Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
109results about How to "Prevents or" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
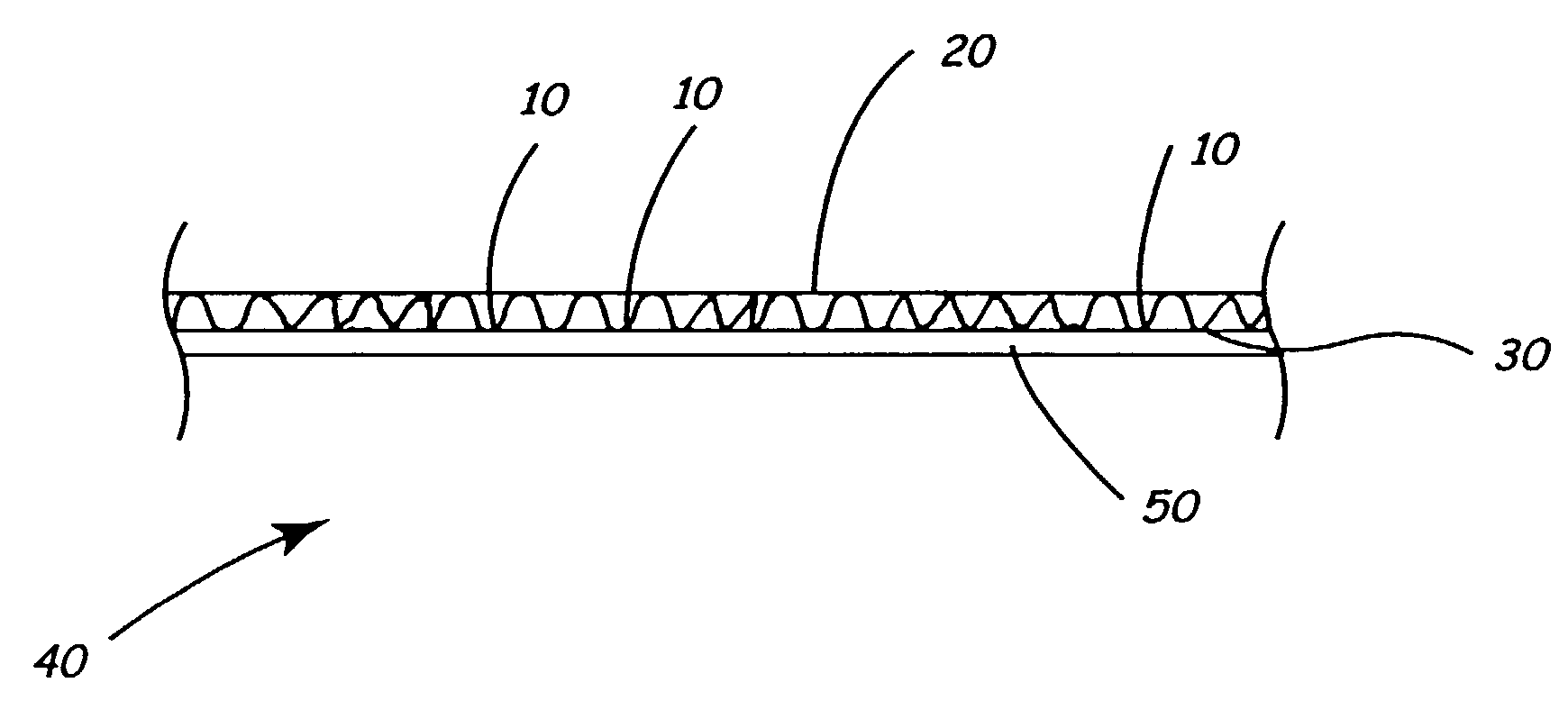
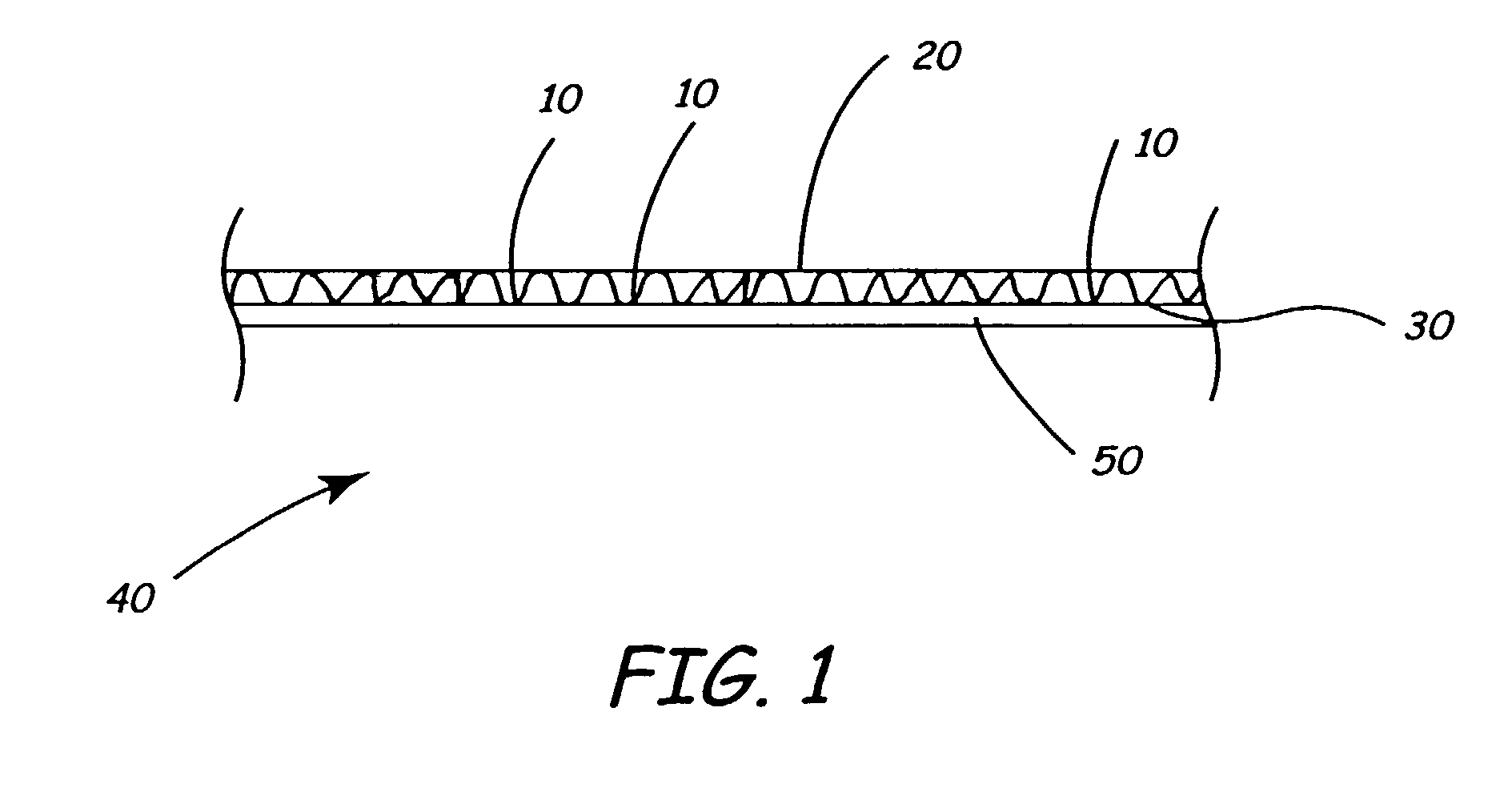
Screen protector
InactiveUS7070837B2Prevents orMinimize formationLiquid crystal compositionsAdhesive processesRefractive indexEngineering
A plastic film screen protector that prevents interference patterns from arising when the film touches the screen is described. The advantages are accomplished by the film having a slightly roughened surface so that the majority of the film facing an electronic device screen does not substantially touch the screen. These physical aberrations prevent Newton ring interference patterns and spots caused by refractive index differences between air and the film material.
Owner:ROSS MARK
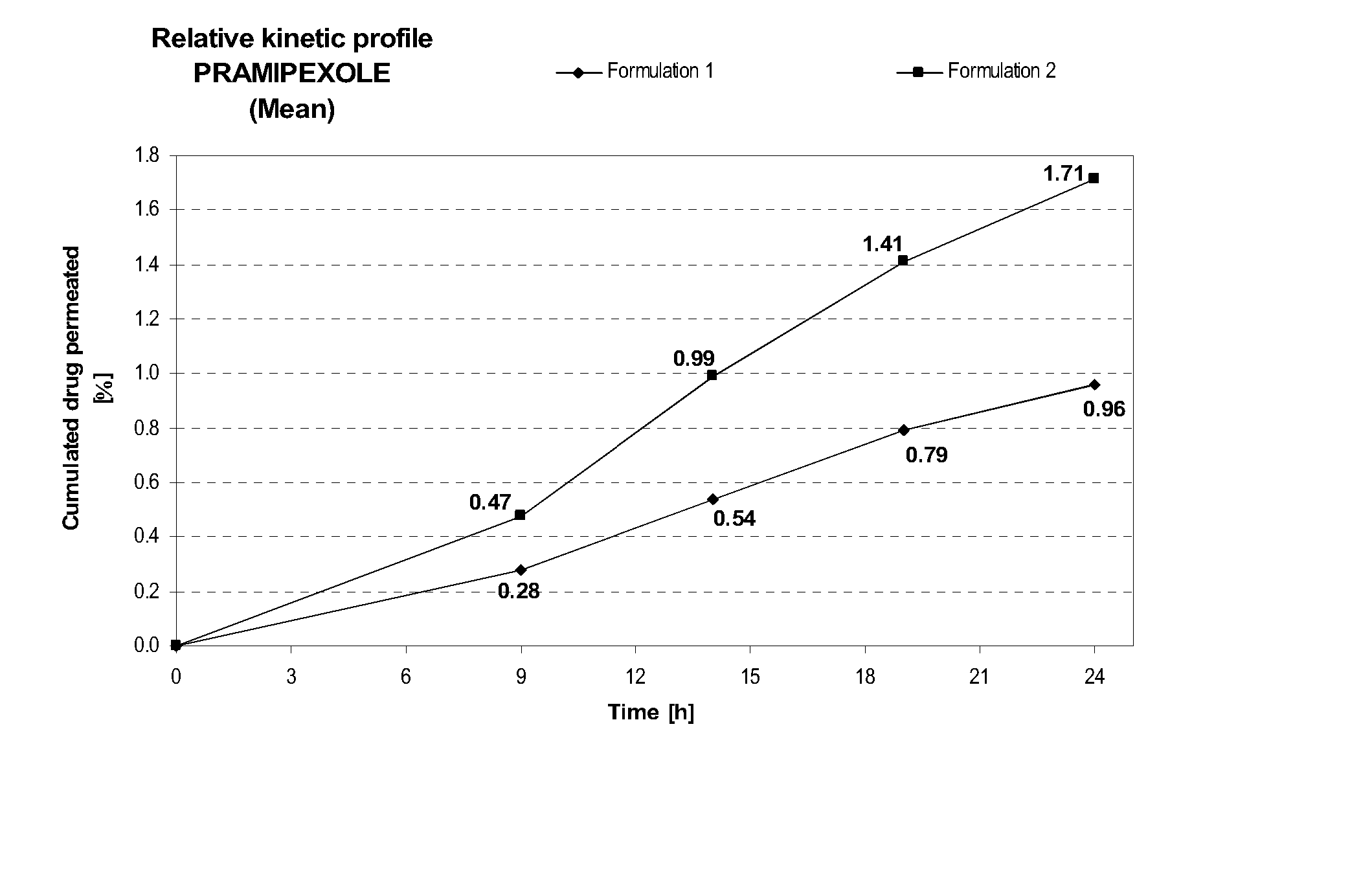
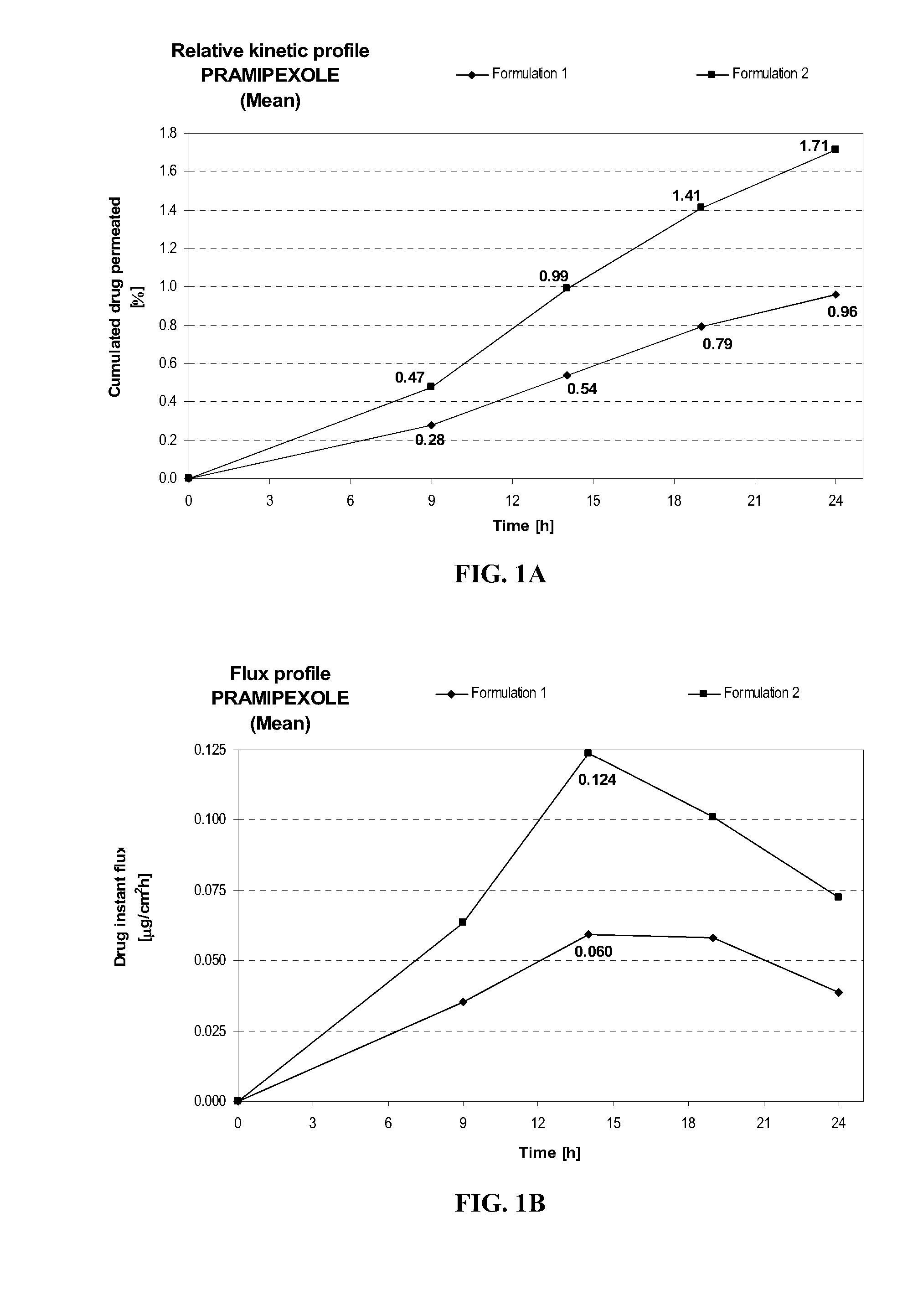
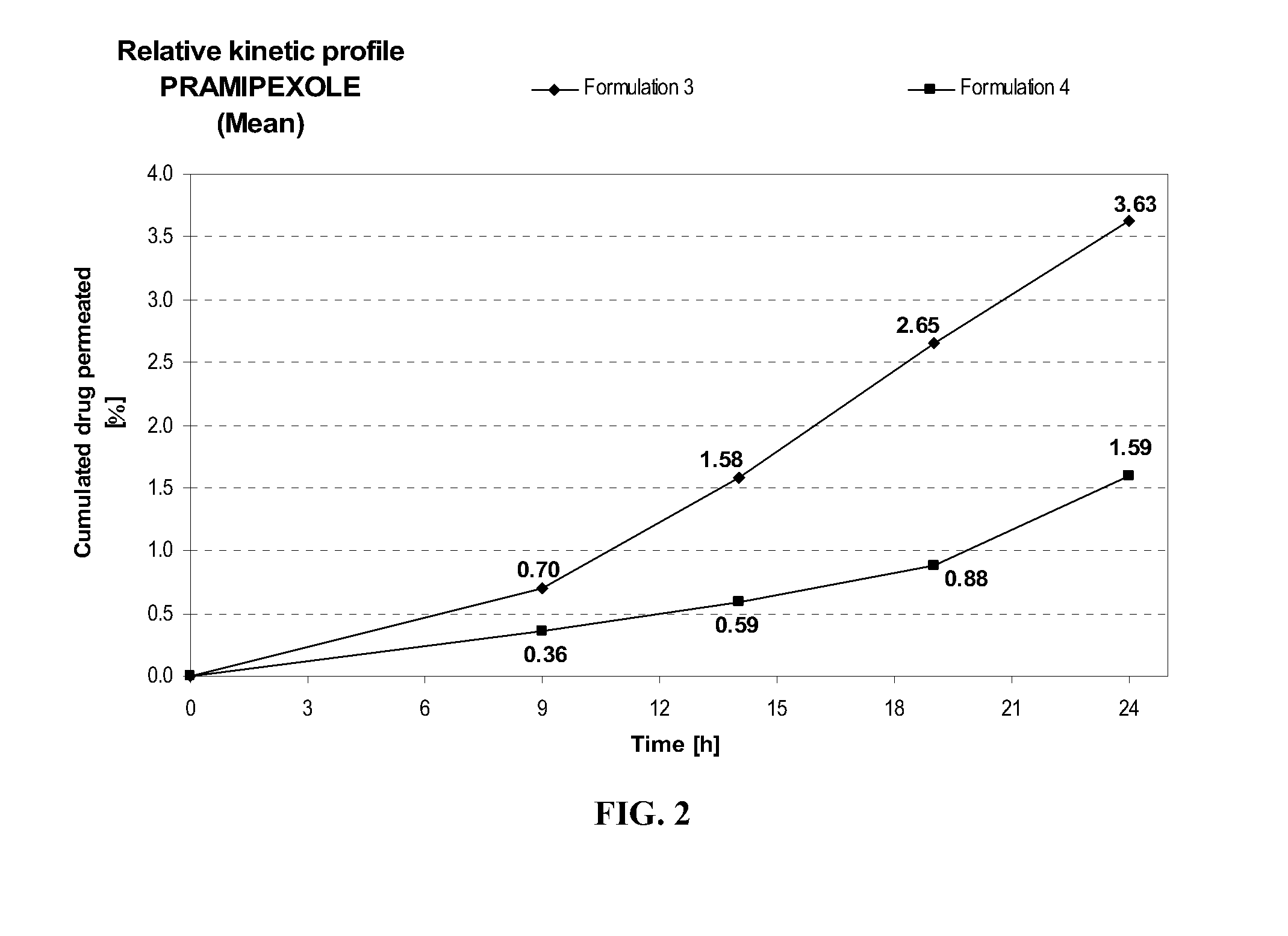
Transdermal delivery of systemically active central nervous system drugs
InactiveUS20070225379A1Reduce transferLoss of therapyBiocideOrganic non-active ingredientsNervous systemActive agent
The invention relates to a transdermal or transmucosal non-occlusive, semi-solid pharmaceutical formulation that includes at least one systemically active agent that acts on the Central Nervous System (CNS) of a mammal; and a permeation enhancing solvent system present in an amount sufficient to solubilize the at least one active ingredient. The permeation enhancing solvent system includes a pharmaceutically acceptable monoalkyl ether of diethylene glycol; a pharmaceutically acceptable glycol; preferably also a fatty alcohol and or a fatty acid; and a mixture of a C2 to C4 alcohol and water so that the permeation enhancing solvent system (a) inhibits crystallization of the at least one active ingredient on a skin or mucosal surface of a mammal, (b) reduces or prevents transfer of the formulation to clothing or to another being, (c) modulates biodistribution of the at least one active agent within different layers of skin, (d) facilitates absorption of the at least one active agent by a skin or a mucosal surface of a mammal, or (e) provides a combination of one or more of (a) through (d).
