPeptide/protein ingredientsPhysiologyCorpus luteum graviditatis
The invention provides a biomarker and a medicament for recurrent implantation failure. The biomarker comprises one or more of pathway related genes with the G0 annotation being GO: 0030286, GO: 0003777, GO: 0007018, GO: 0016887, GO: 0005874, GO: 0005858, GO: 0060285 and GO: 0005871, or / and the KEGG annotation being hsa05016, and one or more of pathway related genes with the G0 annotation being GO: 0030286, GO: 0003777, GO: 0007018, GO: 0016887, GO: 0005874, GO: 0005858, GO: 0060285 and GO: The biomarkers are used for characterizing, diagnosing or classifying'subclinical hypopituitary hypofunction 'patients with repeated implantation failure and predicting the therapeutic reactivity of the patients with repeated implantation failure to luteal support therapy; the invention also provides a medicament for repeated implantation failure, a lutein therapy medicament and a luteinizing activity related medicament, the lutein therapy medicament is a medicament for regulating estrogen and / or progestational hormone, and the luteinizing activity related medicament is a medicament for regulating the estrogen and / or progestational hormone after embryo implantation by applying human chorionic gonadotropin. The repeated implantation failure object has the characteristics that (1) clinical pregnancy is not observed in continuous three or more times of high-quality embryo transplantation, (2) the pathogenesis of RIF is indefinite, and (3) the blood luteinizing hormone level is less than or equal to 5IU / L on the second day (D2 day) after ovulation.
Owner:中国人民解放军总医院第六医学中心
Application of apoptosis-related genes in repeated implantation failure
PendingCN113564242AExcellent diagnostic valueMicrobiological testing/measurementAbnormal expressionTreatment targets
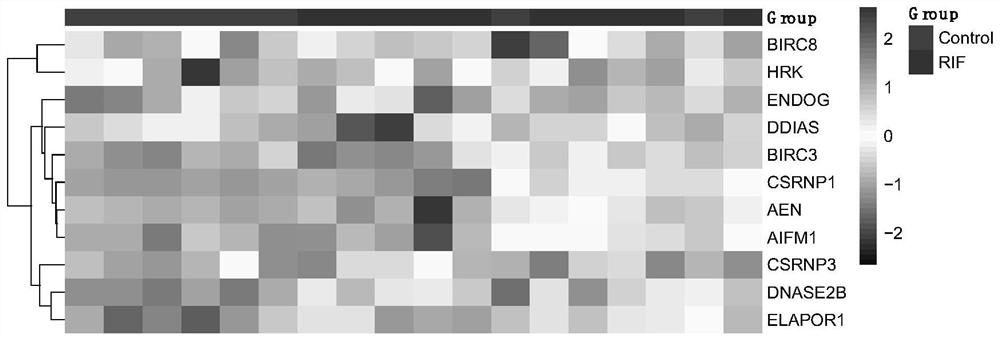
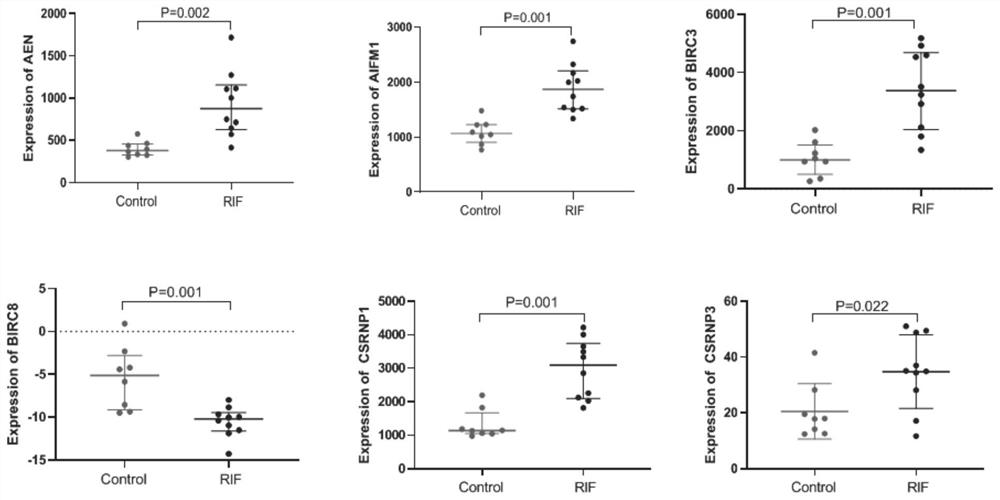
The invention provides application of apoptosis-related genes in repeated implantation failure, and belongs to the technical field of biological medicine and molecular biology. According to the application of the apoptosis-related genes in repeated implantation failure, endometrial RNA-seq related data are called from a GEO database, a series of differential genes are screened out from a GSE92324 queue, finally, it is determined that abnormal expression of three apoptosis related genes, namely, CSRNP1, BIRC8 and ELAPOR1 is closely related to repeated implantation failure, verification is carried out through a verification queue (GSE71835), the apoptosis-related genes are proved to have a good diagnostic value for the repeated implantation failure, so that the apoptosis-related genes can be used as prediction and diagnosis markers and potential treatment targets of the repeated implantation failure, plays an important role in diagnosis and treatment of the repeated implantation failure, and has a good practical popularization and application value.