Owner:ANTARES PHARMA IPL
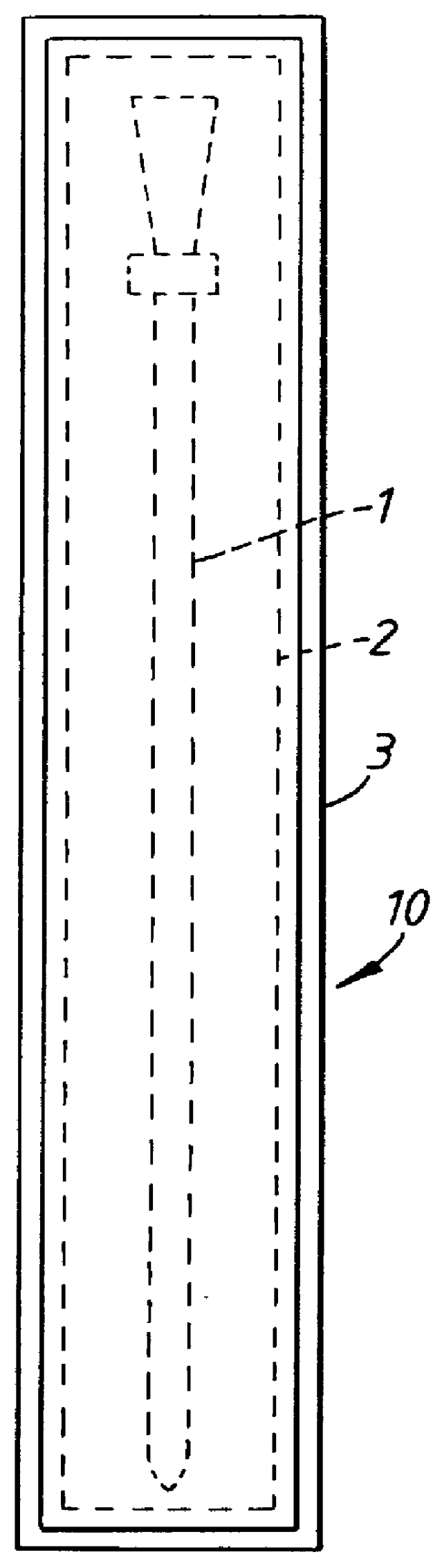
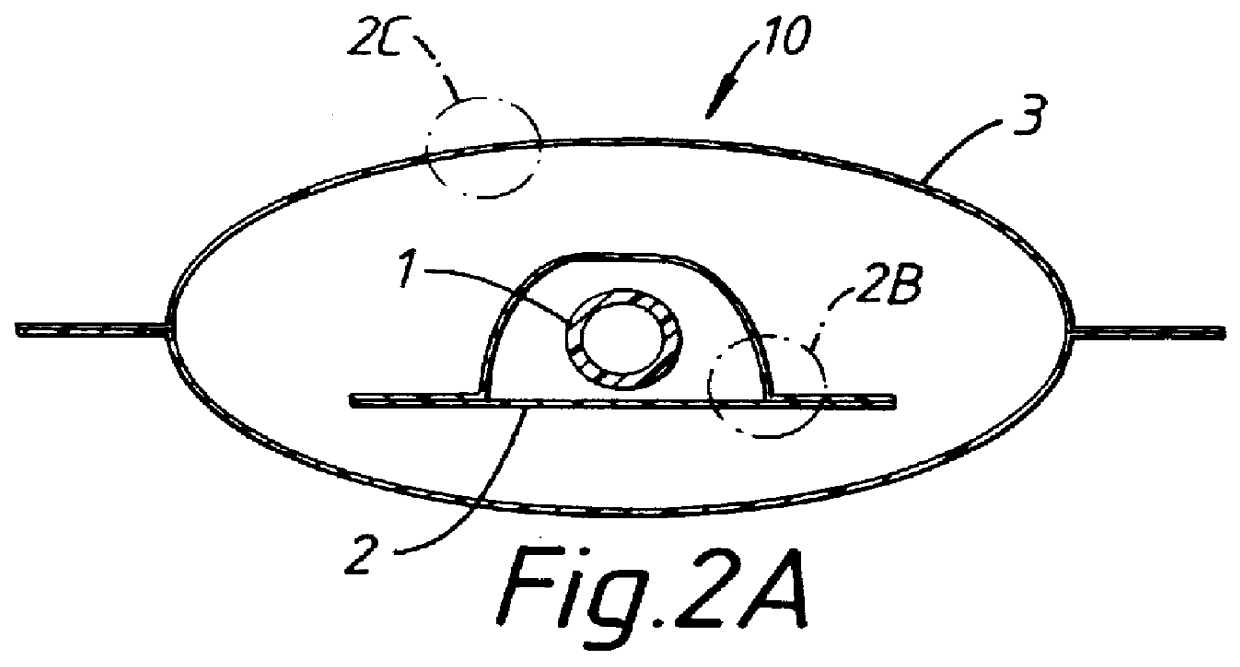
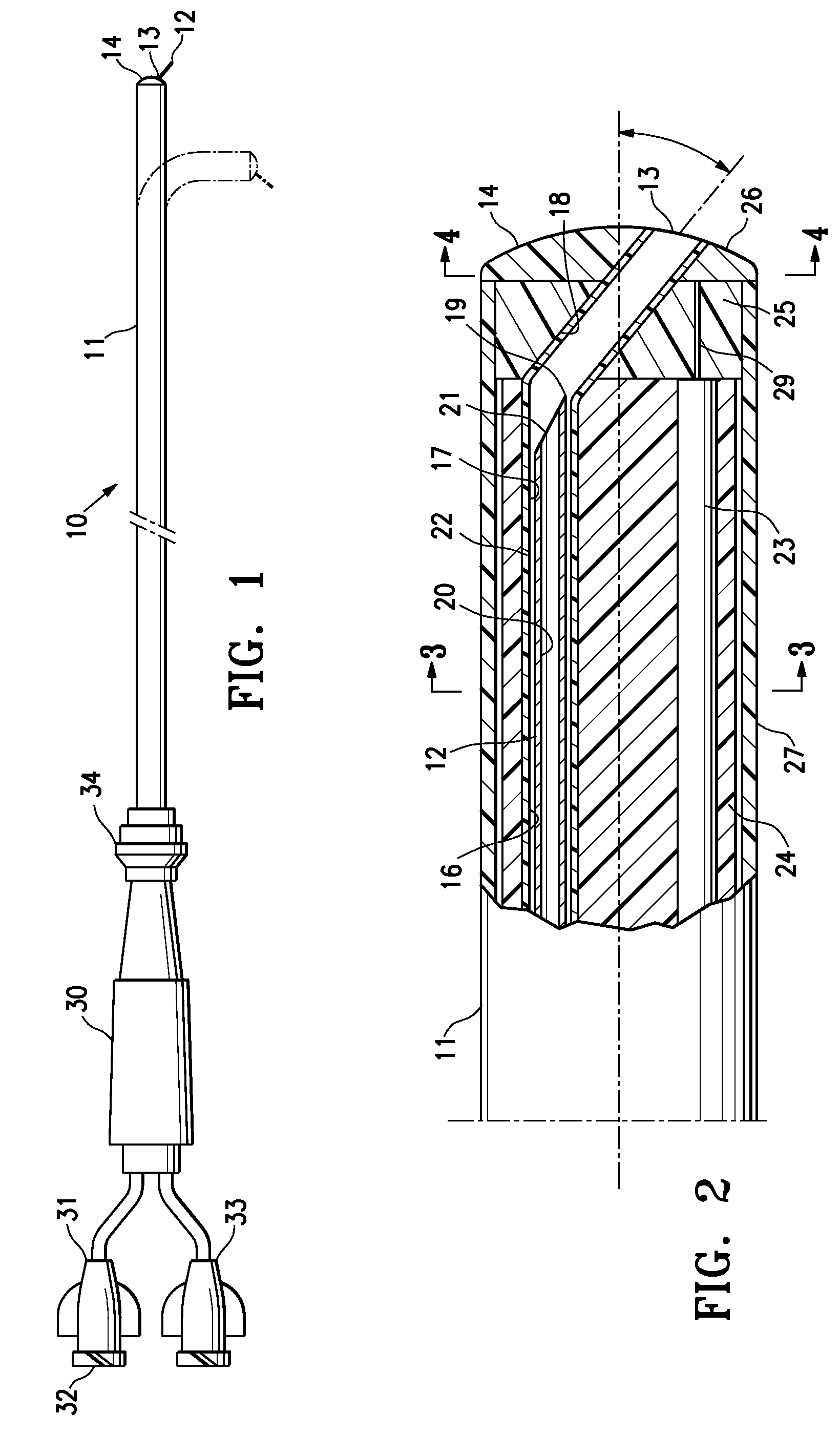
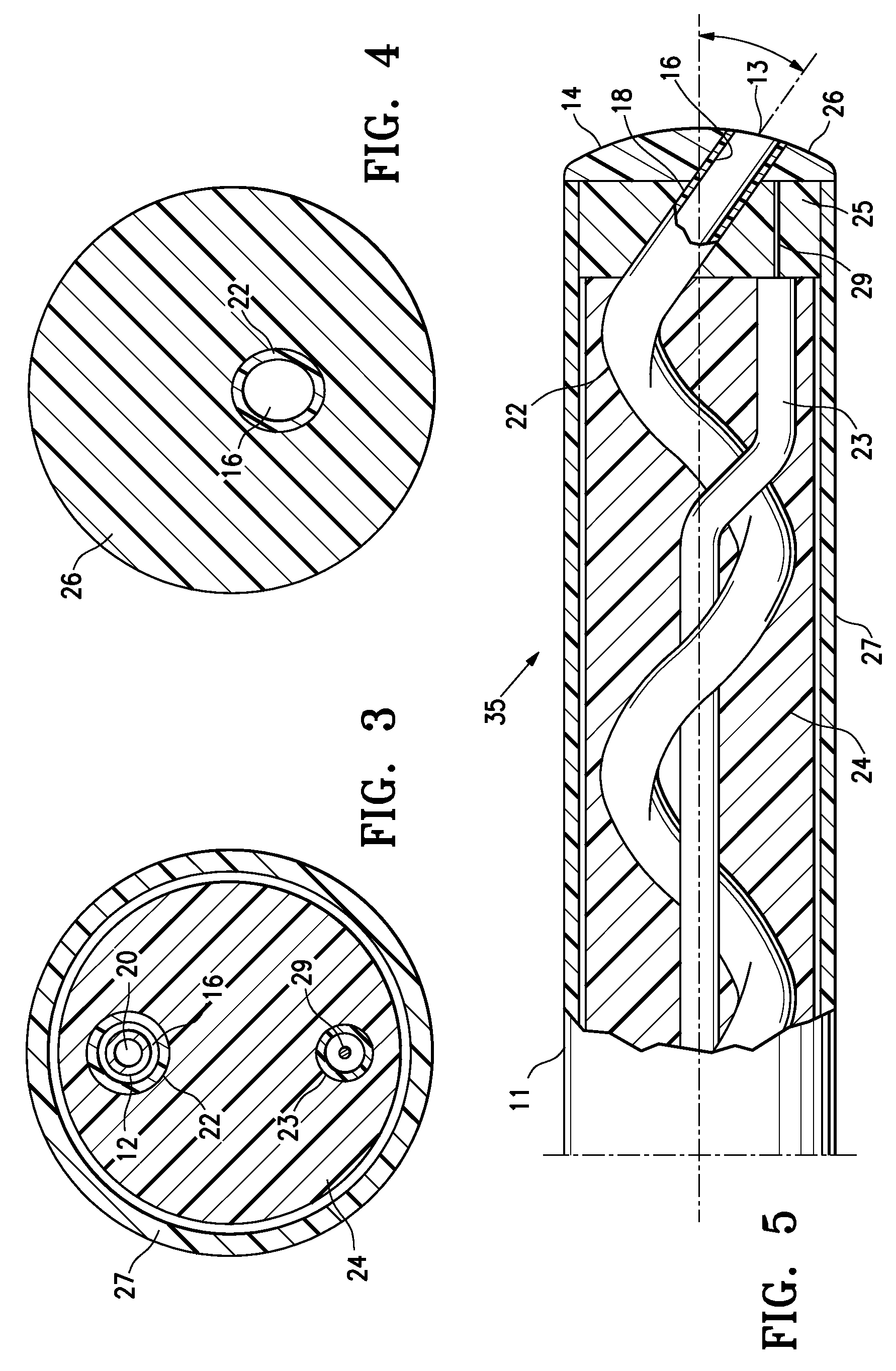
Catheter package
InactiveUS6065597APrevents orAvoid accessDispensing apparatusDiagnosticsBiomedical engineeringEthylene oxide
PCT No. PCT / SE97 / 01033 Sec. 371 Date Jul. 29, 1997 Sec. 102(e) Date Jul. 29, 1997 PCT Filed Jun. 12, 1997 PCT Pub. No. WO97 / 47349 PCT Pub. Date Dec. 18, 1997A catheter package (10; 110; 210) comprising a catheter (1; 101; 201) positioned within an inner container (2; 102; 202) permeable to a sterilizing agent, for example an ethylene oxide gas. An outer container (3; 103; 203) which prevents access of moisture to the interior thereof encloses the inner container and catheter assembly. Two or more catheters may be stored in individual inner containers within the outer container.
Owner:ASTRAZENECA AB
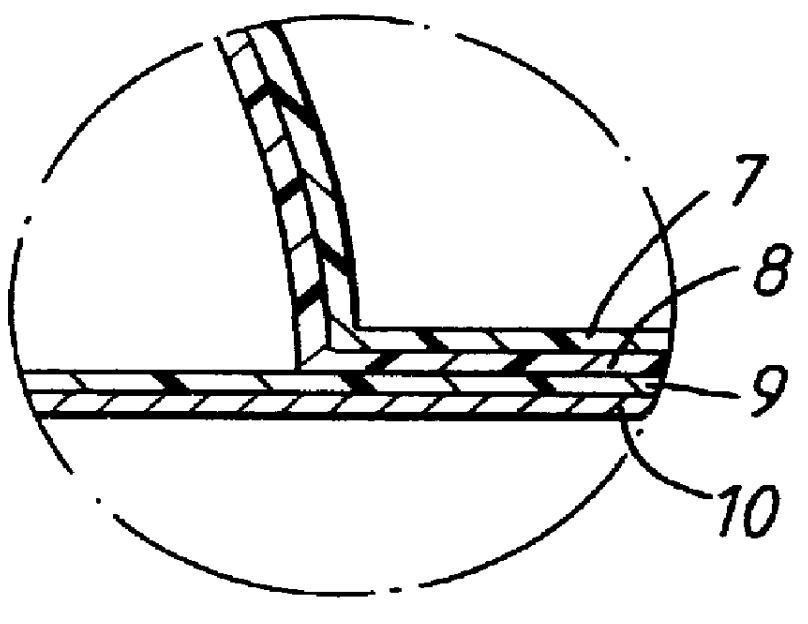
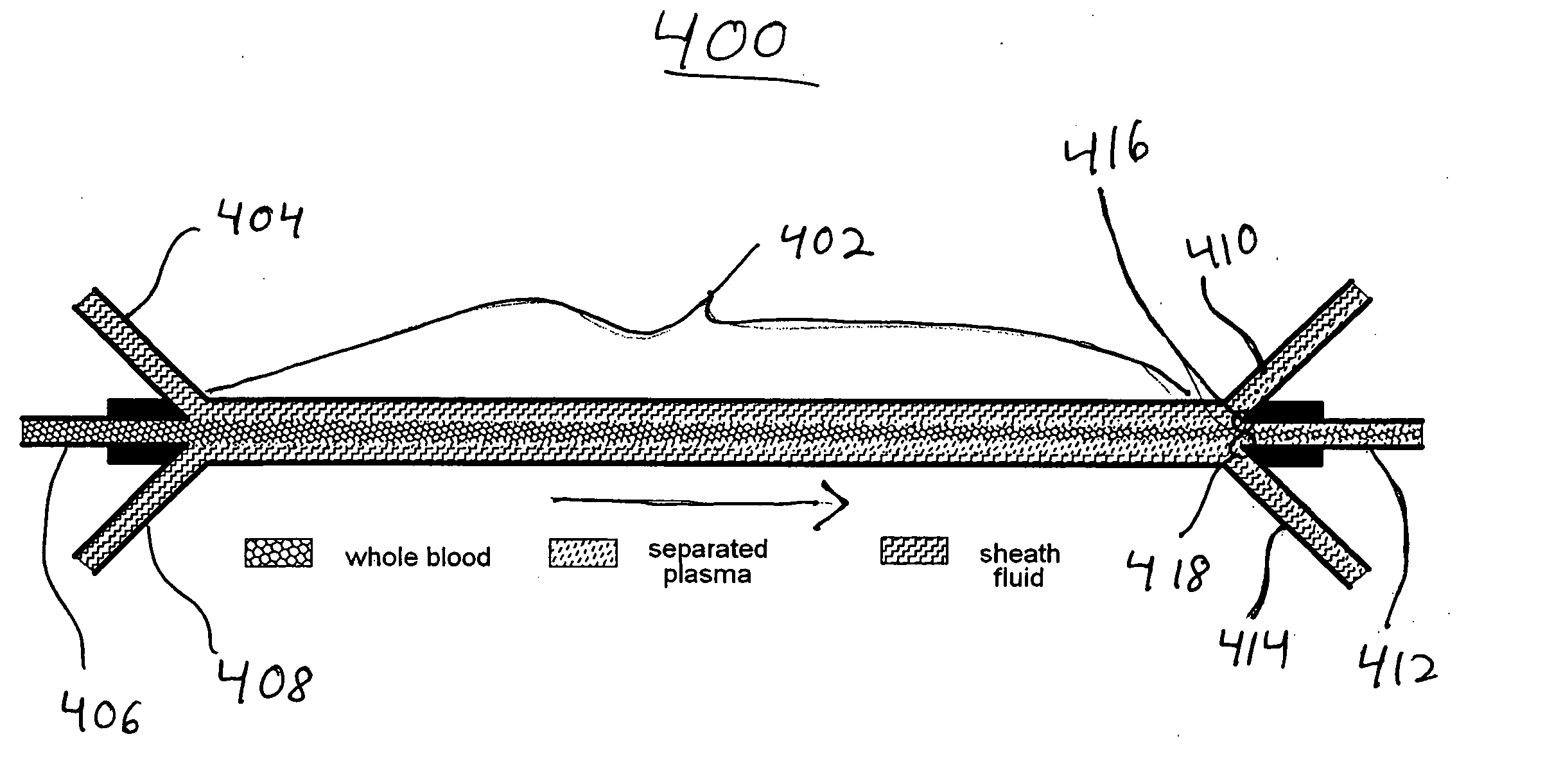
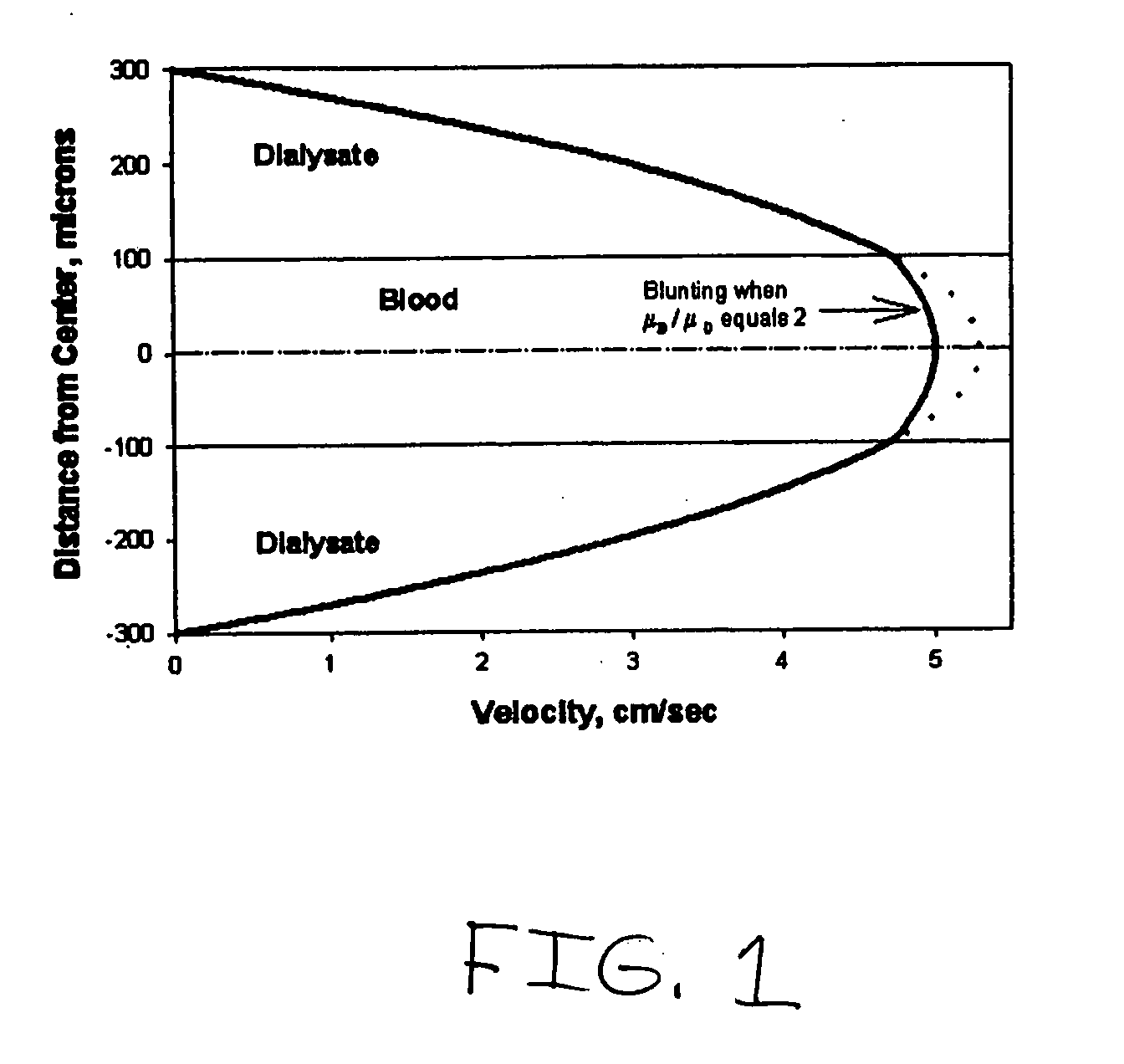
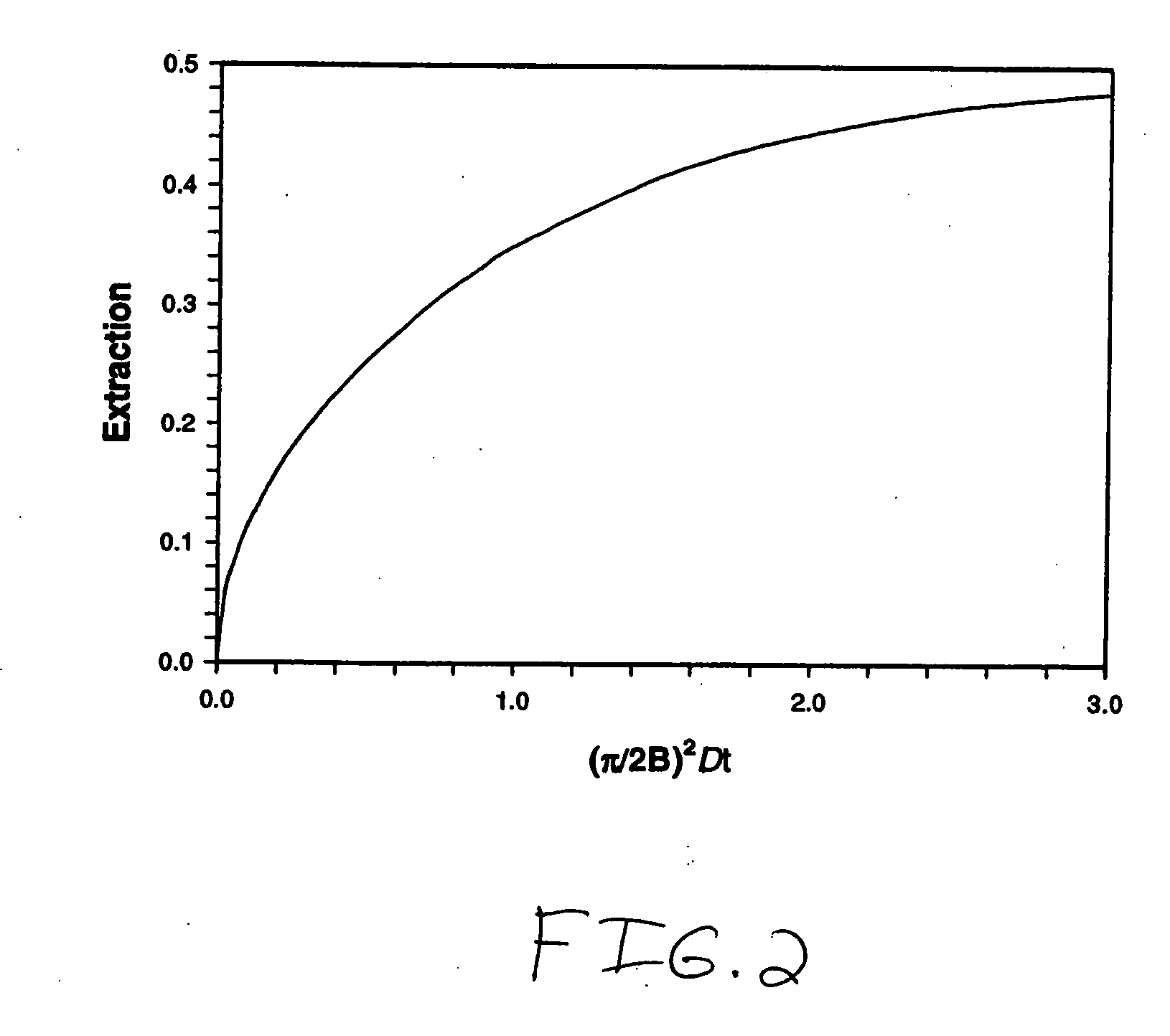
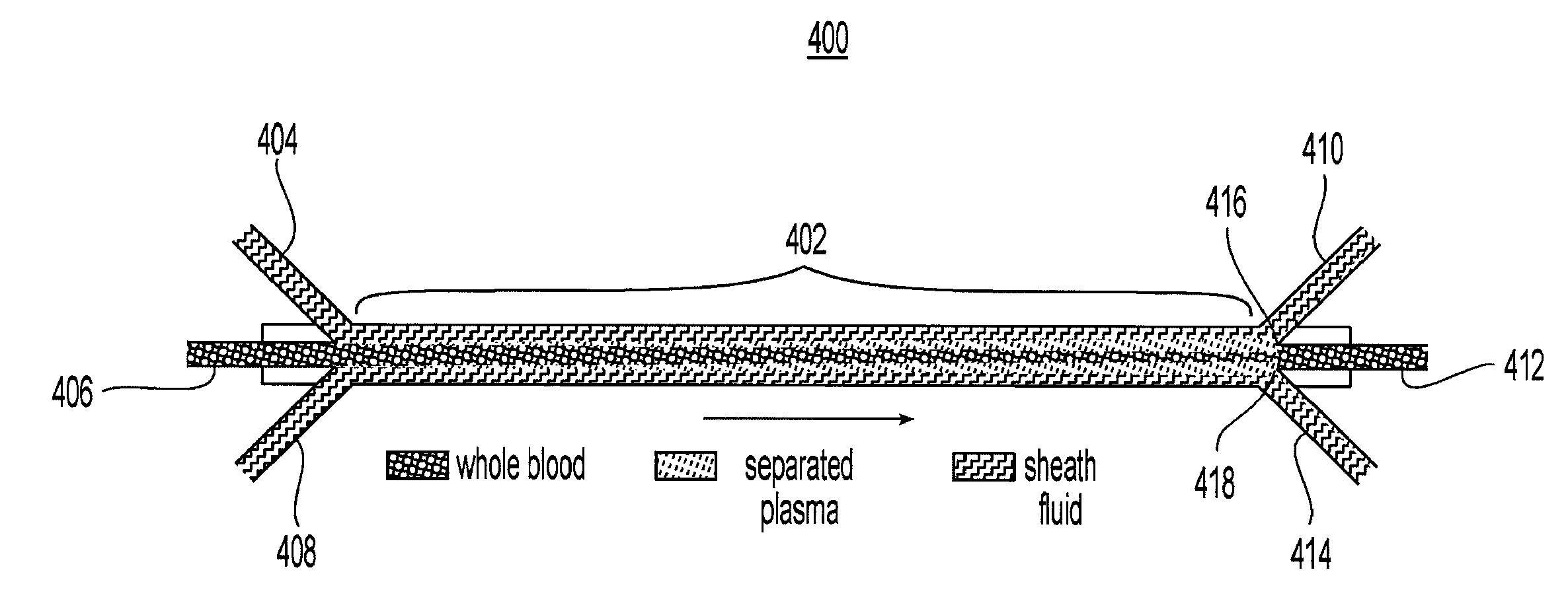
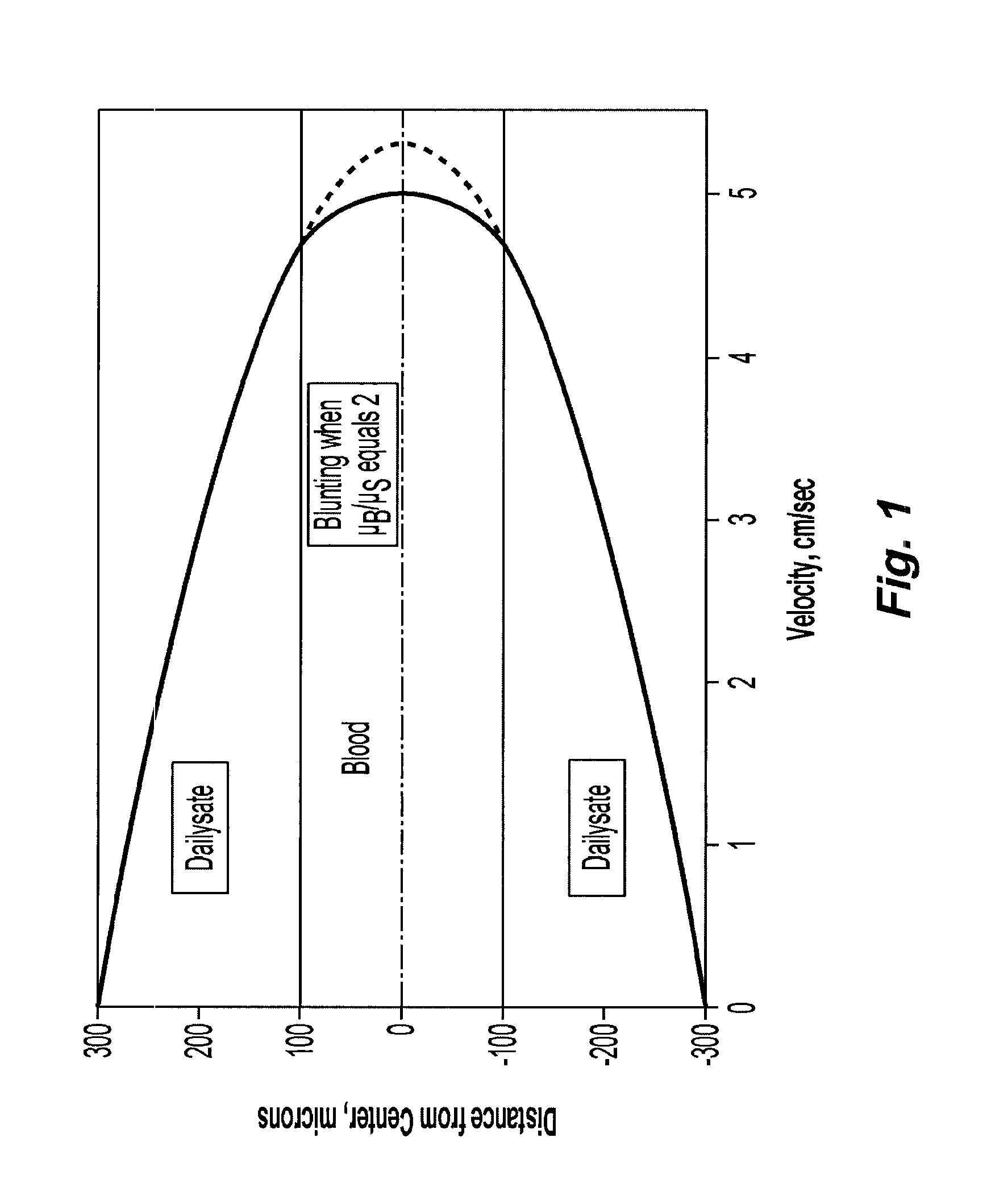
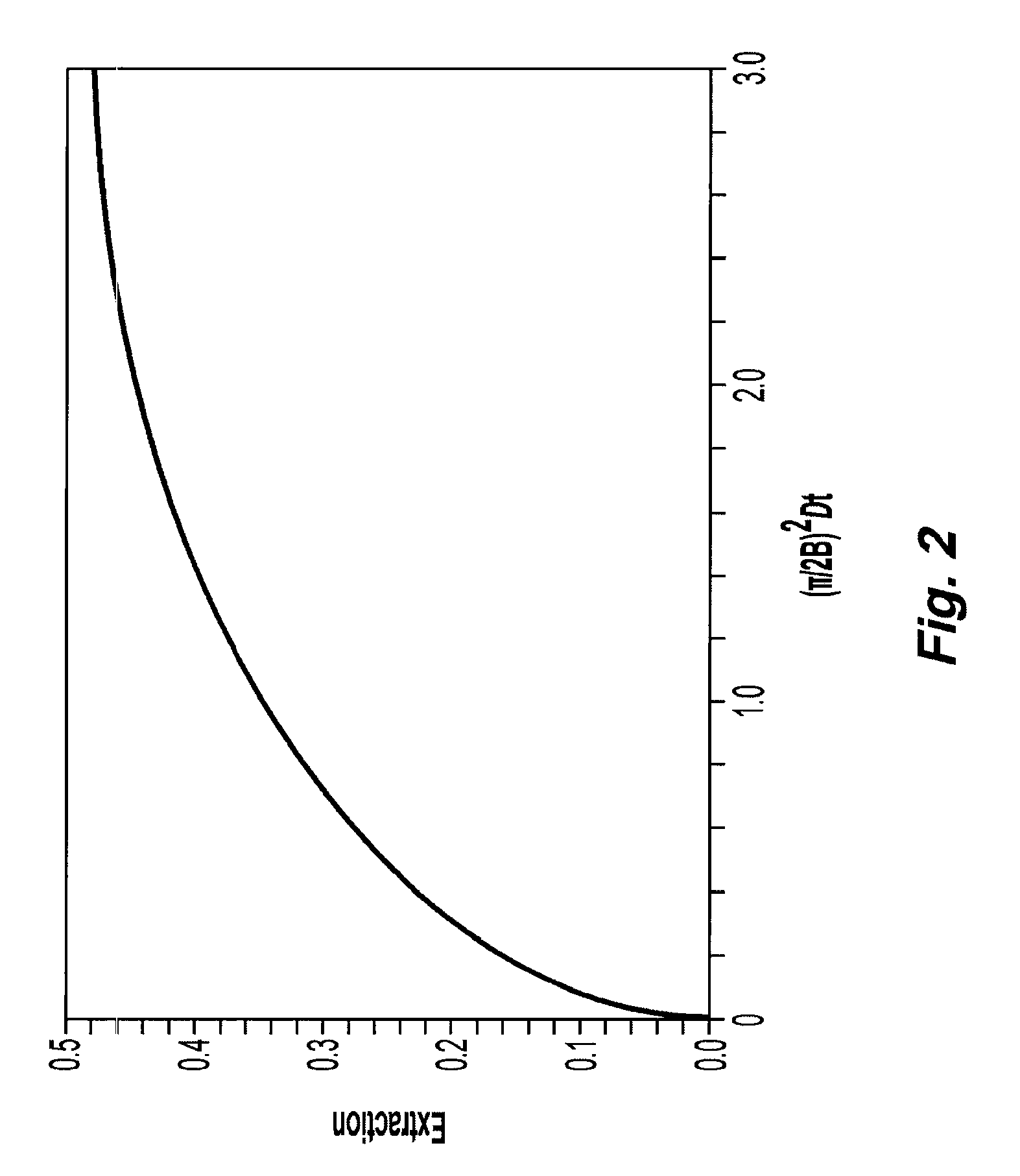
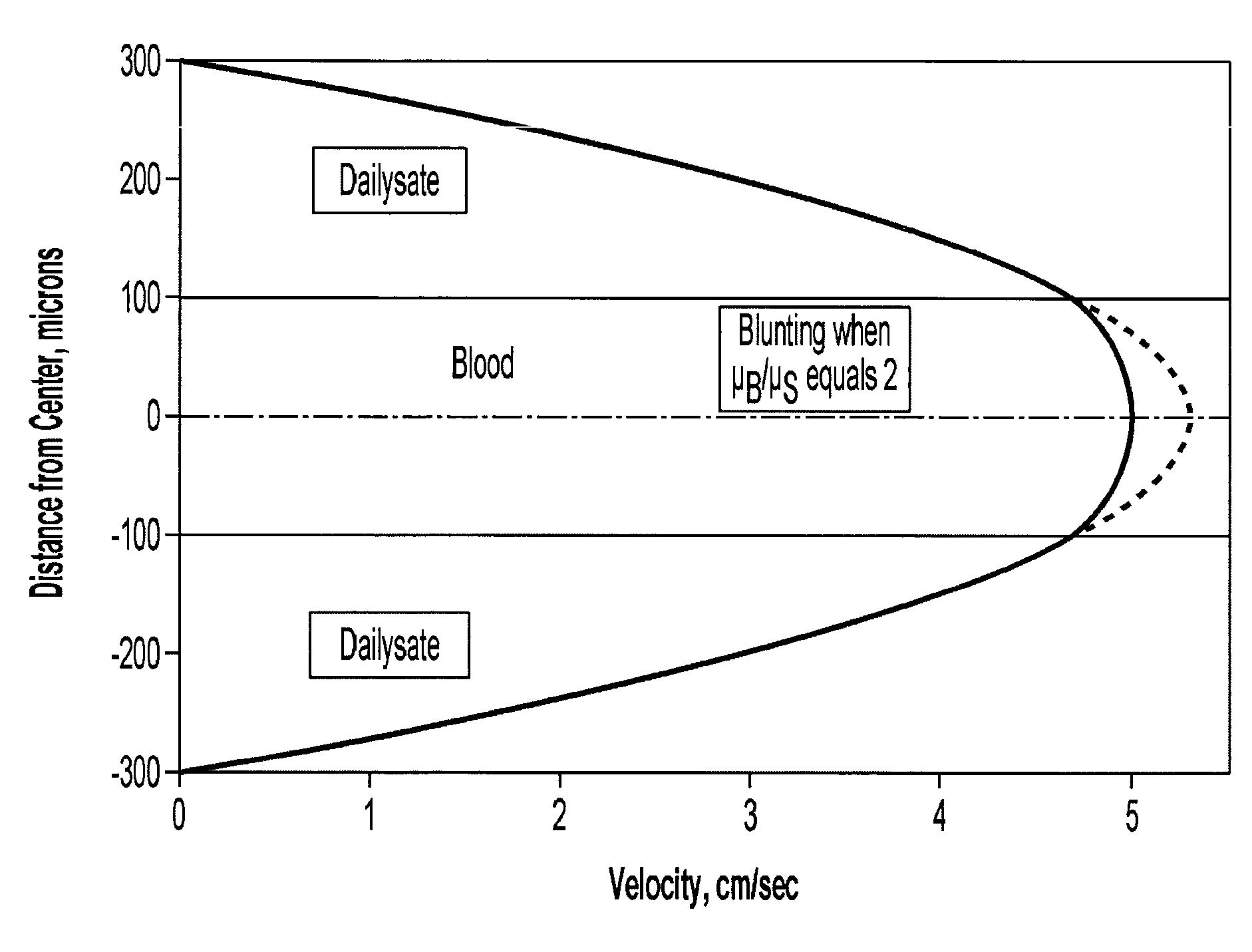
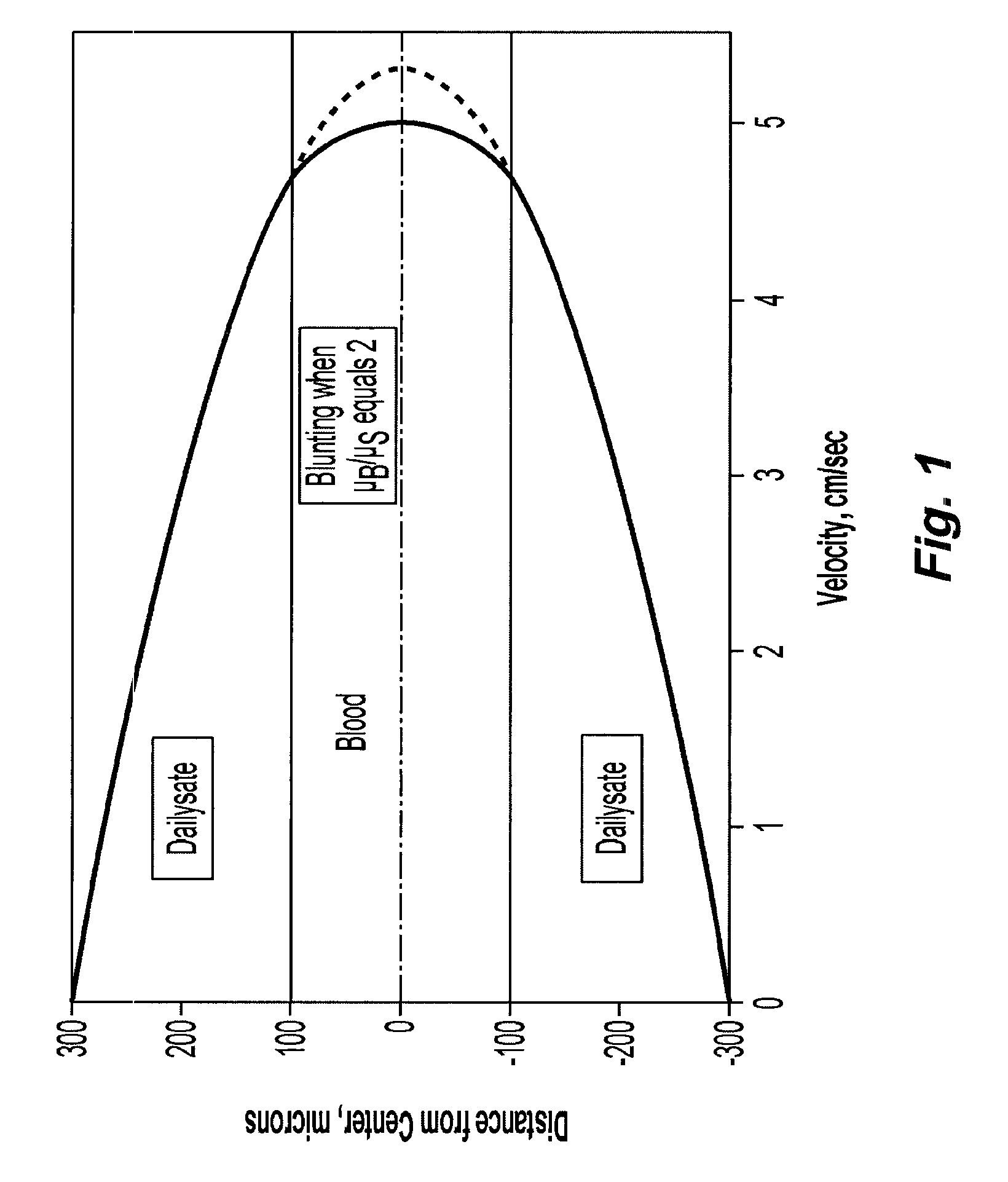
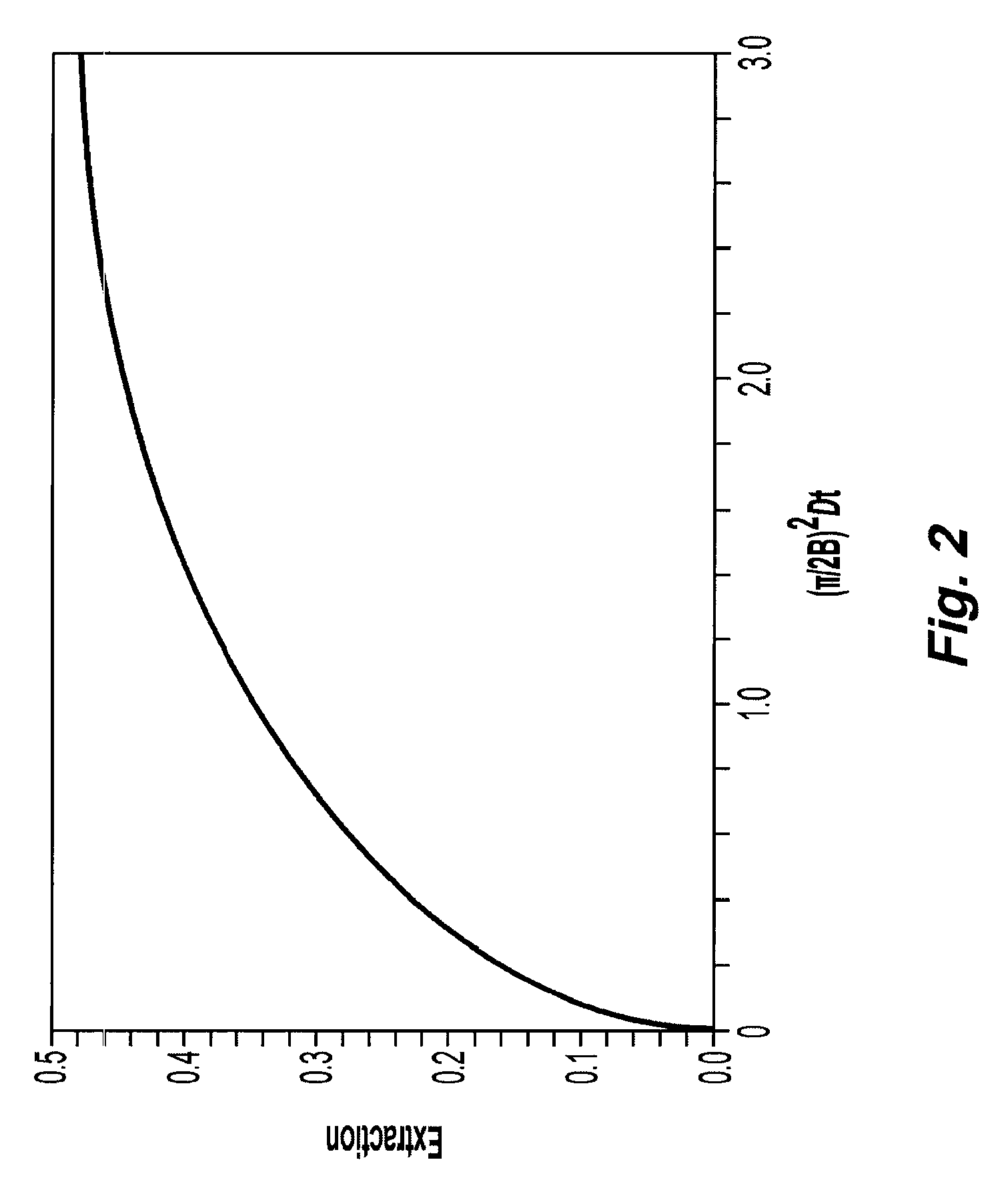
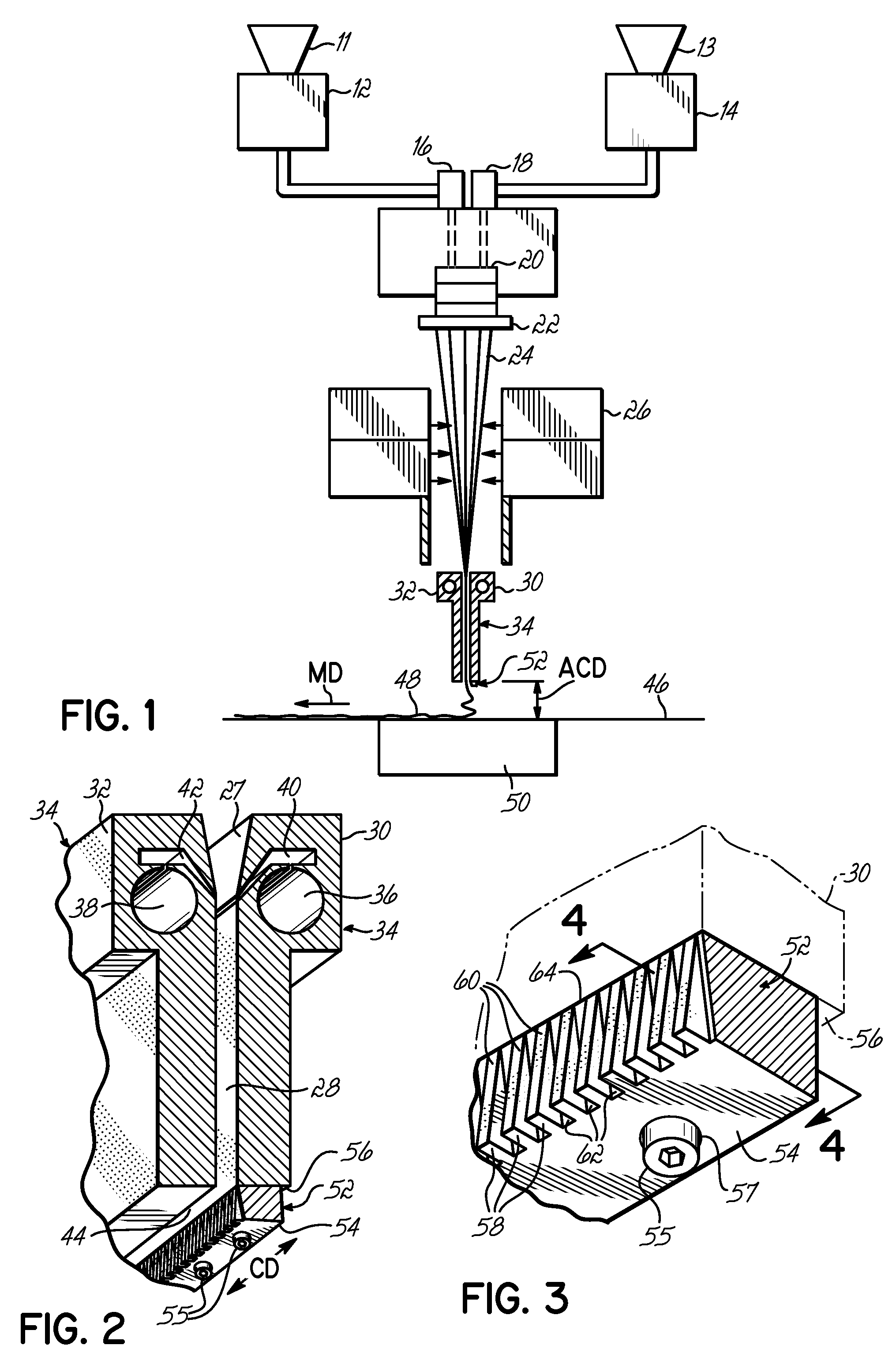
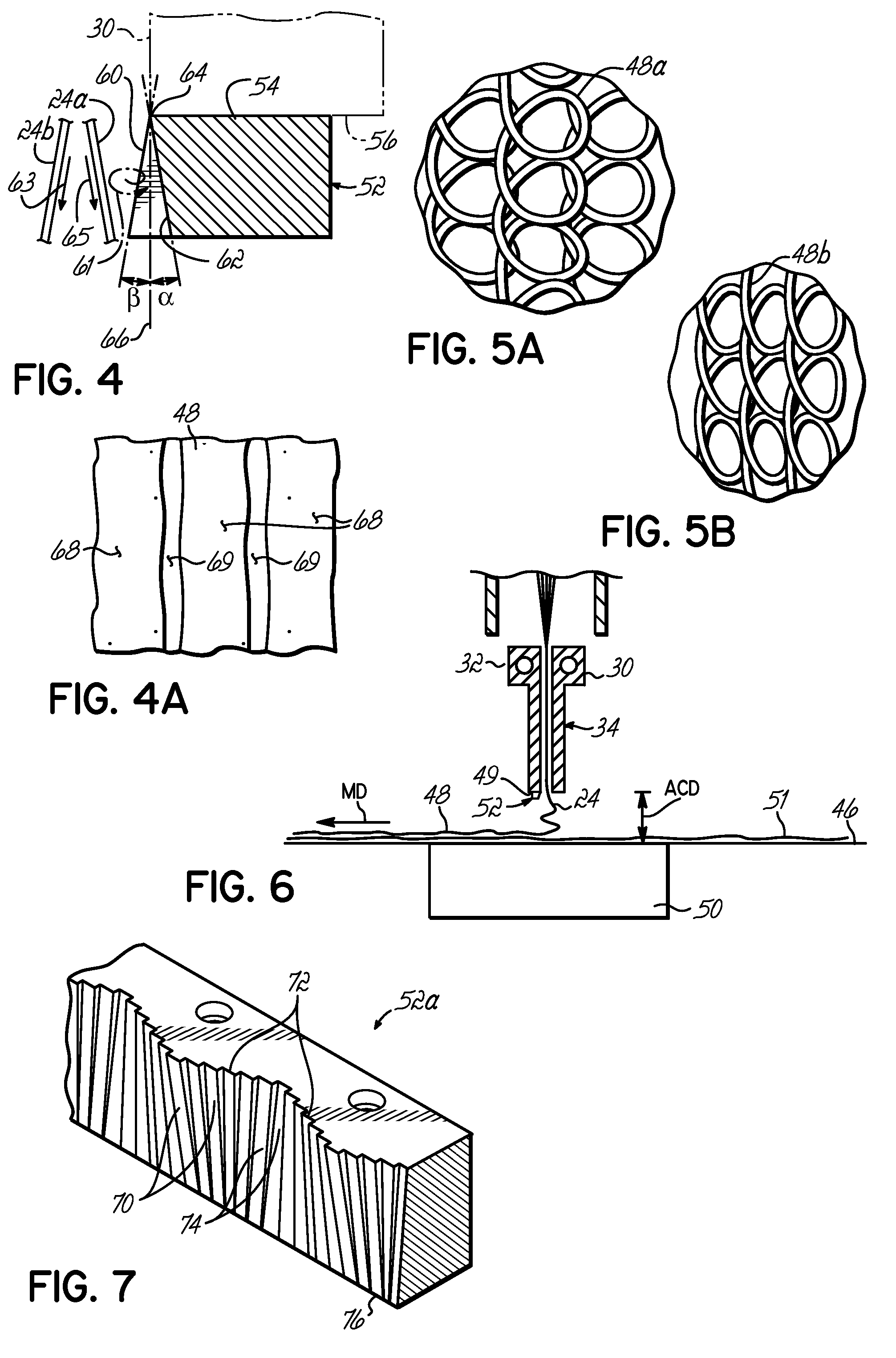
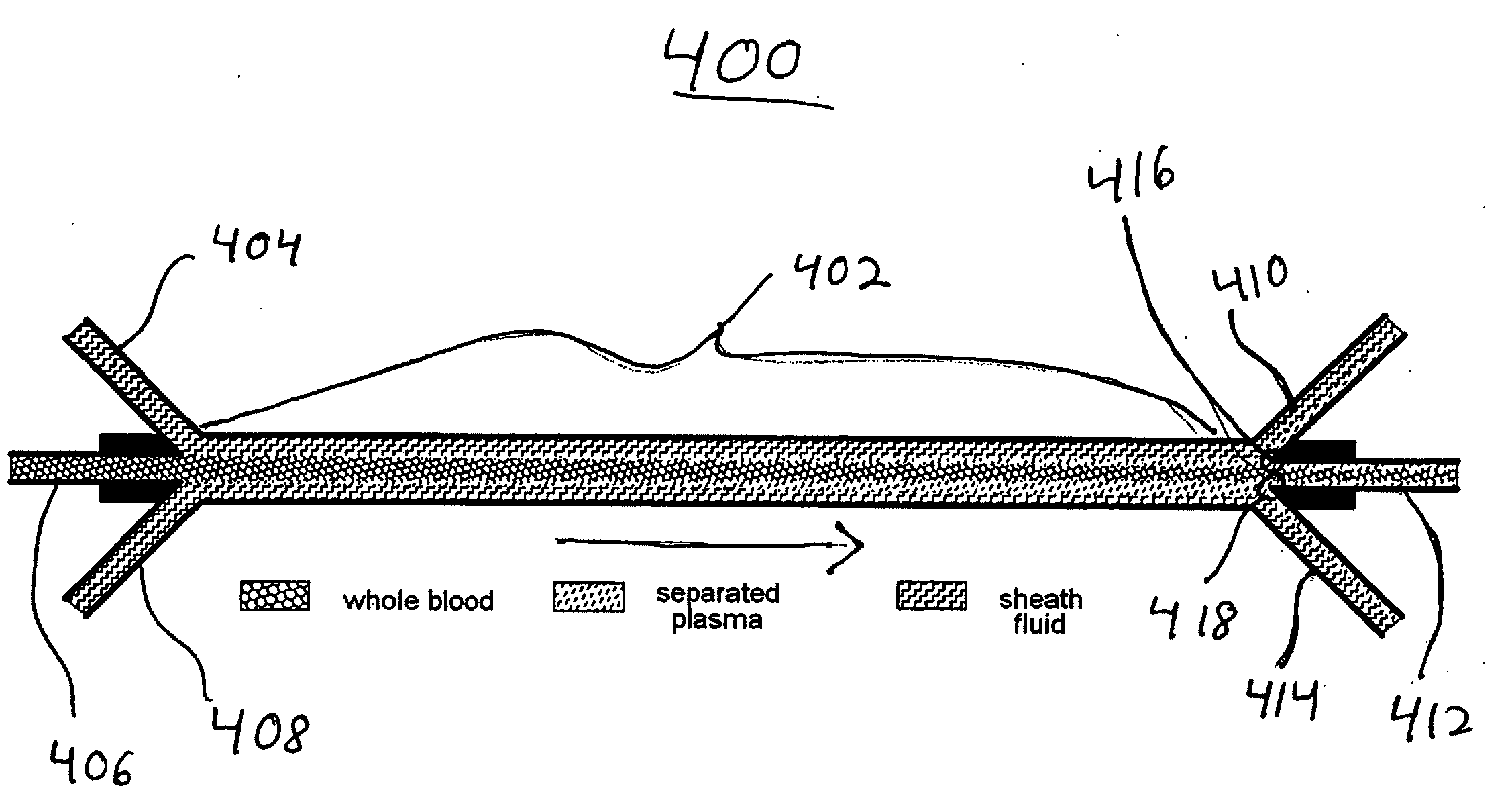
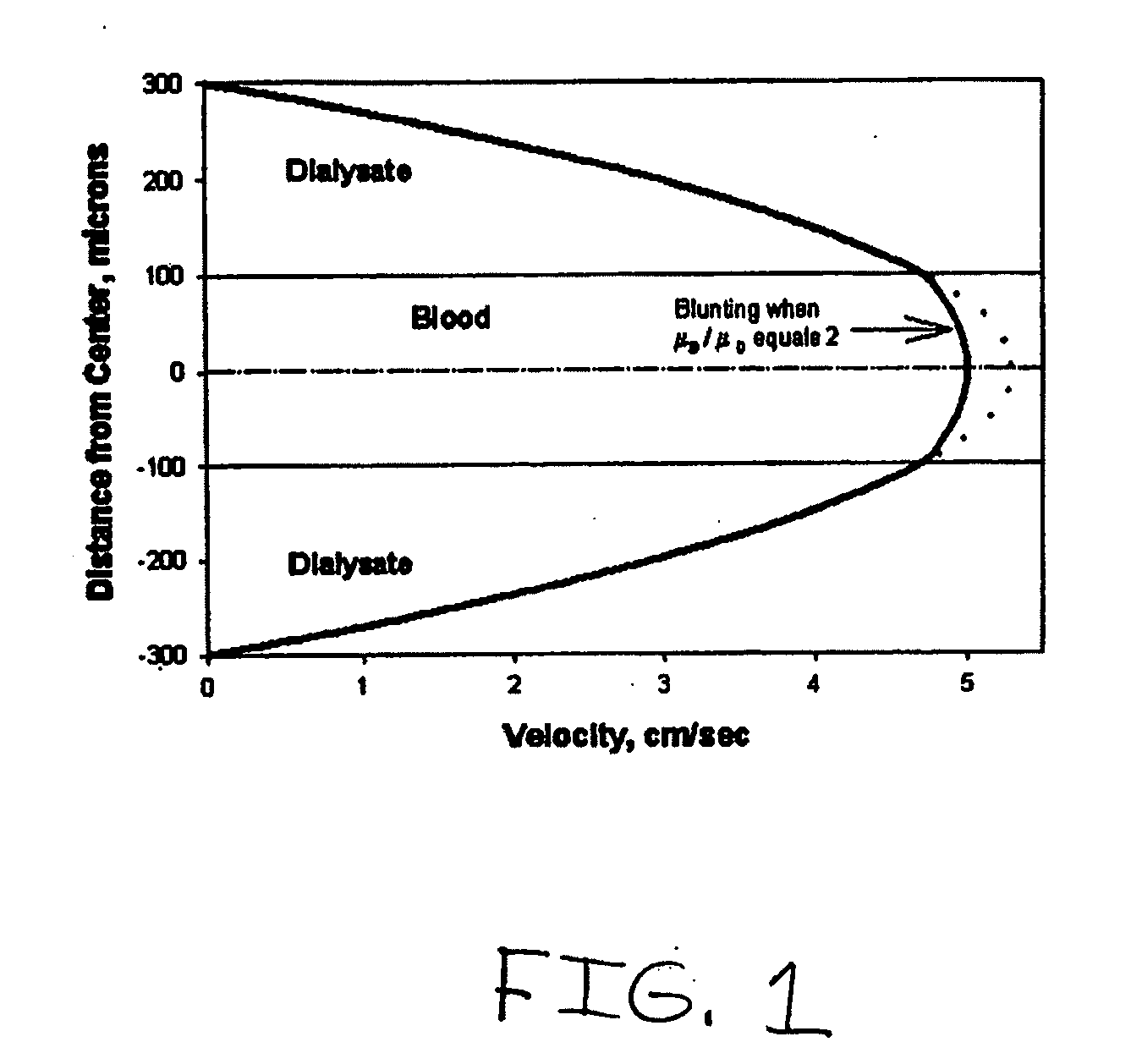
Systems and methods of blood-based therapies having a microfluidic membraneless exchange device
InactiveUS20060076295A1Reduces undesirable activationMinimizing bioincompatibilitiesComponent separationMedical devicesDiffusionThin layer
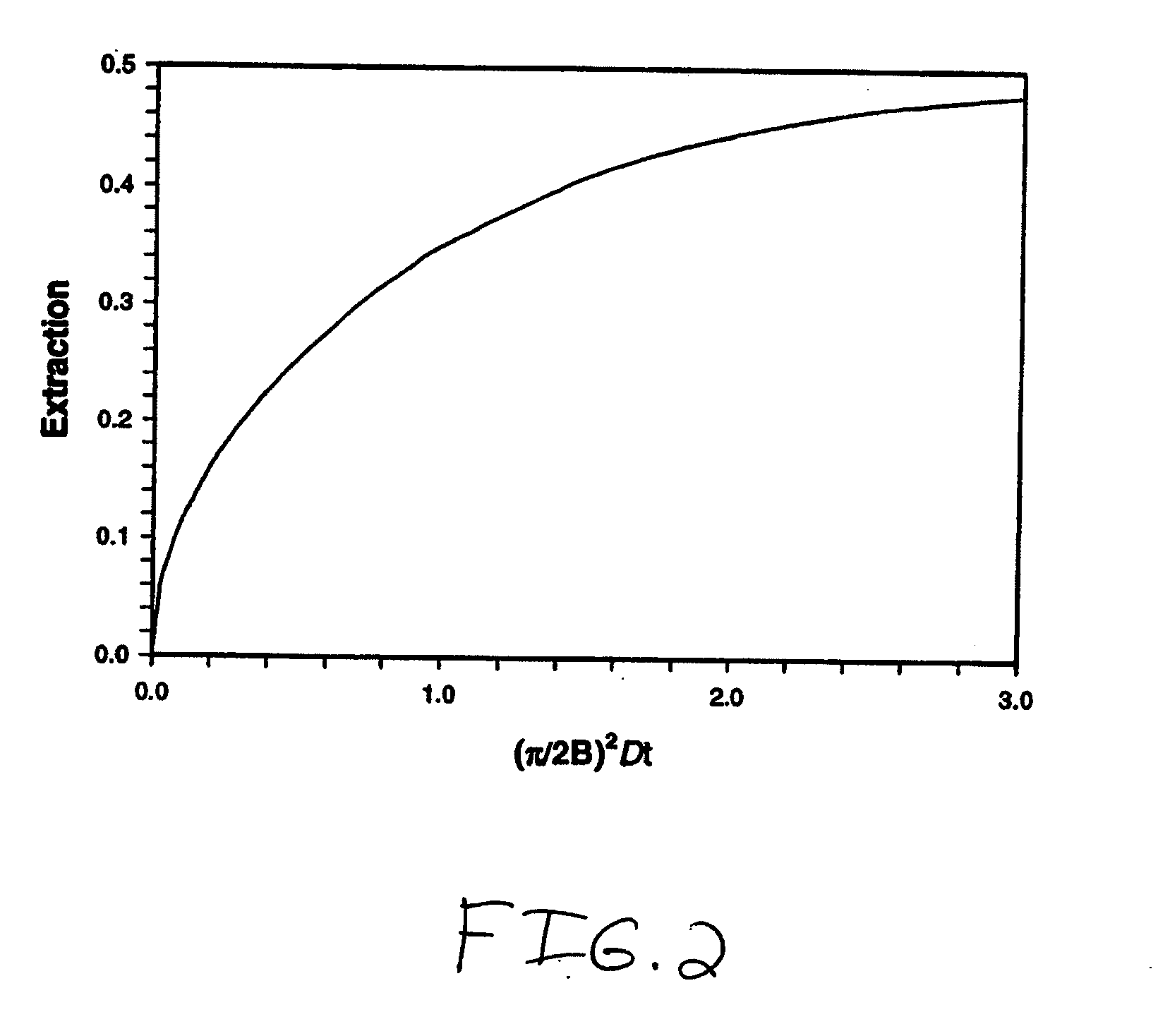
The present invention is directed to devices, systems and methods for removing undesirable materials from a sample fluid by contact with a second fluid. The sample fluid flows as a thin layer adjacent to, or between, concurrently flowing layers of the second fluid, without an intervening membrane. In various embodiments, a secondary separator is used to restrict the removal of desirable substances and effect the removal of undesirable substances from blood. The invention is useful in a variety of situations where a sample fluid is to be purified via a diffusion mechanism against an extractor fluid. Moreover, the invention may be used for the removal of components from a sample fluid that vary in size. When blood is the sample fluid, for example, this may include the removal of ‘small’ molecules, ‘middle’ molecules, macromolecules, macromolecular aggregates, and cells, from the blood sample to the extractor fluid.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Systems and methods of blood-based therapies having a microfluidic membraneless exchange device
InactiveUS20080009780A1PreventsMinimize bioincompatibilitiesSamplingOther blood circulation devicesAmount of substanceThin layer
The present invention is directed to devices, systems and methods for removing undesirable materials from a sample fluid by contact with a second fluid. The sample fluid flows as a thin layer adjacent to, or between, concurrently flowing layers of the second fluid, without an intervening membrane. In various embodiments, a secondary separator is used to restrict the removal of desirable substances and effect the removal of undesirable substances from blood. The invention is useful in a variety of situations where a sample fluid is to be purified via a diffusion mechanism against an extractor fluid. Moreover, the invention may be used for the removal of components from a sample fluid that vary in size. When blood is the sample fluid, for example, this may include the removal of ‘small’ molecules, ‘middle’ molecules, macromolecules, macromolecular aggregates, and cells, from the blood sample to the extractor fluid.