Owner:SHANDONG UNIV QILU HOSPITAL
Detection method for embryo chromosomes of embryo culture solution at blastomere stage
PendingCN111172259AAvoid the sample loss problemAvoid lossMicrobiological testing/measurementMidblastulaZoology
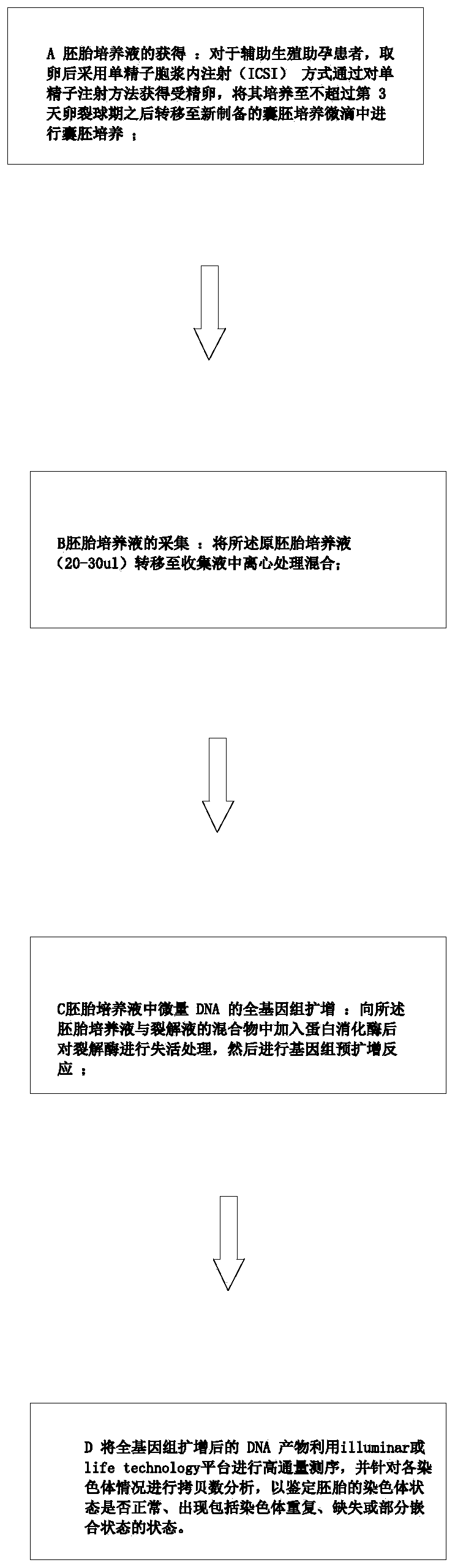
The invention discloses a detection method for embryo chromosomes of an embryo culture solution at the blastomere stage. The embryo culture solution is collected by fertilizing eggs in an ICSI mannerwithout any damage to embryos, the problem of possible sample biopsy trauma in the PGS (preimplantation genetic screening) process is effectively solved, operation is simple, convenient and easy to carry out; and the detection method is suitable for people with high incidence of embryo chromosome abnormality, such as high age, repeated abortion, repeated implantation failure, severe oligozoospermia, chromosome abnormality and the like. According to the detection method, chromosome detection can be carried out at the early stage of embryo culture and prior to the blastula stage, and detection time is brought forward by two days.
Owner:FIRST PEOPLES HOSPITAL OF YUNNAN PROVINCE +1
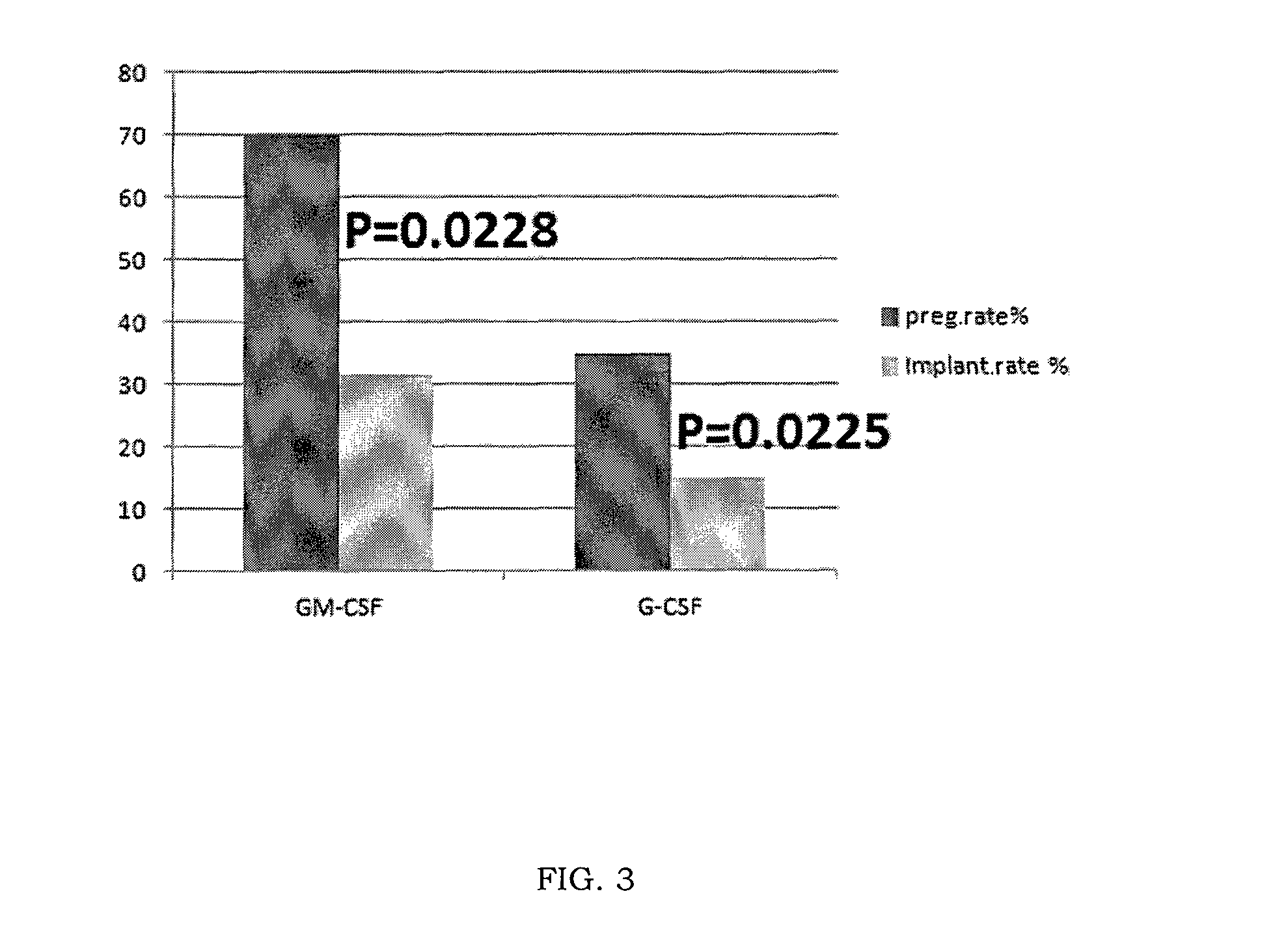
Methods and kits for reducing the likelihood of implantation failure and pregnancy-related disorders in recipients of artificial insemination
InactiveUS20110097382A1Avoid miscarriagePrevent implantation failureOrganic active ingredientsPeptide/protein ingredientsDiseaseGynecology
Methods and kits for preventing or reducing the likelihood of implantation failure and pregnancy-related disorders in a recipient of artificial insemination are provided. The methods include administering into a recipient of artificial insemination in need of such treatment an effective amount of granulocyte colony stimulating factor (G-CSF).