Owner:COLUMBIA UNIV (US)
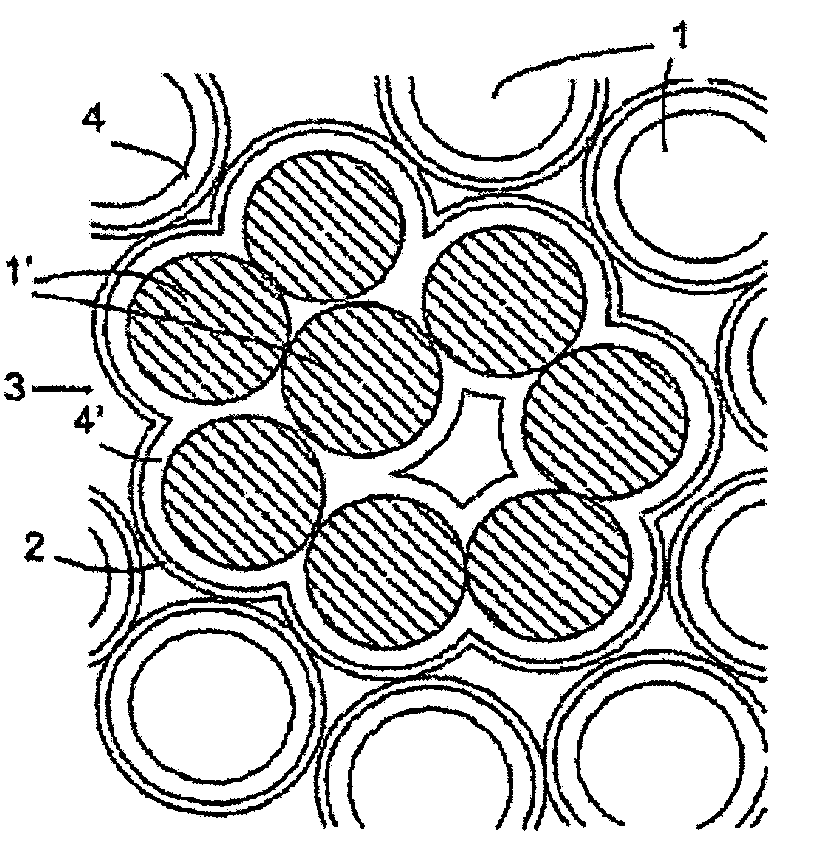
Polyvinyl butyral granular material for 3-D binder printing, production method and uses therefor
InactiveUS7402330B2Flat surfaceFiner and more detailed structureGranule coatingPretreated surfacesSurface layerMaterials science
The invention relates to a granular material for 3D binder printing, said granular material consisting of particles provided with an externally non-polar surface layer (2). The invention also relates to a method for producing a granular material for 3D binder printing, whereby a surface layer (2) having a non-polar outer side is applied to initial particles (1), and to a method for producing an object consisting of the inventive granular material, according to which a layer of the inventive granular material is applied to a base, and pre-determined regions (3) of said layer are moistened with a binding fluid, said binding fluid being selected from fluids in which a surface layer of the particles of the granular material is soluble. The invention further realties to objects consisting of interconnected particles of the inventive granular material. The invention enables a very precise printing process.
Owner:VOXELJET AG
IC chip package structure and underfill process
ActiveUS7148560B2Relieve pressurePrevents orSemiconductor/solid-state device detailsSolid-state devicesMultiple injectionInjection point
A novel integrated circuit (IC) chip package structure and underfill process which reduces stress applied to corners of a flip chip in an IC package structure during the application of an adhesive material between the flip chip and a carrier substrate is disclosed. The process includes providing a dam structure on a carrier substrate; attaching solder bumps of an inverted flip chip to the carrier substrate; injecting an adhesive material between the flip chip and the carrier substrate at multiple injection points located along adjacent edges of the flip chip; and injecting a sealant material around the adhesive material. During application of the adhesive material and the sealant material to the IC package structure in the underfill process, the dam structure reduces stress applied to the corners of the flip chip. This prevents or at least reduces de-lamination of dielectric layers on the flip chip.
Owner:TAIWAN SEMICON MFG CO LTD
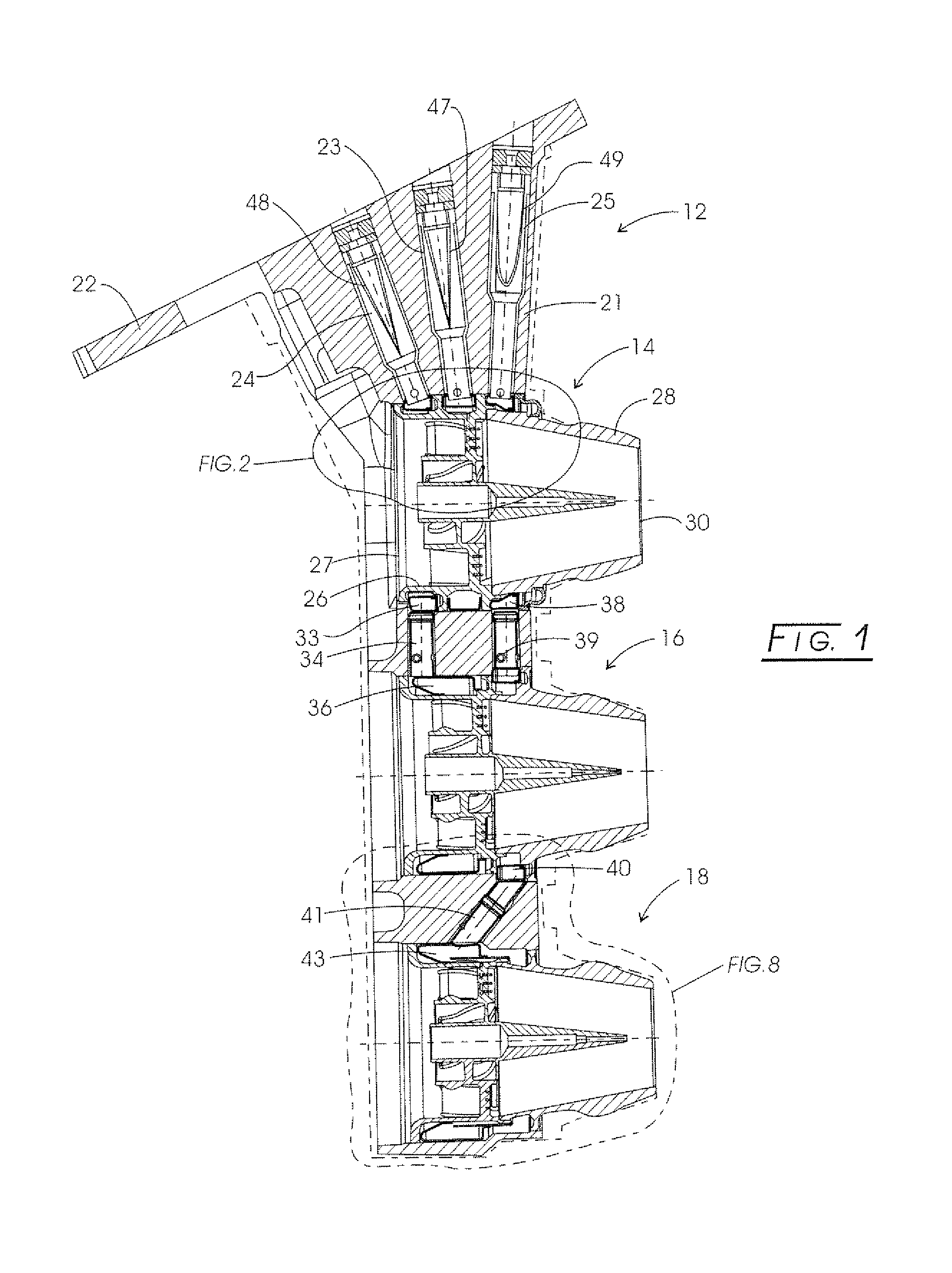
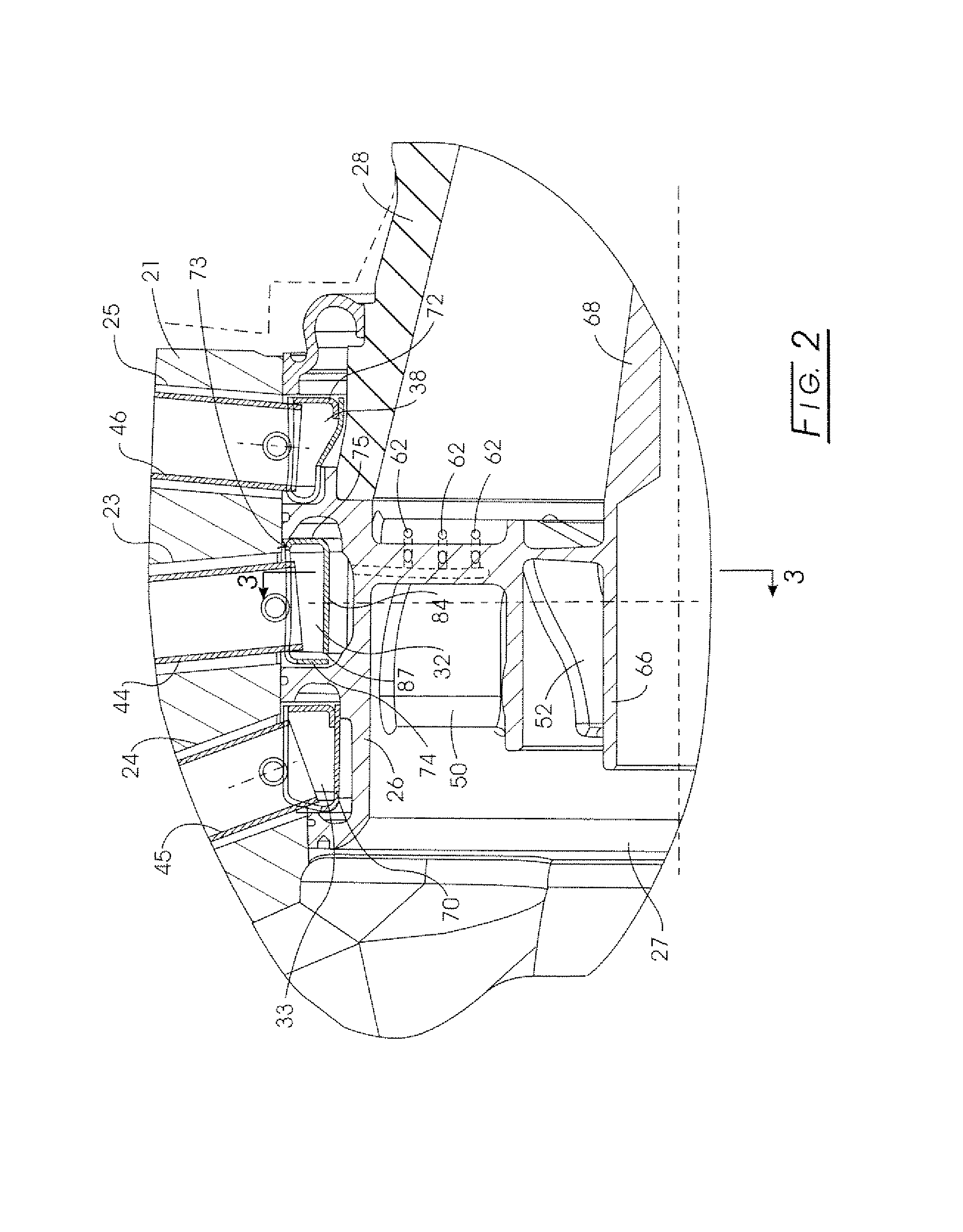
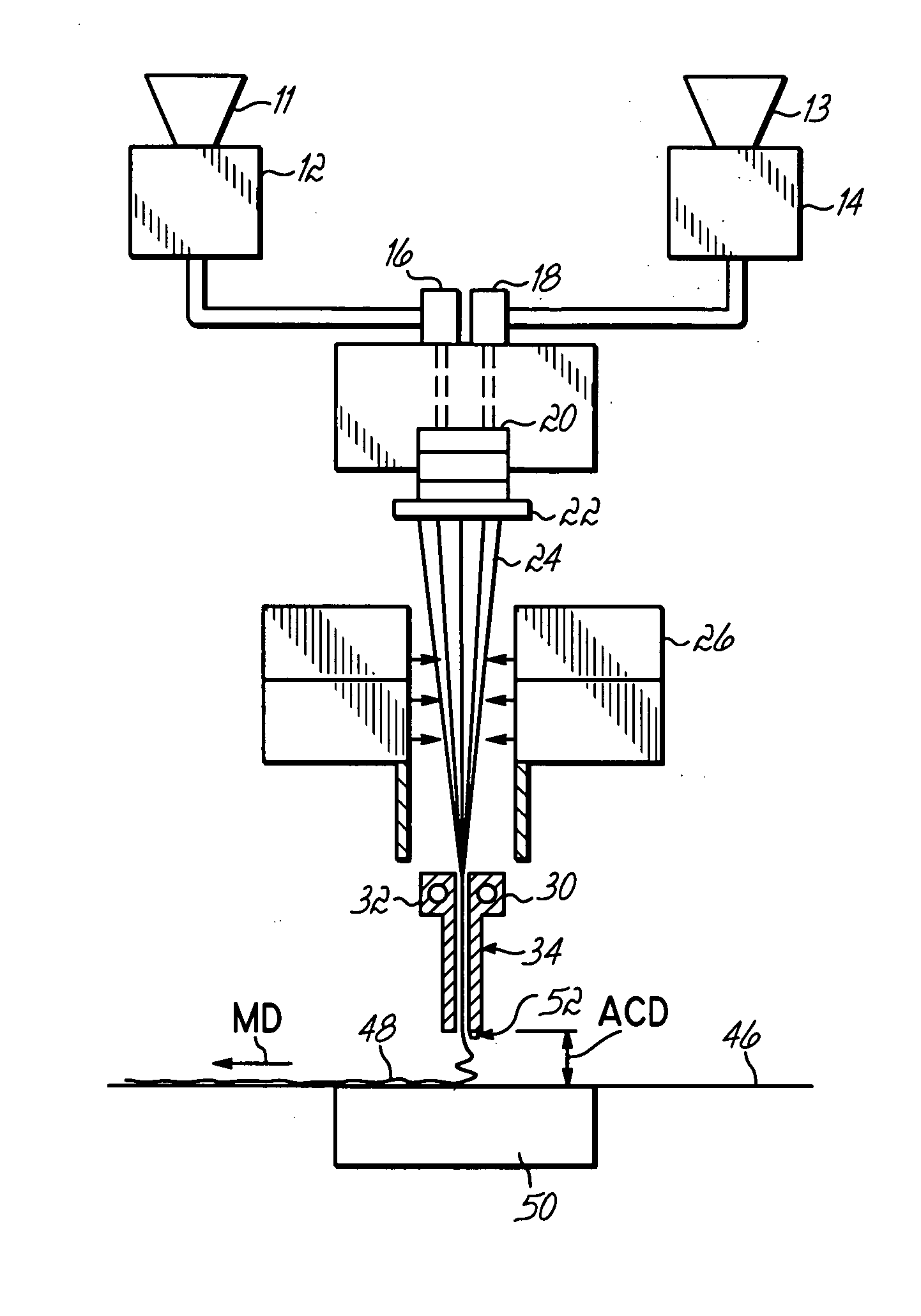
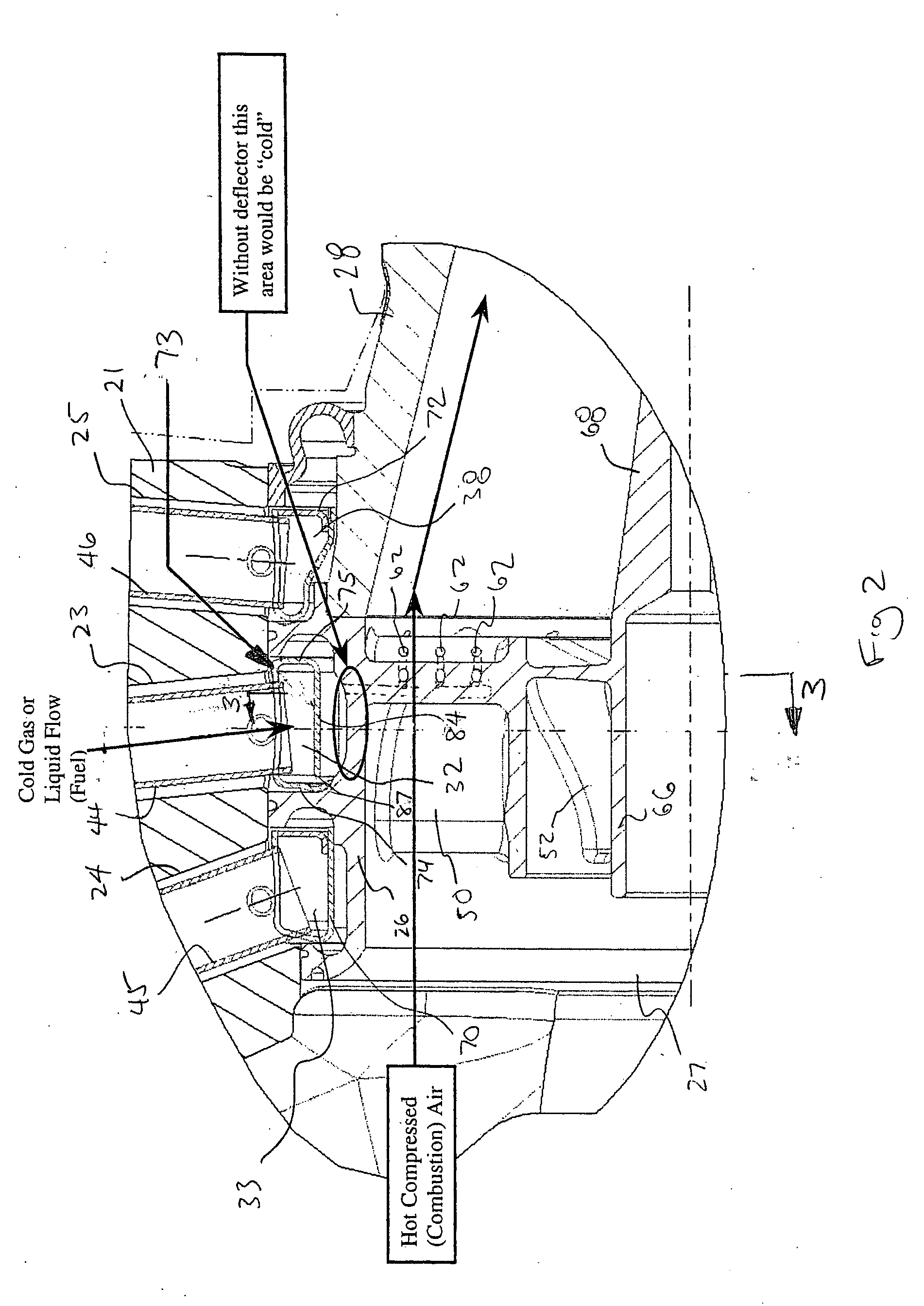
Nozzle assembly with fuel tube deflector
ActiveUS7140560B2Prevents orReduce thermal stressLiquid surface applicatorsContinuous combustion chamberEngineeringFuel line
A deflector at the downstream end of a fuel tube deflects incoming fuel into an annular fuel channel in a fuel swirler of an injector. The deflector prevents direct impact of the cooler fuel on the exposed walls of the swirler body. The deflector can be formed by a tab or other integral portion of the heatshield, or as a separate piece fixed to the heatshield or swirler body. The deflector minimizes disruption of flow through any flow slots that are covered by the deflector, and may have i) a relatively small circumferential extent, such that the deflector covers only a few flow slots, ii) flow openings or slots that allow a portion of the fuel to flow inwardly of the deflector into the otherwise covered flow slot(s), or iii) a space or gap between the side(s) of the deflector and the inner walls of the annular channel.