Owner:NORA THERAPEUTICS
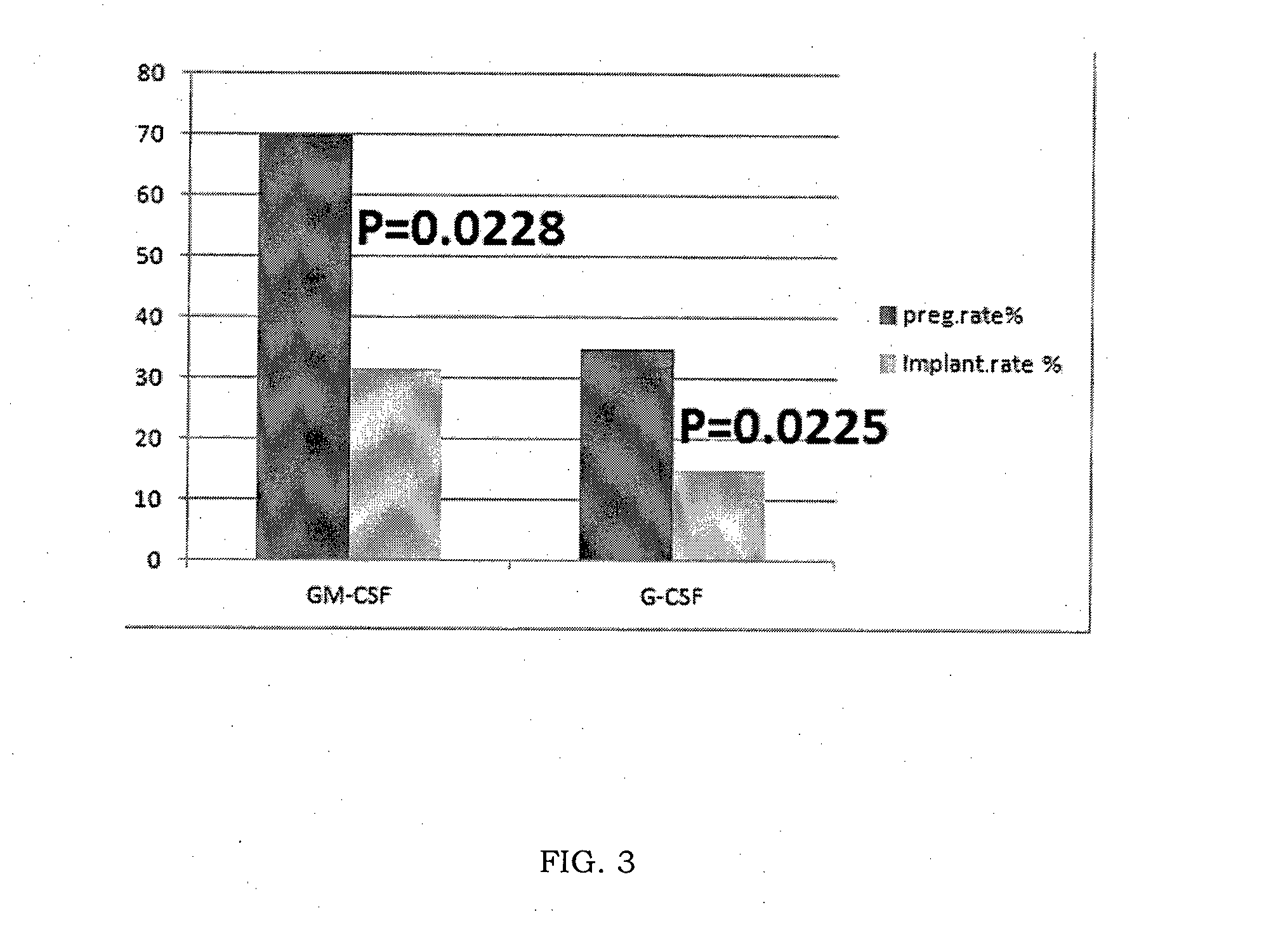
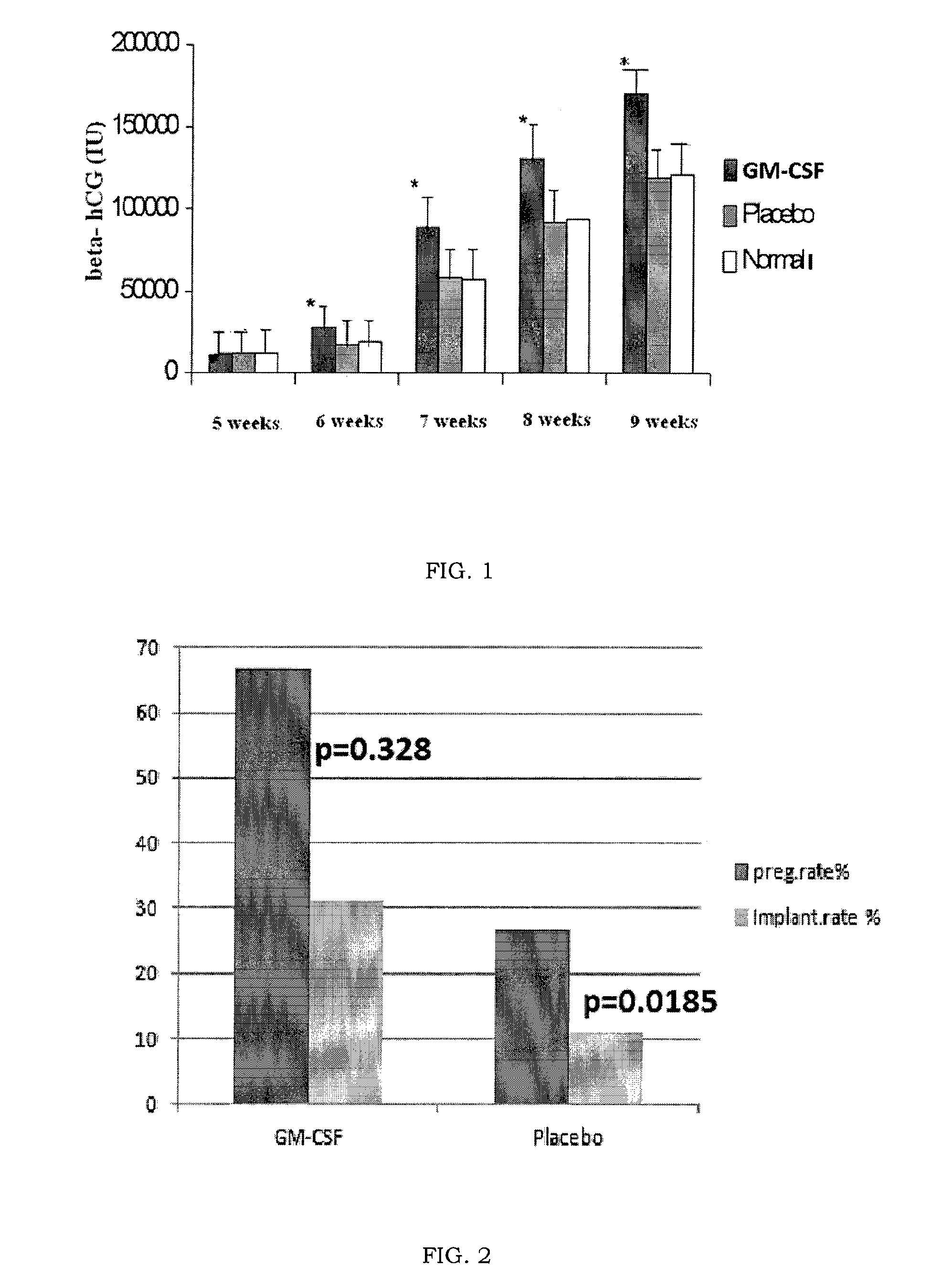
Gm-csf for use in the prevention of spontaneous abortion and embryo implantation failure
ActiveUS20150306180A1Reduction in spontaneous abortion rateAvoid problemsPeptide/protein ingredientsSexual disorderRecurrent miscarriageGynecology
The invention relates to GM-CSF for use in a method for the prevention of spontaneous abortion in a subject suffering from recurrent miscarriage, comprising administering to said subject an effective amount of GM-CSF as sole active substance, wherein said method also prevents or reduces the likelihood of embryo implantation failure in the subject undergoing an assisted reproduction procedure.
Owner:SCARPELLINI FABIO +1
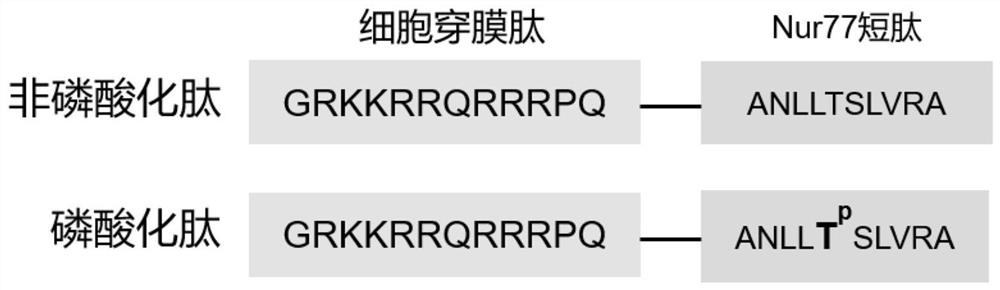
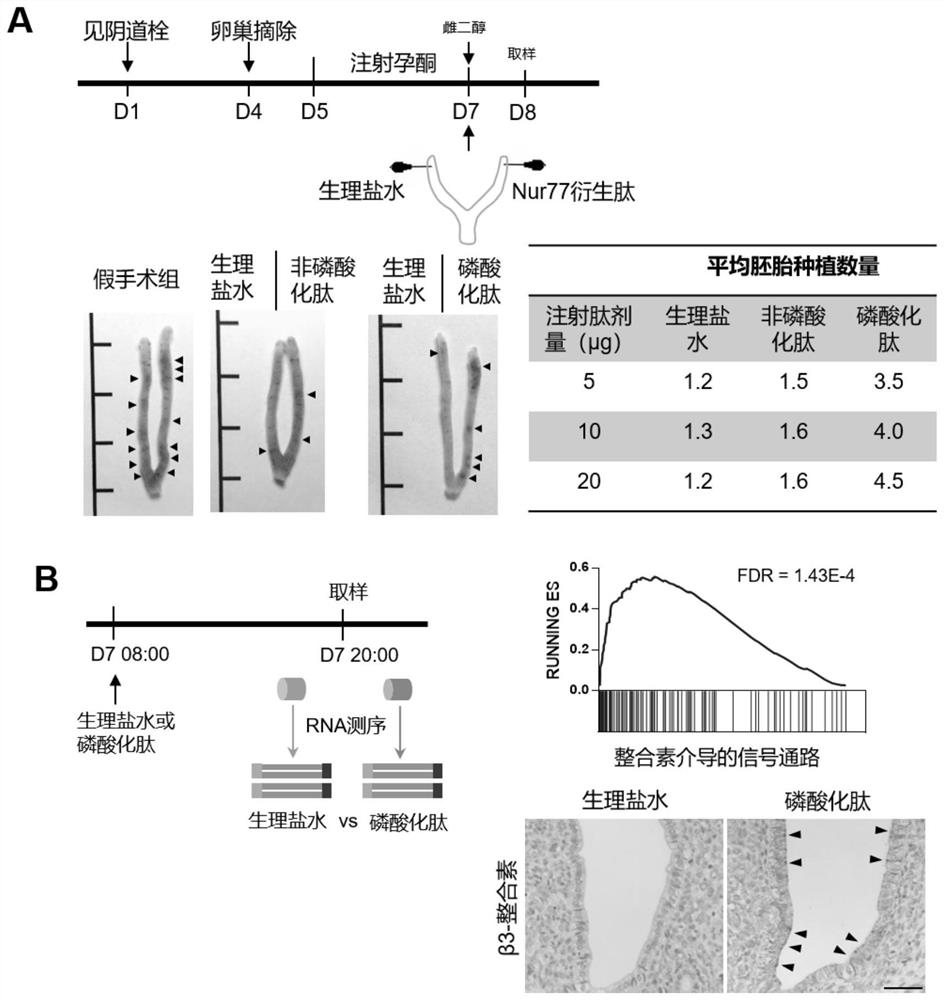
Nur77 phosphorylated derived peptide and application thereof in preparation of medicine for promoting embryo implantation
ActiveCN114426573AEasy to implantImprove adhesionPeptide/protein ingredientsImmunological disordersUterine epitheliumCell adhesion
The invention discloses an Nur77 phosphorylated derived peptide and application thereof in preparation of a medicine for promoting embryo implantation. The sequence of the phosphorylated derived peptide is shown as SEQ ID NO.1. Through a mouse in-vitro embryo adhesion test, an in-vivo epithelium-embryo adhesion test for delaying implantation into a mouse model and correlational research on the molecular cell level, the Nur77 phosphorylated derived peptide can be used for preparing a medicine for promoting embryo implantation. It is proved that the Nur77 phosphorylated derived peptide can enhance the uterine epithelium-embryo adhesion effect by promoting the beta 3 integrin mediated cell adhesion effect, so that embryo implantation is promoted; the medicine prepared by using the phosphorylated derived peptide can improve repeated implantation failure (RIF) caused by unknown reasons of women.
Owner:NANJING DRUM TOWER HOSPITAL
Use of N-acetyl-5-methoxytryptamine, and related compositions and culture media
InactiveCN111035637AInhibit synthesisSmall shrinkageOrganic active ingredientsEnemata/irrigatorsUterusZoology
The present invention refers to the use of N-acetyl-5-methoxytryptamine (melatonin) and / or an analogue thereof, for use in the medical or veterinary field in the assisted reproduction for promoting the mechanism of implantation of the embryo, and in particular for the prevention of implantation failure into the uterus, by topical administration of an effective amount in a mammalian subject femalein need of such treatment, and related compositions, culture media and medical devices.
Owner:ARES TRADING SA
Application of CFTR enhancer in medicine for predicting and treating recurrent abortion
ActiveCN114073768AAchieve forecastingFluorescence/phosphorescenceSexual disorderRecurrent AbortionsDisease
The invention discloses a method for measuring intracellular chlorine concentration or chloride channel gene CFTR expression of human endometrial stromal cells in a menstrual cycle so as to predict prognosis of endometrial deciduation related diseases of the human endometrial stromal cells. Intracellular chlorine concentration up-regulation and chlorine channel gene CFTR expression down-regulation in endometrial stromal cells in a menstrual cycle can hinder deciduation of the endometrial cells, and finally, repeated abortion / repeated implantation failure is caused. Restoration of CFTR expression and / or reduction of chloride ion concentration in endometrial stromal cells in a menstrual cycle can improve uterine decidualization and improve the outcome of recurrent abortion / recurrent implantation failure. The invention develops a new field for clinical prediction and treatment of recurrent abortion / recurrent implantation failure, and has a good application prospect.