Owner:PARKER INTANGIBLES LLC
Systems and methods of blood-based therapies having a microfluidic membraneless exchange device
InactiveUS7588550B2Reduces undesirable activationPrevents orSamplingOther blood circulation devicesDiffusionThin layer
The present invention is directed to devices, systems and methods for removing undesirable materials from a sample fluid by contact with a second fluid. The sample fluid flows as a thin layer adjacent to, or between, concurrently flowing layers of the second fluid, without an intervening membrane. In various embodiments, a secondary separator is used to restrict the removal of desirable substances and effect the removal of undesirable substances from blood. The invention is useful in a variety of situations where a sample fluid is to be purified via a diffusion mechanism against an extractor fluid. Moreover, the invention may be used for the removal of components from a sample fluid that vary in size. When blood is the sample fluid, for example, this may include the removal of ‘small’ molecules, ‘middle’ molecules, macromolecules, macromolecular aggregates, and cells, from the blood sample to the extractor fluid.
Owner:COLUMBIA UNIV (US)
Composition for treating skin lesions
InactiveUS20110064826A1Reduce burstSpeed up recovery timeBiocideInorganic active ingredientsTopical treatmentCommon St. Johnswort
The present invention provides a composition for topical treatment of skin and mucosal membrane lesions comprising a synergistic combination of copper compound and hypericum perforatum extract.
Owner:DYNAMICLEAR
IC chip package structure and underfill process
ActiveUS20060163749A1Relieve pressureReduce layeringSemiconductor/solid-state device detailsSolid-state devicesMultiple injectionSealant
A novel integrated circuit (IC) chip package structure and underfill process which reduces stress applied to corners of a flip chip in an IC package structure during the application of an adhesive material between the flip chip and a carrier substrate is disclosed. The process includes providing a dam structure on a carrier substrate; attaching solder bumps of an inverted flip chip to the carrier substrate; injecting an adhesive material between the flip chip and the carrier substrate at multiple injection points located along adjacent edges of the flip chip; and injecting a sealant material around the adhesive material. During application of the adhesive material and the sealant material to the IC package structure in the underfill process, the dam structure reduces stress applied to the corners of the flip chip. This prevents or at least reduces de-lamination of dielectric layers on the flip chip.
Owner:TAIWAN SEMICON MFG CO LTD
Lyophilized platelet rich plasma for the use in wound healing (chronic or acute) and bone or tissue grafts or repair
InactiveUS20050191286A1Simple compositionPrevent flow awayBiocidePeptide/protein ingredientsWhite blood cellAntibiotic Y
This invention relates to an improved Lyophilized platelet rich plasma used to make a platelet gel wound healant, and methods of preparation and use thereof for healing wounds are disclosed. The improved wound healant comprises therapeutically effective amounts of activated growth factors, platelet ghost, plasma (know as the plasma back bone), white blood cells with optional none, one or more additional anti-oxidant such as vitamin A and / or C and / or E, and / or none one or more antibiotics and / or GHK-Cu (produced by ProCyte Inc.)
Owner:WALKER MACKIE J JR
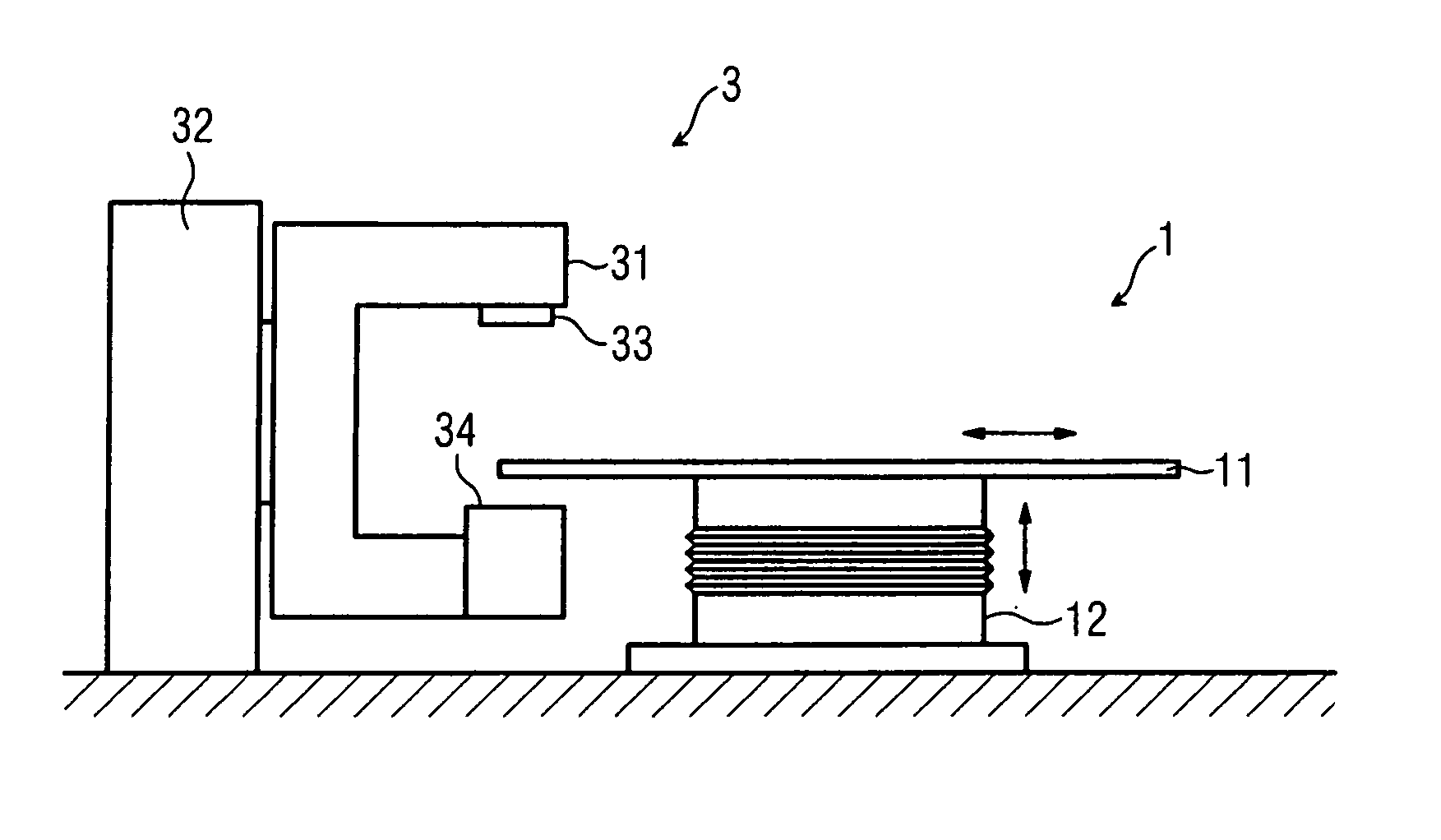
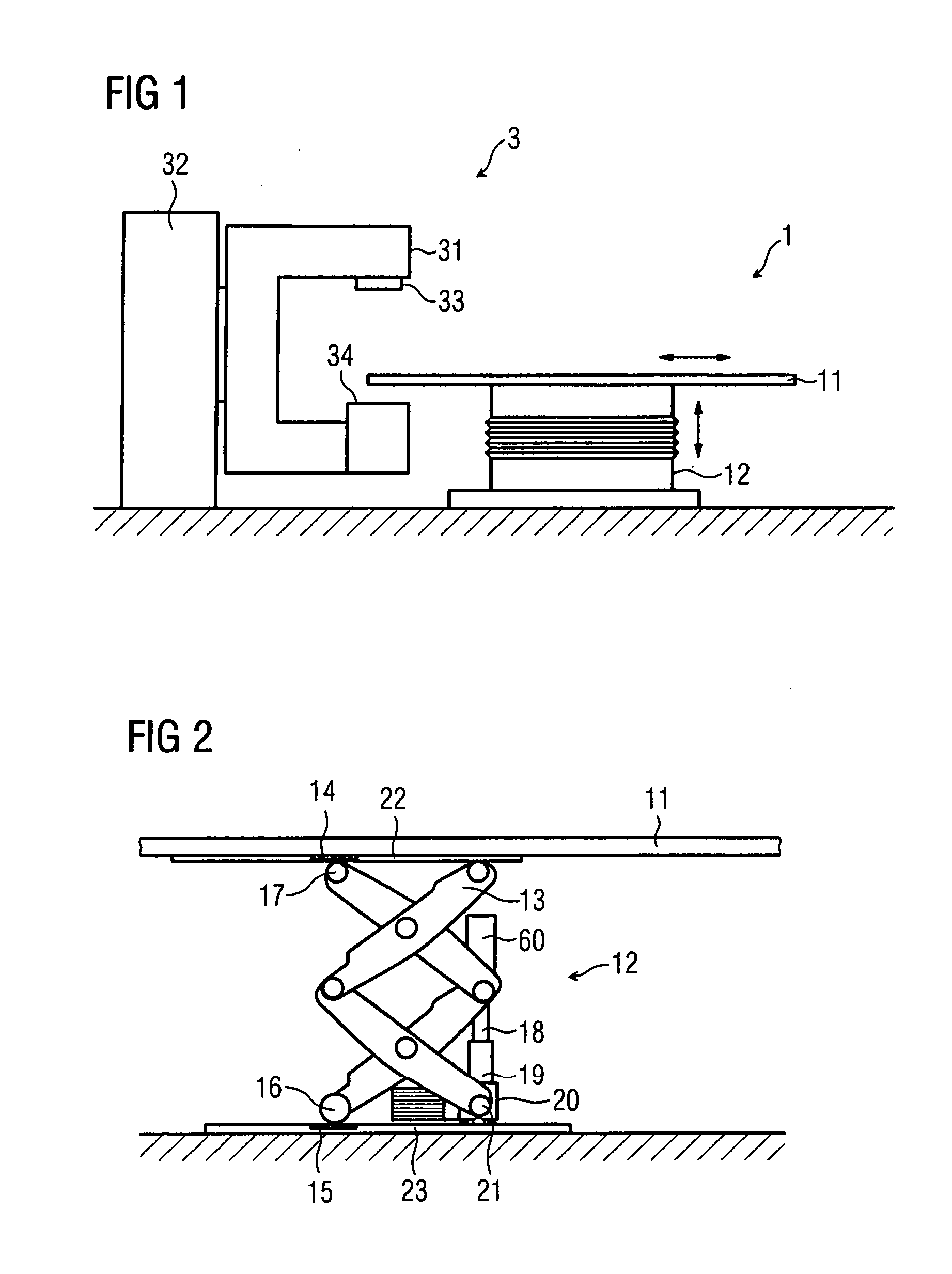
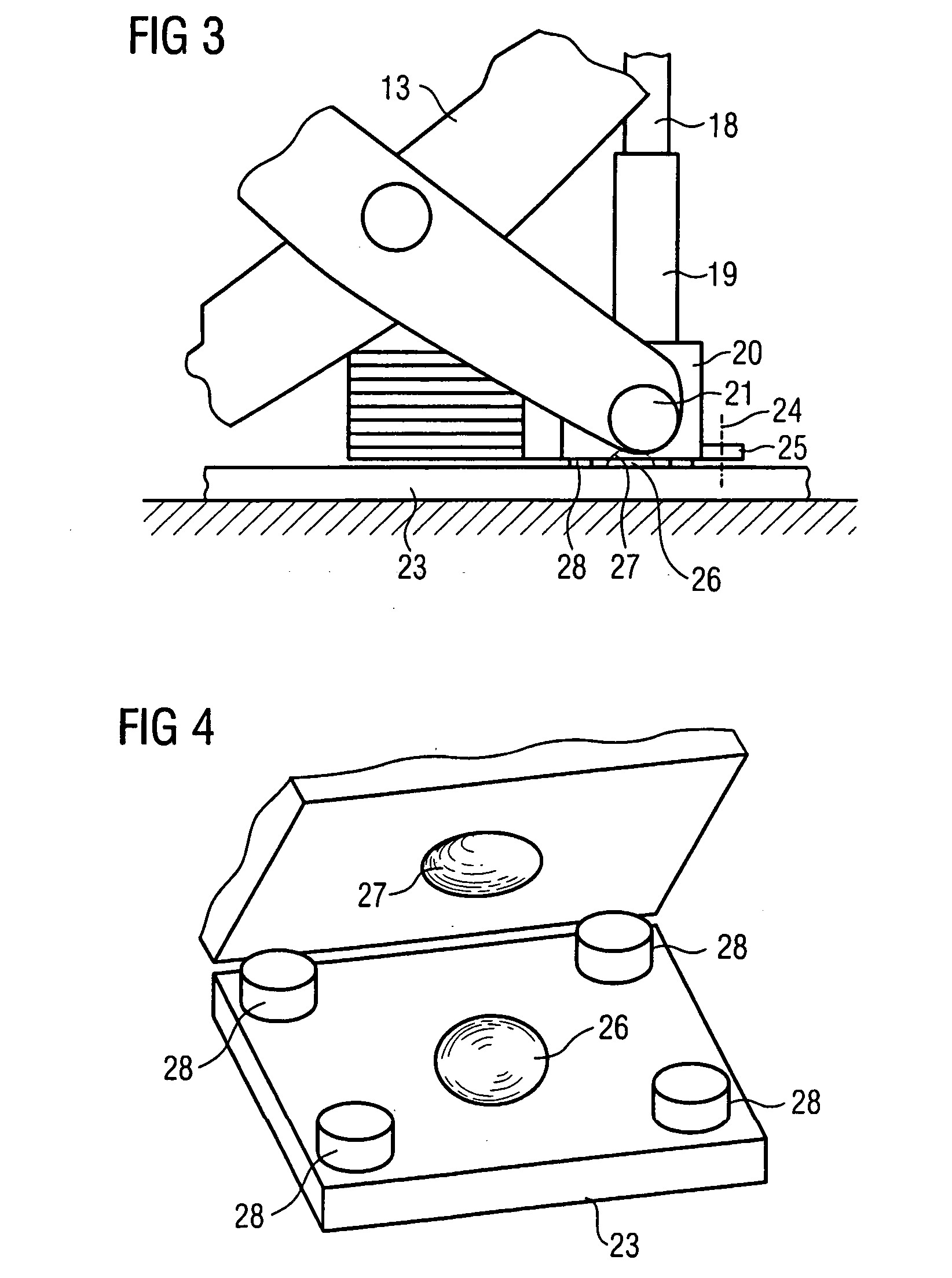
Lifting unit
InactiveUS20070079443A1Avoid fatigueReduce wearOperating tablesPatient positioning for diagnosticsLinear motionEngineering
A medical lifting device is provided. The medical lifting device comprises a lifting unit that is operable in a linear motion. A spindle drive is operable to adjust the lifting unit. A first ball joint is disposed in the lifting unit and a second ball joint is disposed where the spindle drive is respectively supported.