Owner:SUN YAT SEN UNIV
Oxytocin antagonist dosing regimens for promoting embryo implantation and preventing miscarriage
PendingUS20190247361A1Enhance endometrial receptivityReduce the possibilityPeptide/protein ingredientsMammal material medical ingredientsRegimenMiscarriage
The invention provides compositions and methods for the use of oxytocin antagonists, such as substituted pyrrolidin-3-one oxime derivatives, among other compounds, in the treatment of subjects undergoing embryo transfer therapy. The compositions and methods of the invention can be used to dose subjects with oxytocin antagonists, including (3Z,55)-5-(hydroxymethyl)-1-[(2′-methyl-1,1′-biphenyl-4-yl)carbonyl]pyrrolidin-3-one O-methyloxime, among others, so as to improve endometrial receptivity and reduce the likelihood of embryo implantation failure and miscarriage following, for example, in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) embryo transfer procedures.
Owner:OBSEVA
Topical liquid composition comprising melatonin
InactiveUS20180021308A1Easy to optimizeImproving embryonic implantationOrganic active ingredientsInorganic non-active ingredientsPhysiologyImplantation failure
The present invention relates to a novel melatonin-based formulation, particularly a topical liquid composition of melatonin (or analog thereof) suitable for use in intrauterine washing performed during medically assisted reproduction (e.g. in vitro fertilisation—IVF). Melatonin is notoriously unstable, especially in solution. The compositions of the invention exhibit high stability, which allows them to be kept for prolonged periods before their eventual use in the inhibition or prevention of embryonic implantation failure.
Owner:ARES TRADING SA
Carrier status of annexin A5 M2 haplotype and obstetric risks
ActiveUS10925891B2Increase ratingsReduce riskOrganic active ingredientsMicrobiological testing/measurementPhysiologyFoetal growth
The present invention relates to determining the carrier status of Annexin A5 M2 haplotype of parents (both male and female) prior to and / or after pregnancy to minimize the risk of pregnancy complications, including, but not limited to, recurrent pregnancy loss (RPL), infertility, miscarriage, in vitro fertilization (IVF) failure, IUI failure, implantation failure, foetal growth restriction (FGR), small for gestational age (SGA) newborn, intra-uterine foetal death (IUFD), gestational hypertension (GH), pre-eclampsia (PE) and / or venous thromboembolism (VTE). Once M2 carrier status is determined, methods of intervention, including administration of low molecular weight heparin (LMWH) and / or other anti-coagulants can be administered either prior to and / or after pregnancy. Methods of detecting the carrier status as well as method of diagnosing and or predicting prognosis based on the M2 carrier status of a patient and / or couple is also contemplated.
Owner:IHG PHARMACO LTD
Uterine endometrial fluid for prediction of success in fertility treatment
Provided herein are methods, systems, and kits for improving success rates in assisted reproductive technologies such as in vitro fertilization, frozen embryo transfer, and intrauterine insemination. These methods, systems, and kits rely on levels of protein, metabolite, and microRNA markers determined herein to be linked to uterine toxicity and embryo implantation failure.
Owner:FERTILITY LAB SCI
GM-CSF for use in the prevention of spontaneous abortion and embryo implantation failure
ActiveUS9522172B2Avoid problemsPrevents orPeptide/protein ingredientsSexual disorderRecurrent miscarriageGynecology
The invention relates to GM-CSF for use in a method for the prevention of spontaneous abortion in a subject suffering from recurrent miscarriage, comprising administering to said subject an effective amount of GM-CSF as sole active substance, wherein said method also prevents or reduces the likelihood of embryo implantation failure in the subject undergoing an assisted reproduction procedure.
Owner:SCARPELLINI FABIO +1
Method for preventing sundry fungus infection in fully-open edible fungus inoculation
InactiveCN110024625AImprove survival rateAvoid Planting FailureCultivating equipmentsMushroom cultivationBiotechnologyPollution
The invention relates to the technical field of edible fungus inoculation, and discloses a method for preventing sundry fungus infection in fully-open edible fungus inoculation. The method includes the steps: S1 filling special cultivation bags with various raw and auxiliary materials needed by cultivation, and sterilizing the raw and auxiliary materials by a pressure cooker at the temperature of100 DEG C to form a cultivation bag collectively referred to cultivation materials; S2 before a cooker door is opened, cleaning and sterilizing transportation tools, cleaning personal hygiene of workers, performing atomized dust settling on front space of a steam pot door, cleaning the ground to remove dust to reach a dustless and aseptic safety cooling standard, instantly covering surface layersof fungus bags after the fungus bags are stacked to prevent dust from directly falling onto the fungus bags, transporting the fungus bags discharged from the cooker in a covering manner, and performing pot translation and transportation at the temperature when the temperature of the bags falls to 70 DEG C. The method for preventing sundry fungus infection in the fully-open edible fungus inoculation can solve the problem of implantation failure caused by pollution of sundry fungi without successful operating procedures and technical measures in the prior art.
Owner:辛集市万隆食用菌有限责任公司
Biomarkers and methods of diagnosis and treatment for recurrent implant failure
PendingCN114292907AOrganic active ingredientsPeptide/protein ingredientsImplant failureBiologic marker
The present invention relates to methods of luteal support therapy for recurrent implantation failure, as well as methods and biomarkers for predicting the responsiveness of luteal support therapy for recurrent implantation failure patients.
Owner:YIKON GENOMICS (SUZHOU) CO LTD +1
Topical liquid composition comprising melatonin
InactiveUS10188631B2Easy to implantImproving embryonic implantationOrganic active ingredientsInorganic non-active ingredientsPhysiologyImplantation failure
Owner:ARES TRADING SA
Methods for assessing pregnancy outcome