Owner:SIEMENS HEALTHCARE GMBH
Stabilized filament drawing device for a meltspinning apparatus and meltspinning apparatus including such stabilized filament drawing devices
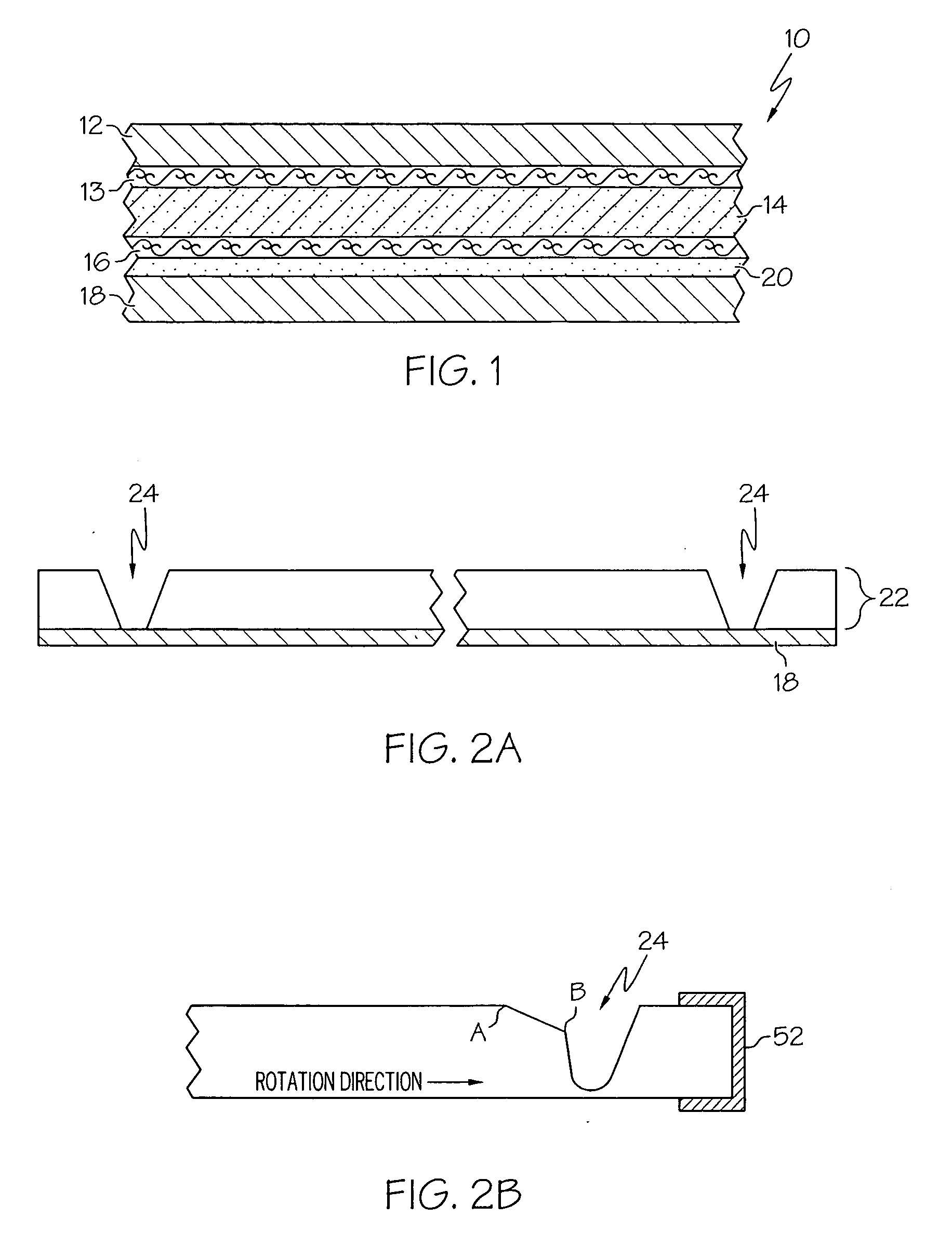
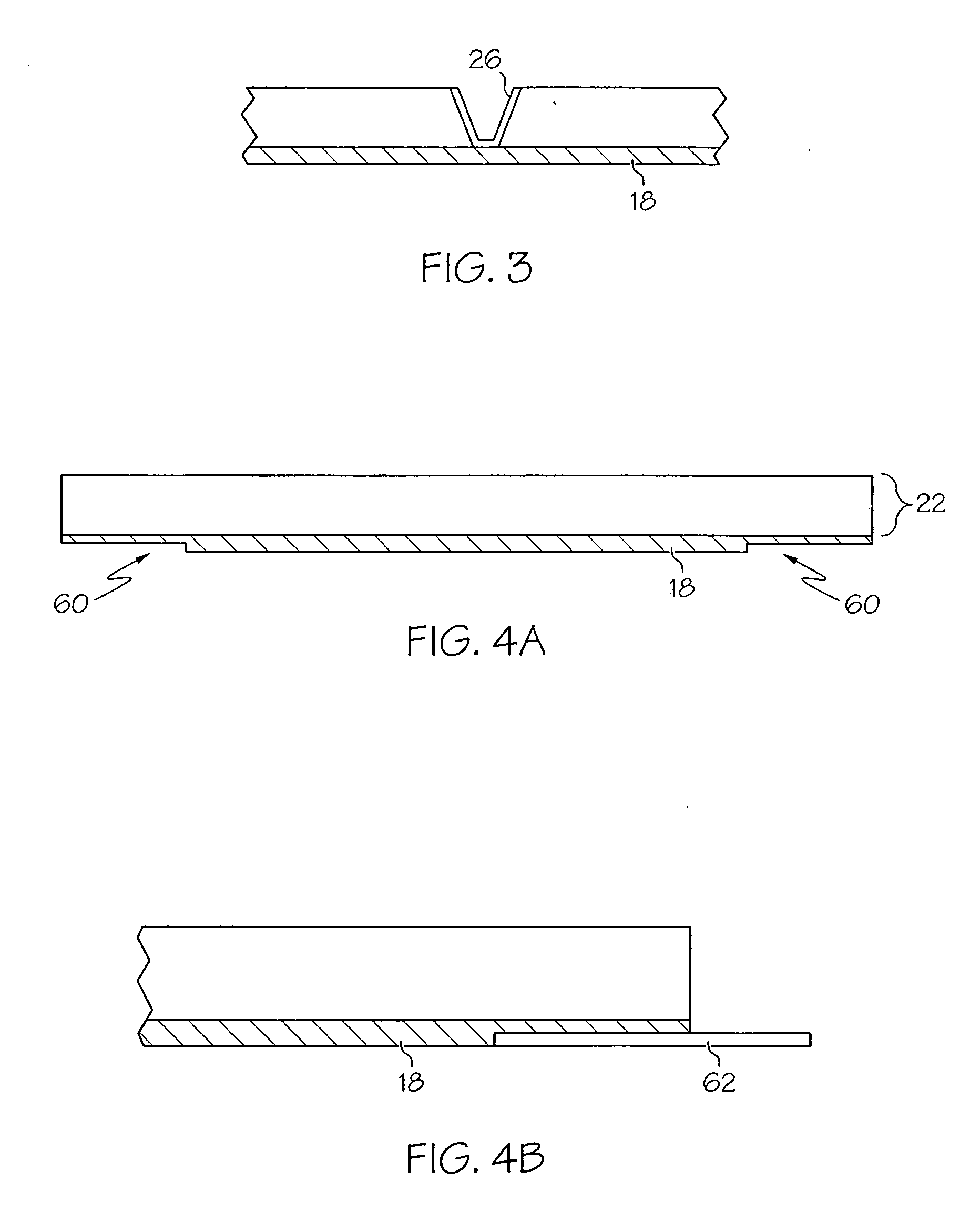
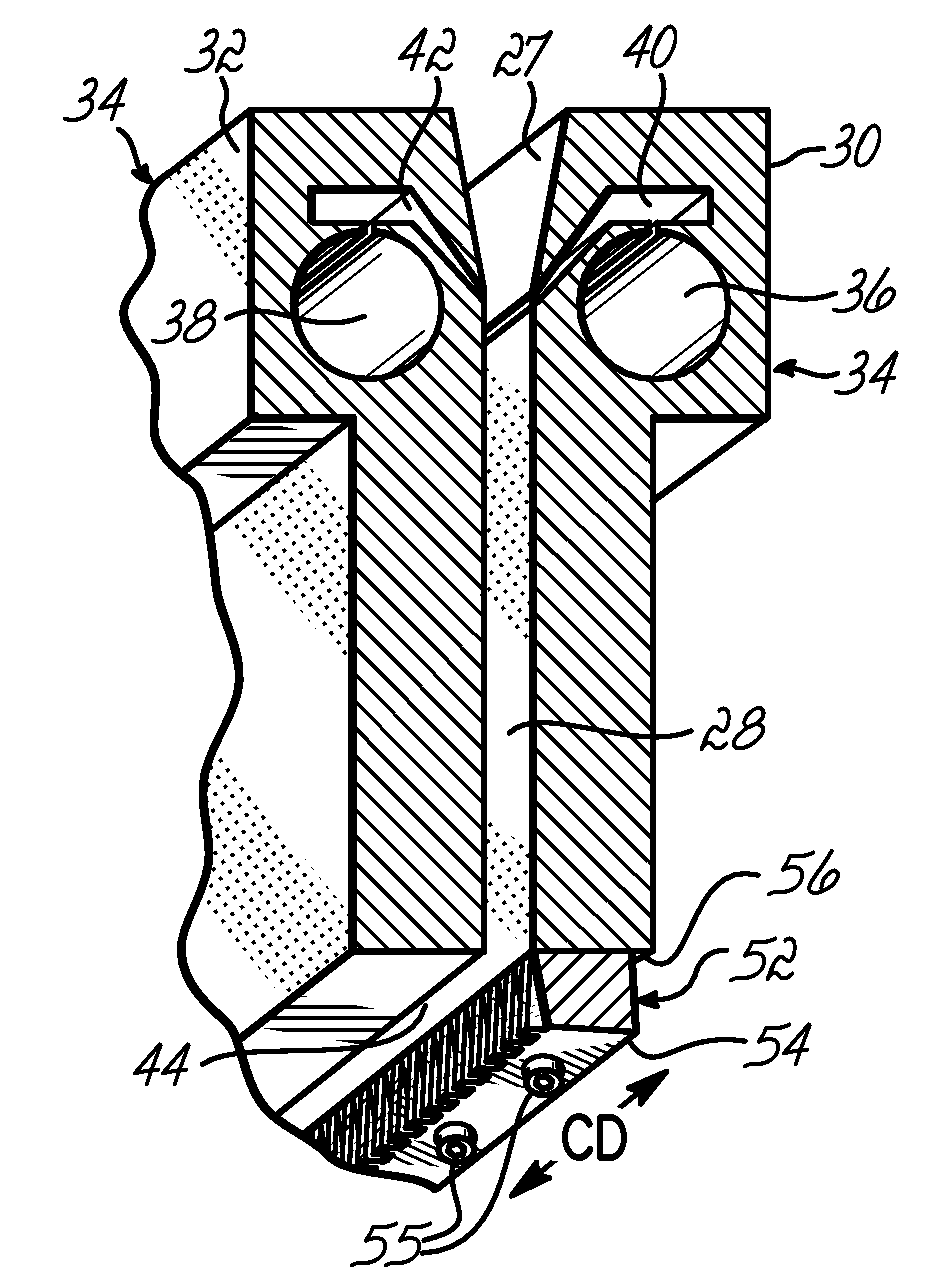
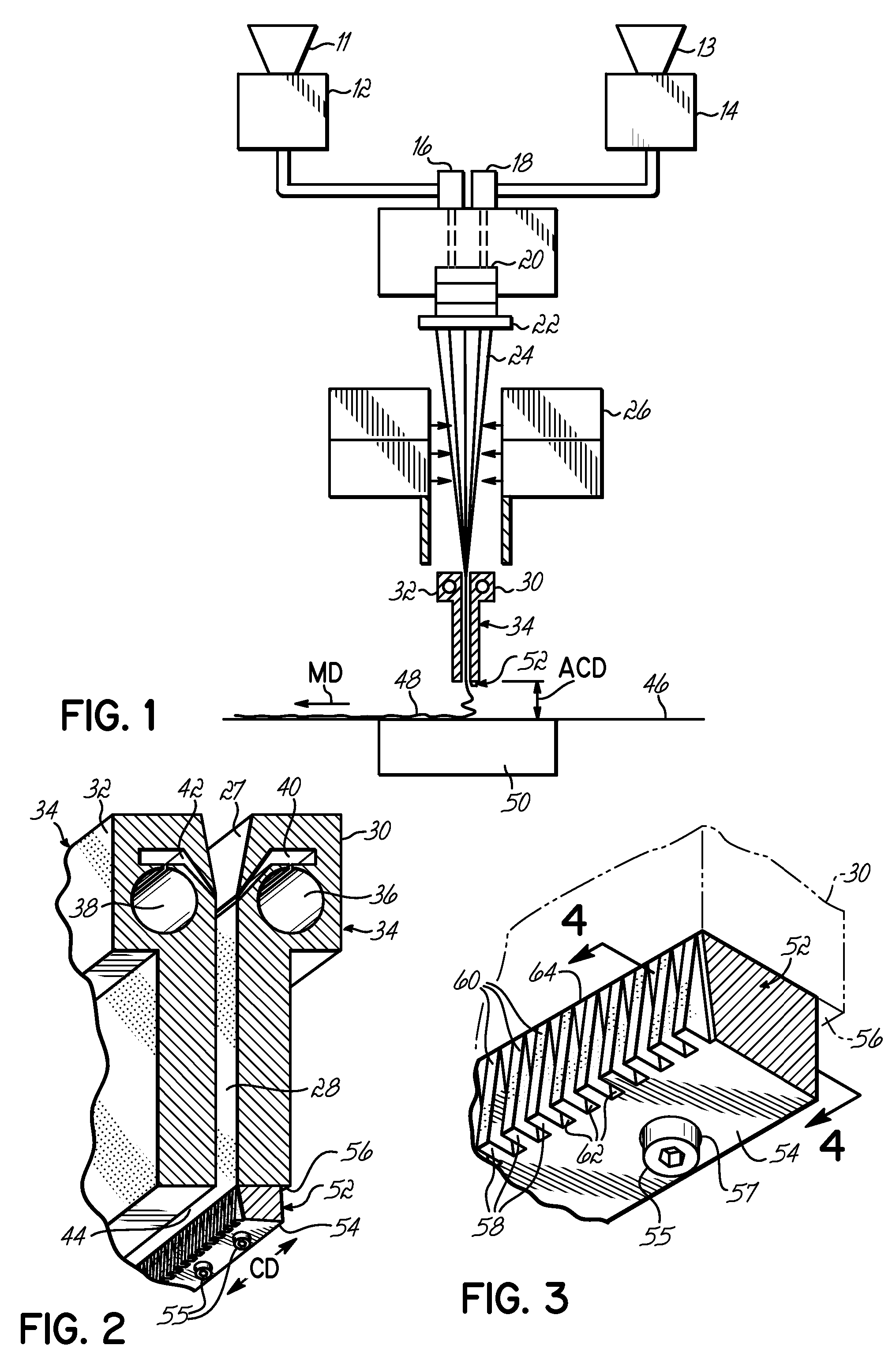
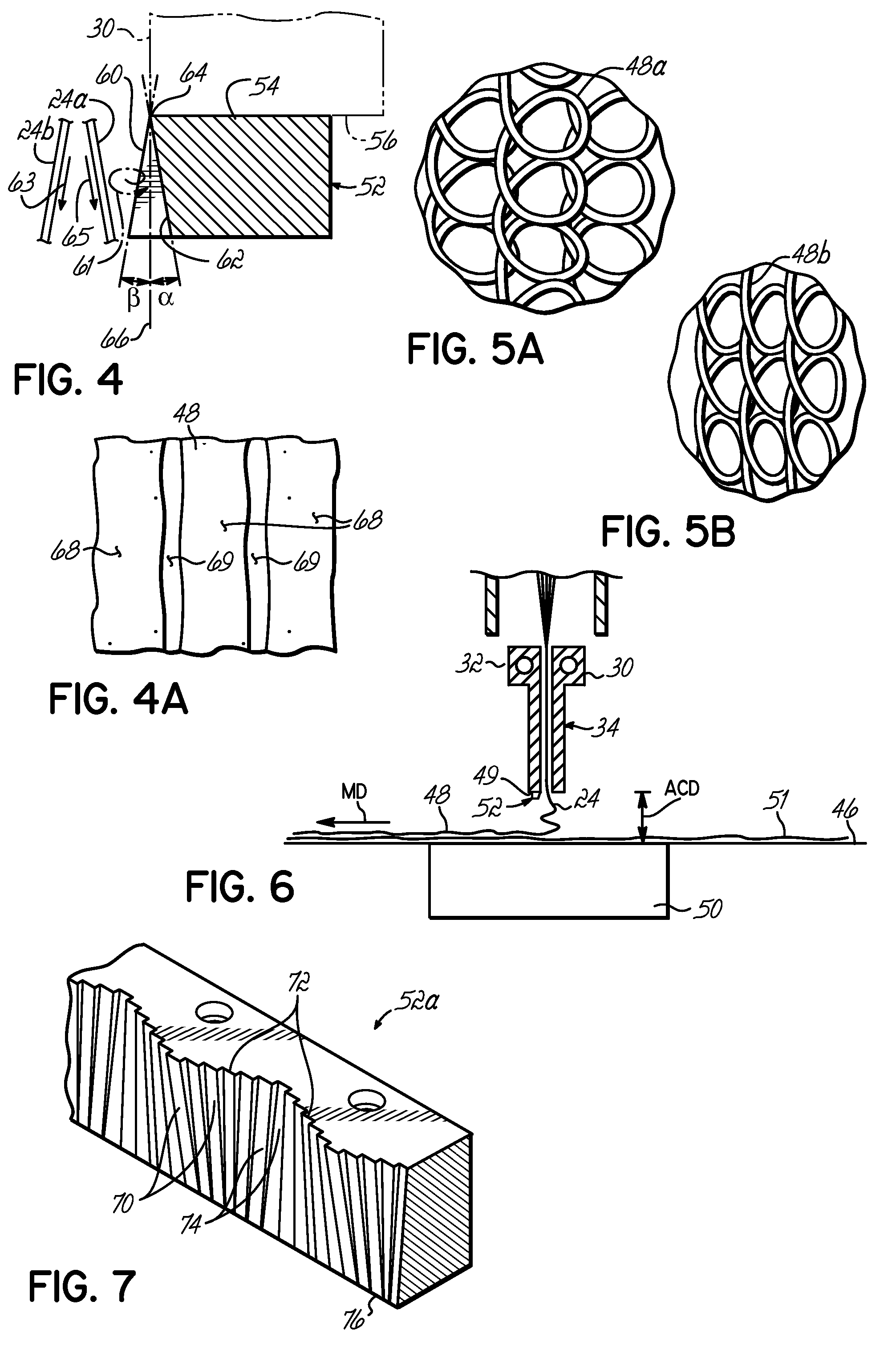
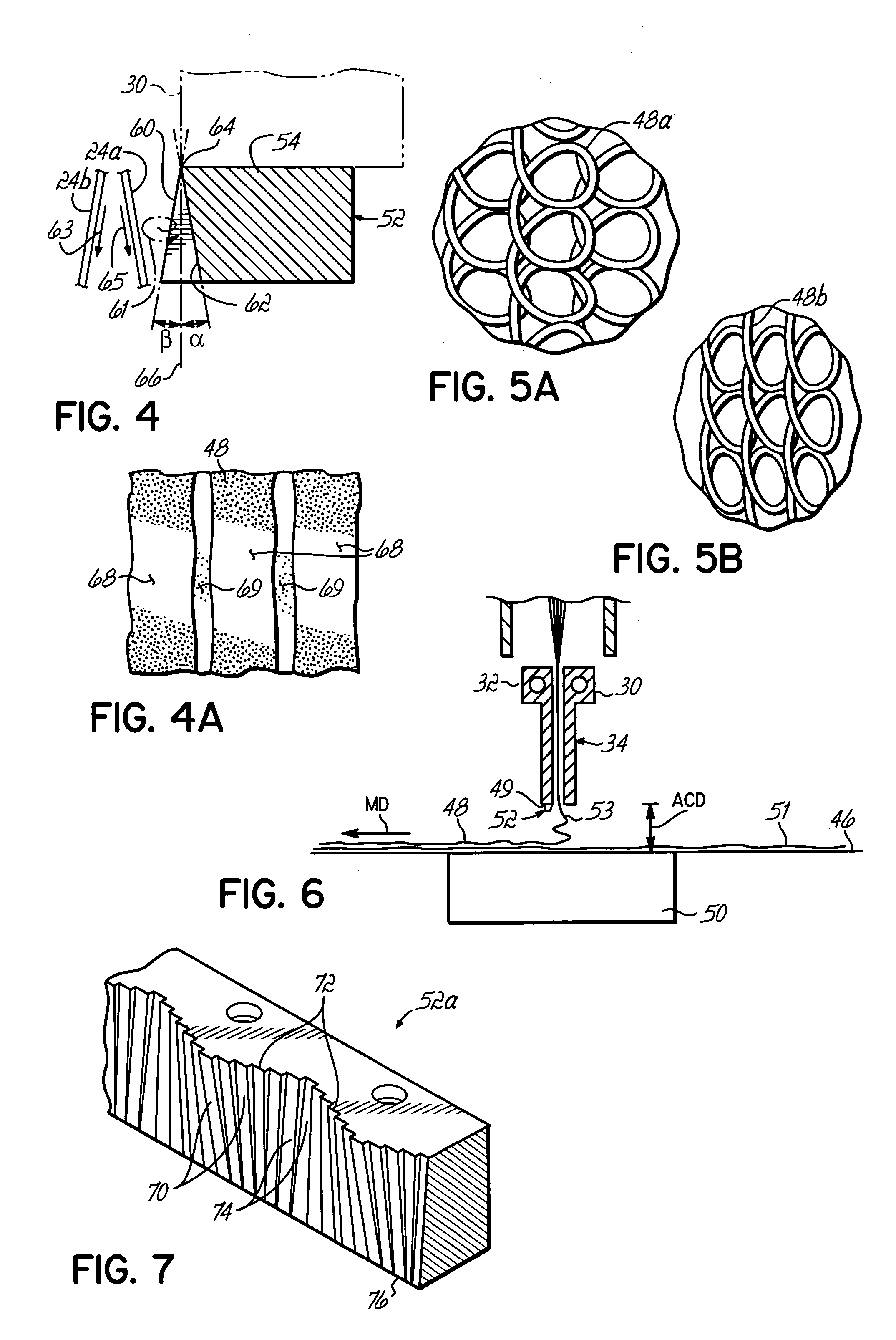
InactiveUS7172398B2Hinders its propagationReduce randomnessCeramic shaping apparatusMelt spinning methodsMechanical engineeringHigh velocity
A stabilized filament drawing device for a meltspinning apparatus and a meltspinning apparatus including the stabilized filament drawing device. The stabilized filament drawing device applies a high-velocity flow of air to attenuate the filaments, which are discharged from a device outlet in a discharge direction. The filament drawing device includes multiple inclined guides adjacent to the outlet that cause the filaments and high-velocity flow of air to deviate from the discharge direction. Each of the guides has a major surface that is angled relative to a common plane containing the discharge direction and a cross-machine direction of the device outlet of the filament drawing device. The guides are oriented such that the major surface is also inclined relative to the discharge direction.