The invention relates to a method of assessing pregnancy outcome. The inventors investigated endometrial miRNAs associated with pregnancy outcome by studying miRNAs associated with endometrial receptivity, implantation failure and embryo miscarriage. They performed a miRNomic study to find miRNAs that are differentially expressed according to the endometrial receptivity status, compared the endometrial miRNome between receptive patients with negative beta-hCG and receptive patients with positive beta-hCG, and compared the endometrial miRNome between receptive patients with a miscarriage between 8-12 weeks of amenorrhoea and receptive patients with a live birth. They demonstrated miRNA differential expression in endometrial samples according to the pregnancy outcome. Thus, the invention relates to a method of assessing pregnancy outcome of a patient, comprising a step of measuring in a biological sample obtained from the patient the expression level of at least one miRNA selected from the group consisting of miR-455-3 p, miR-4423-3p, miR-4445-3p, miR-3128, miR-3201, let-7b-5p, let-7c-5p, miR-4534, miR-214-3p, miR-15b-5p, miR-424-3p, miR-181a-5p, miR-574-3p, miR-92a-3 p, miR-320c, let-7d-5p, miR-125a-5p, miR-320a, miR-320b and let-7f-5p.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
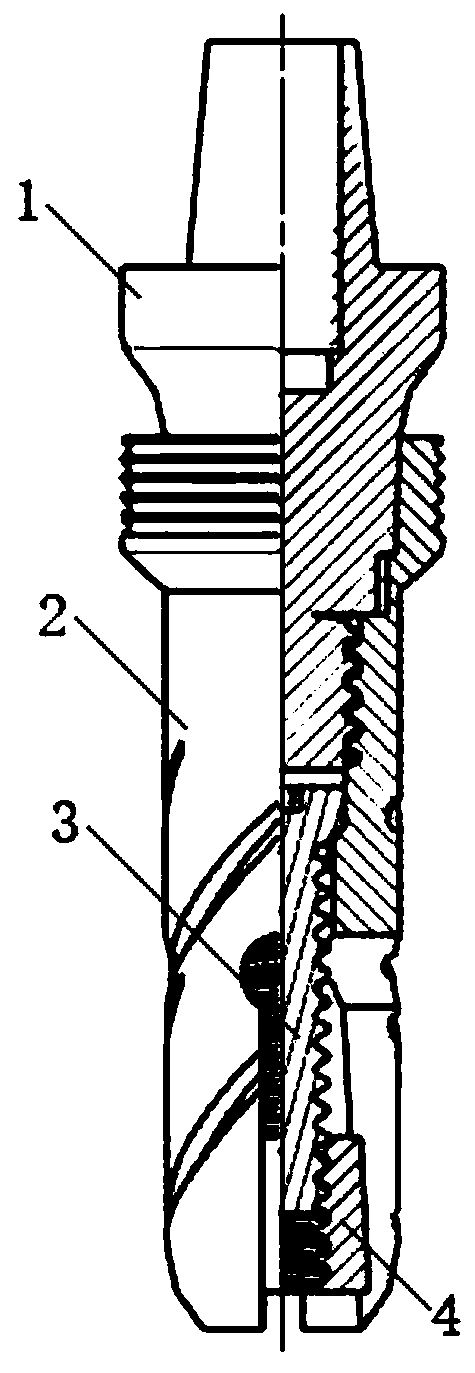
Inclined wedge-type expansion-type dental implantation device with expansion block
The invention relates to an inclined wedge-type expansion-type dental implantation device with an expansion block. The device comprises a base station with threads, an implant, an adjusting screw rodand an expansion block, wherein the interior of the implant is provided with an expansion conical hole penetrating the lower end of the implant, and the interior of the implant is also provided with ascrew rod mounting hole communicated with the expansion conical hole; an expansion cut groove and a micro cut groove are distributed from the lower end to the neck of the implant; the adjusting screwis connected to a threaded base and inserted into the screw rod mounting hole of the implant, and the expansion block is embedded into the expansion conical hole of the implant through the adjustingscrew of the adjusting screw rod. The device is simple in structure and good in implantation effect. The expansion block and the adjusting screw are mutually matched, and the expansion block move upwards along the central axis of the implant so as to achieve the effect of expanding the implant; and the contact area between the implant and the bone is improved through the micro cut groove, so thatthe secondary stability is improved, the implantation failure rate can be effectively reduced, the implantation efficiency is improved, on the other hand, the friction force between the implant and the bone can be improved, and the implant is prevented from loosening in the initial stage of implantation.
Owner:WUYI UNIV
Method for increasing embryo implantation rate in a female subject suffering polycystic ovary syndrome
ActiveUS20200330495A1Improve pregnancy rateReduce riskHydroxy compound active ingredientsCapsule deliverySoftgelPhysiology
The present invention relates to methods for increasing embryo implantation rate in an uterus, for preventing embryo implantation failure in a female subject suffering PCOS and for improving pregnancy rate comprising administering a composition comprising myo-inositol and D-chiro-Inositol in a weight ratio between 1:1 to 9:1 respectively to a female subject suffering PCOS. The invention also relates to a composition comprising myo-inositol and D-chiro-Inositol in a weight ratio between 1:1 to 9: lrespectively for use in the treatment or prevention of PCOS in a female subject, for use in the treatment or prevention of infertility in a female subject suffering polycystic ovary syndrome, or for use in preventing or reducing the risk of ovarian hyperstimulation syndrome in a female subject suffering polycystic ovary syndrome and subjected to ovary stimulation treatment. The invention also relates to a soft capsule comprising a) a soft capsule shell and b) a pharmaceutical composition comprising myo-inositol and D-chiro-Inositol in a weight ratio between 1:1 to 9:1 respectively.
Owner:BIOSEARCH SA
Carrier status of annexin a5 m2 haplotype and obstetric risks
ActiveUS20170027981A1Increase ratingsReduce riskOrganic active ingredientsMicrobiological testing/measurementFoetal growthIntrauterine fetal demise
The present invention relates to determining the carrier status of Annexin A5 M2 haplotype of parents (both male and female) prior to and / or after pregnancy to minimize the risk of pregnancy complications, including, but not limited to, recurrent pregnancy loss (RPL), infertility, miscarriage, in vitro fertilization (IVF) failure, IUI failure, implantation failure, foetal growth restriction (FGR), small for gestational age (SGA) newborn, intra-uterine foetal death (IUFD), gestational hypertension (GH), pre-eclampsia (PE) and / or venous thromboembolism (VTE). Once M2 carrier status is determined, methods of intervention, including administration of low molecular weight heparin (LMWH) and / or other anti-coagulants can be administered either prior to and / or after pregnancy. Methods of detecting the carrier status as well as method of diagnosing and or predicting prognosis based on the M2 carrier status of a patient and / or couple is also contemplated.
Owner:IHG PHARMACO LTD
Combined application of sequential infusion CD19 CAR-T and BCMA CAR-T cells in immune-mediated platelet infusion invalidation of acute leukemia patients
PendingCN114558126AInfusion invalidInfusion ImprovementAntibody medical ingredientsAntineoplastic agentsAllo hsctWhole blood product
The invention discloses a combined application of sequential infusion of CD19 CAR-T and BCMA CAR-T cells in immune-mediated platelet infusion invalidation (PTR) of acute leukemia patients, and is characterized in that the combined application is as follows: the infusion dose of the CD19 CAR-T cells is (1.0-2.0) * 10 < 7 > / kg, the infusion dose of the BCMA CAR-T cells is (1.0-2.0) * 10 < 7 > / kg, the infusion dose of the BCMA CAR-T cells is (1.0-2.0) * 10 < 7 > / kg, and the infusion dose of the BCMA CAR-T cells is (1.0-2.0) * 10 < 7 > / kg. According to the BCMA CAR-T cells, the infusion dosage is (1.0-2.0) * 10 < 7 > / kg. The CD19 CAR-T and BCMA CAR-T cells are sequentially infused, so that the platelet infusion ineffectiveness of an immune-mediated PTR patient is improved, the platelet usage amount is reduced, and the death risk caused by bleeding is reduced; the average hospitalization day is shortened; the economic burden of patients is relieved; the social problem that blood product resources are seriously deficient is relieved; meanwhile, the generation of HLA related antibodies and donor specific HLA antibodies can be reduced, the death possibility of patients caused by major hemorrhage complications caused by hemorrhage in subsequent chemotherapy and transplantation due to invalid platelet infusion is reduced, and implantation failure after allogenic hematopoietic stem cell transplantation is prevented.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV +1
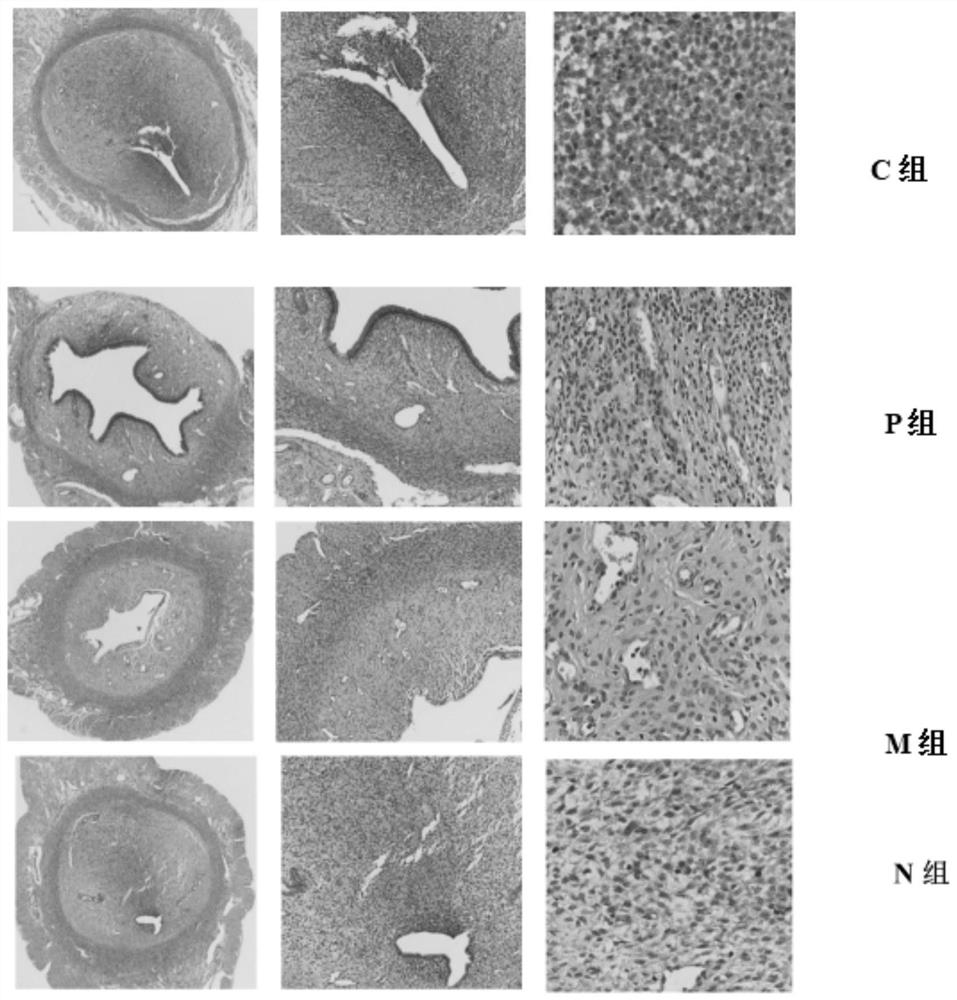
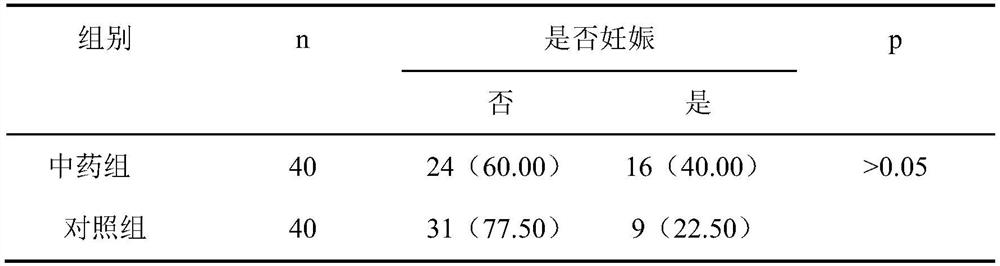
A traditional Chinese medicine composition for treating embryo recurrent implantation failure diseases and its application
ActiveCN112546118BNot easy to skidImprove microcirculationSexual disorderAlkali/alkaline-earth metal chloride active ingredientsBiotechnologyAngelica Sinensis Root
The invention relates to a traditional Chinese medicine composition for treating the disease of repeated embryo implantation failure. The traditional Chinese medicine composition is made of the following raw materials in parts by weight: 29-31 parts of amethyst, 14-16 parts of dodder, and 14 parts of rehmannia glutinosa ‑16 parts, Millipeda 11‑13 parts, Sangji 14‑16 parts, Chishao 8‑10 parts, Angelica 14‑16 parts, Baishao 14‑16 parts, Chuanxiong 8‑10 parts, Atractylodes macrocephala 14‑16 parts , Codonopsis 14‑16 parts, Poria cocos 14‑16 parts, Eucommia 11‑13 parts, Dipsacus 11‑13 parts, tangerine peel 8‑10 parts. The invention also provides the application of the traditional Chinese medicine composition. The traditional Chinese medicine composition of the invention has the advantages of fast curative effect, short treatment period and remarkable effect on the disease of repeated embryo implantation failure; in addition, it has no toxic and side effects on human body.
Owner:SHANGHAI FIRST MATERNITY & INFANT HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com