Owner:AKTIENGES ADOLPH SAURER
Systems and methods of blood-based therapies having a microfluidic membraneless exchange device
InactiveUS20090292234A1Reduces undesirable activationPrevents orSamplingMedical devicesThin layerMembrane configuration
The present invention is directed to devices, systems and methods for removing undesirable materials from a sample fluid by contact with a second fluid. The sample fluid flows as a thin layer adjacent to, or between, concurrently flowing layers of the second fluid, without an intervening membrane. In various embodiments, a secondary separator is used to restrict the removal of desirable substances and effect the removal of undesirable substances from blood. The invention is useful in a variety of situations where a sample fluid is to be purified via a diffusion mechanism against an extractor fluid. Moreover, the invention may be used for the removal of components from a sample fluid that vary in size. When blood is the sample fluid, for example, this may include the removal of ‘small’ molecules, ‘middle’ molecules, macromolecules, macromolecular aggregates, and cells, from the blood sample to the extractor fluid.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Hem flange joint
ActiveUS20100266809A1Inhibits bubble formationPrevents orLaminationAdhesive processes with adhesive heatingAdhesiveEngineering
The present invention relates to a method of producing an edging-fold bond. For this purpose use is made more particularly of high-viscosity adhesives. The method features reduced bubble formation within the edging fold and also within the sealant (where present) that seals the edging-fold seam. More particularly the adhesive comprises spacers which further reinforce this effect.
Owner:SIKA TECH AG
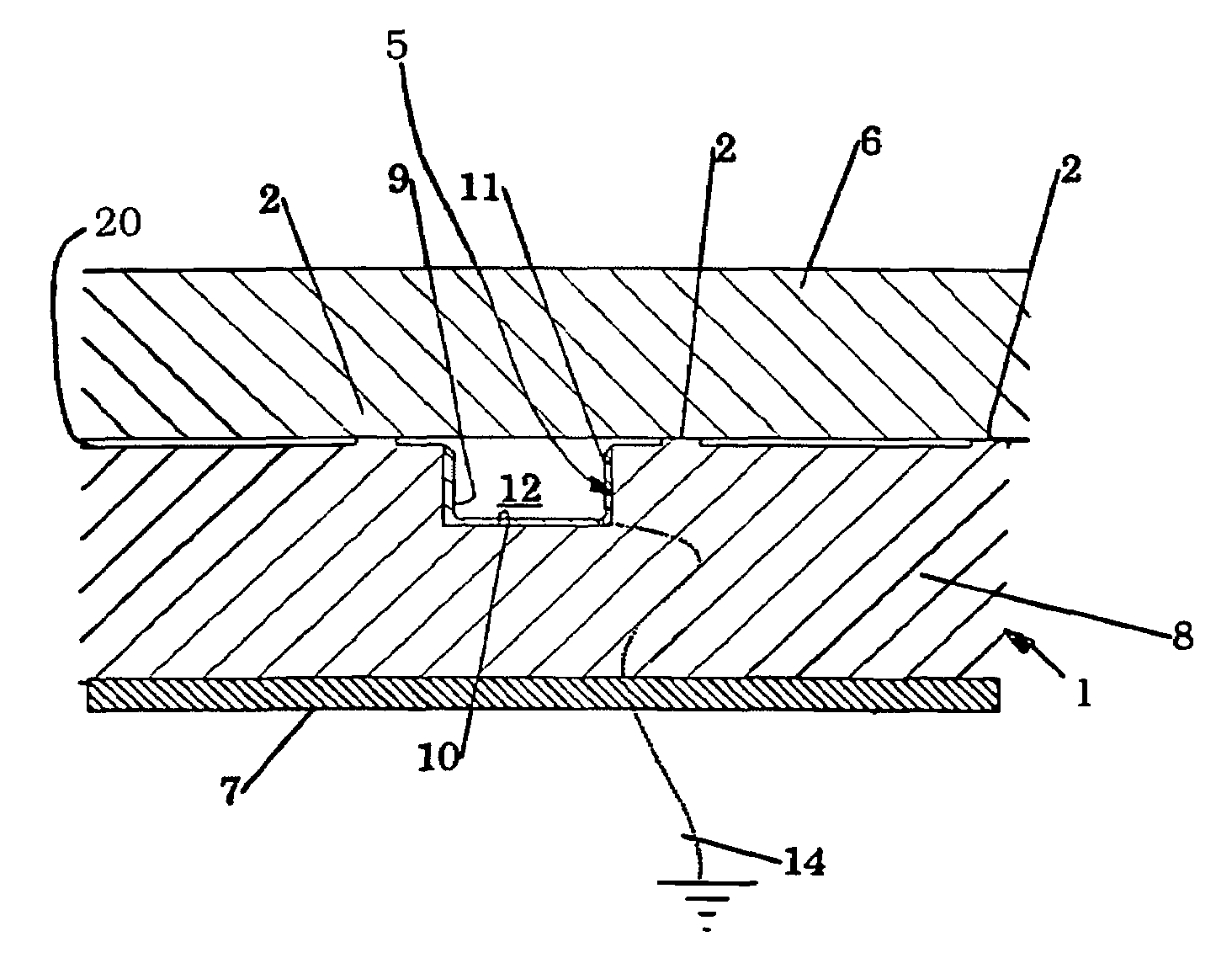
Lithographic apparatus and device manufacturing method
ActiveUS7088431B2Negatively contributeBreakthrough in the channel is effectively preventedSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusEngineeringLighting system
Owner:ASML NETHERLANDS BV
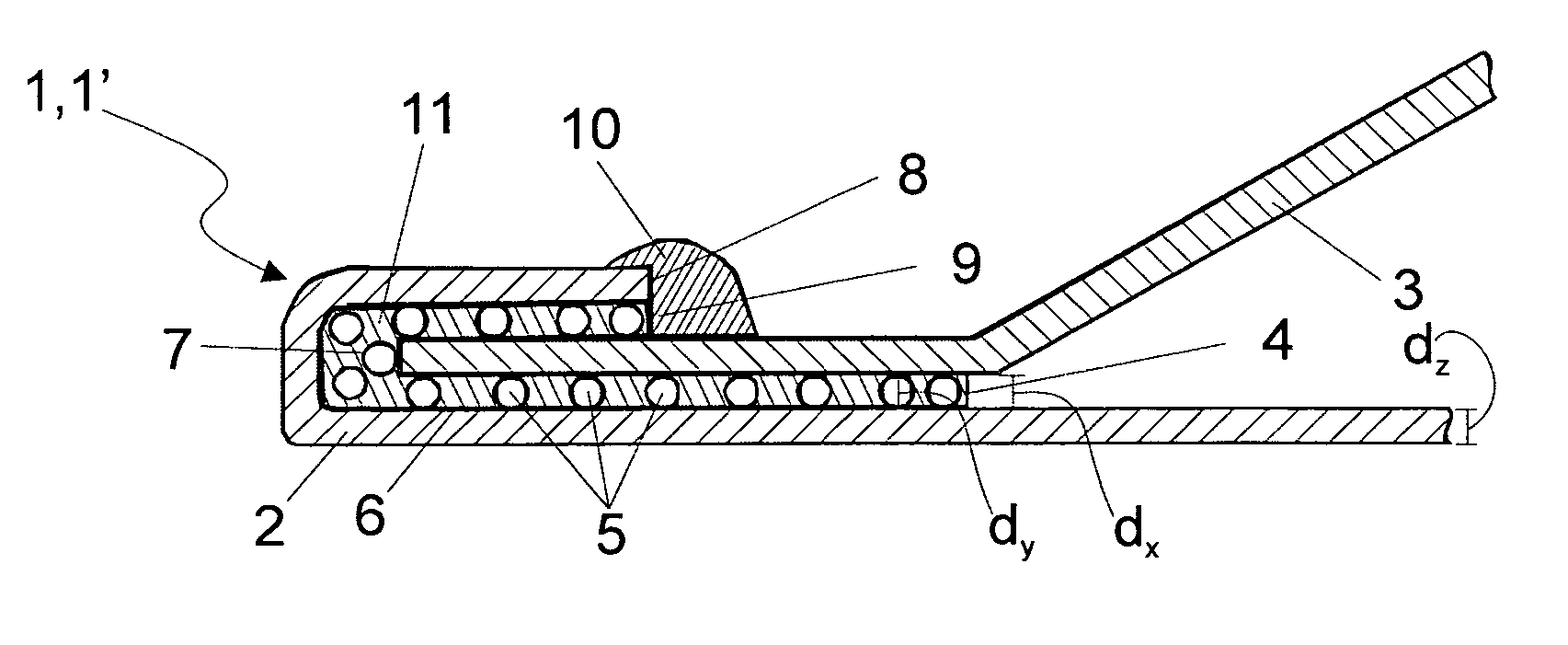
Needle catheter with an angled distal tip lumen
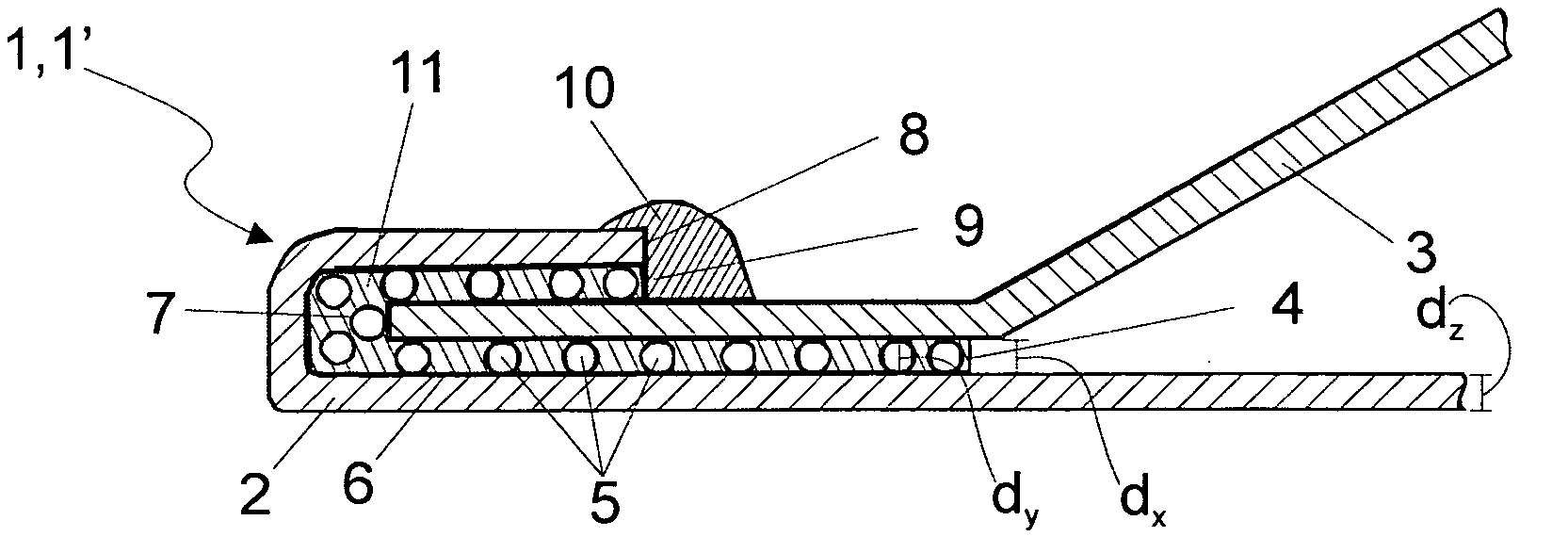
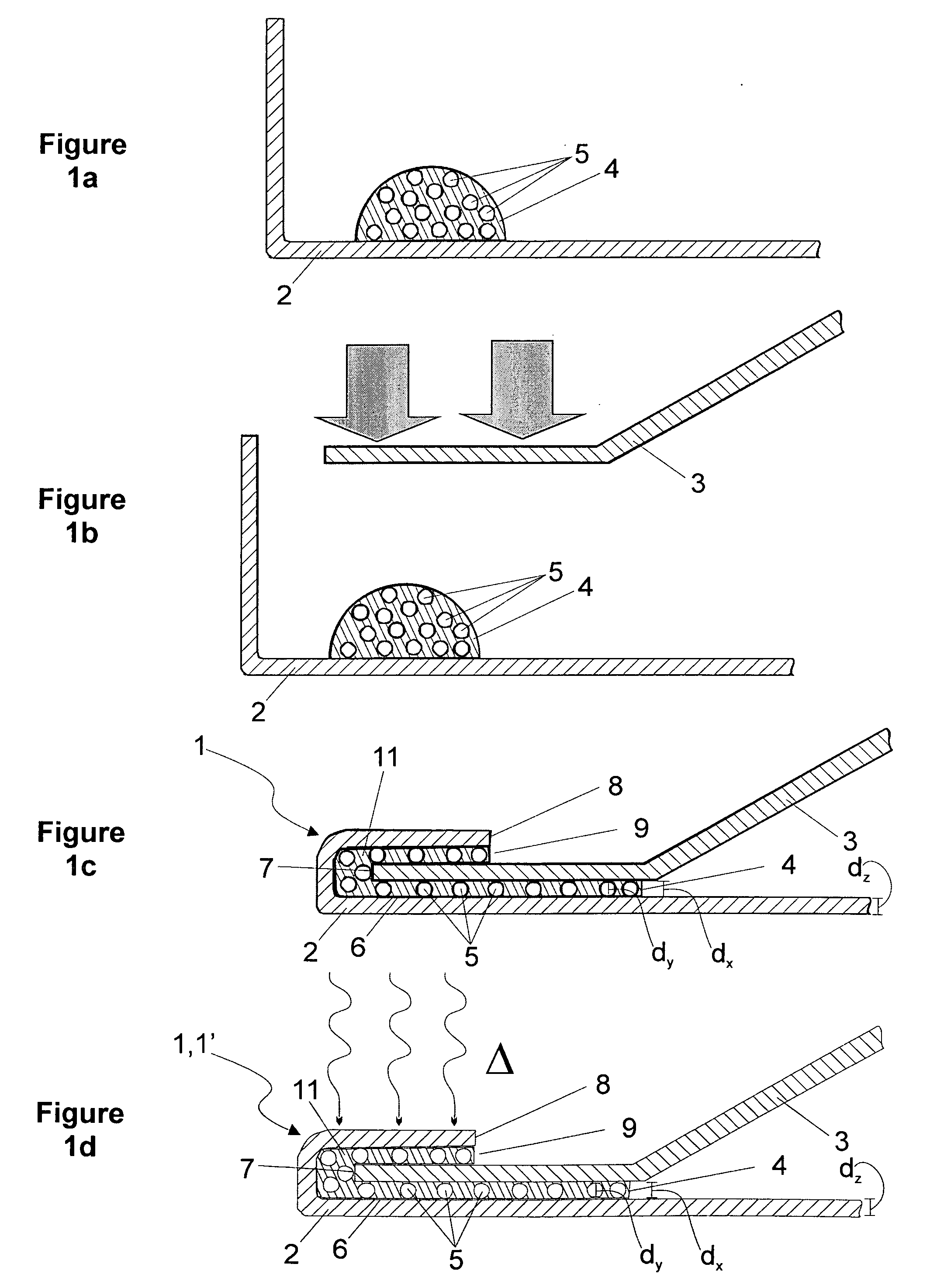
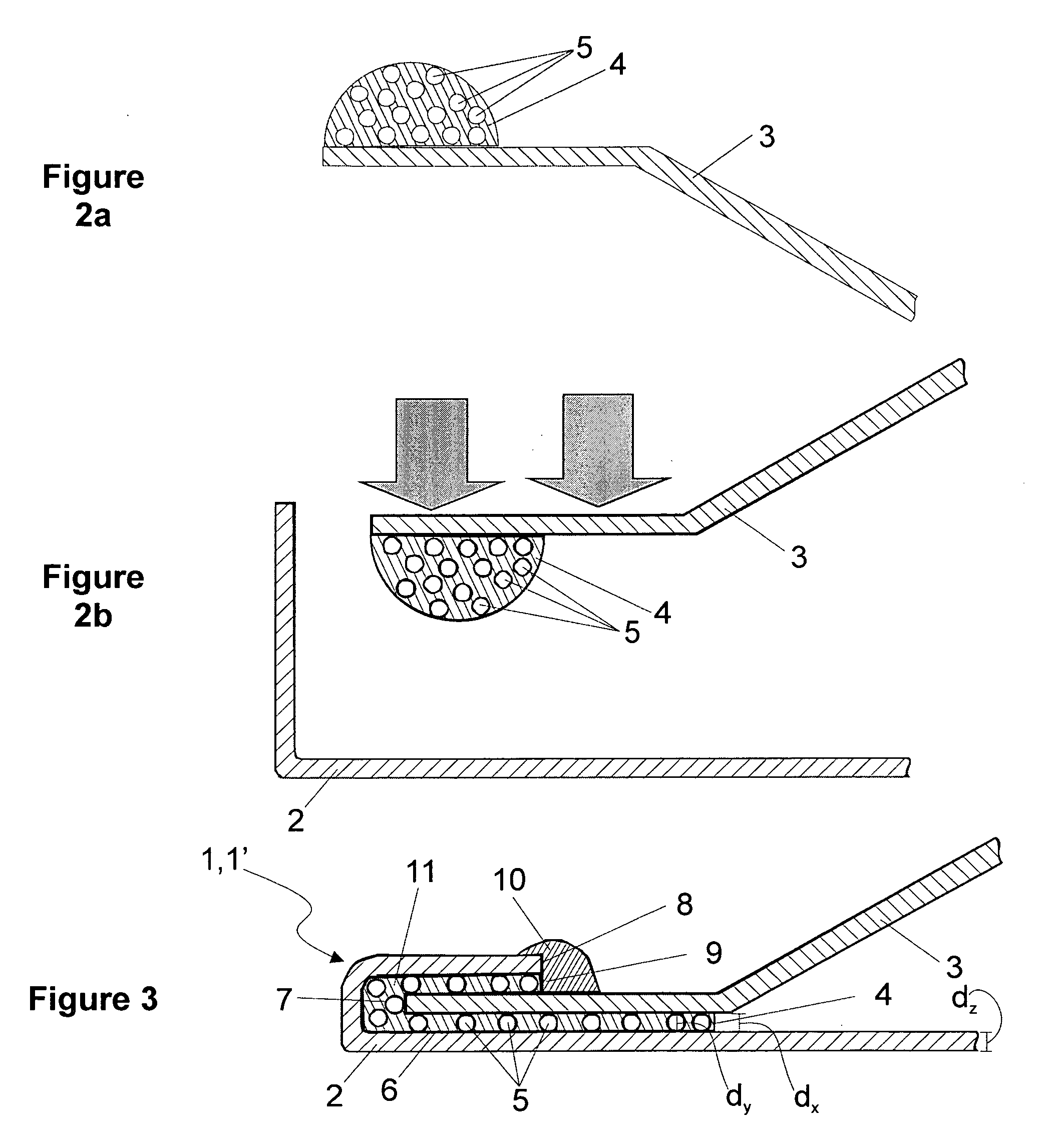
ActiveUS8486022B2High retention rateEasy to pushInfusion syringesMedical devicesInjection siteNeedle catheter
A needle catheter configured for injecting an agent into a wall of a patient's body cavity, which directs a needle from the distal tip of the catheter into the wall of the body cavity at an angle relative to the axis of the shaft. The resulting angled injection pathway improves the retention of the agent in the body cavity wall, while keeping a distal section of the catheter substantially perpendicular to the body cavity wall for optimal push against the tissue at the injection site.
Owner:ABBOTT CARDIOVASCULAR
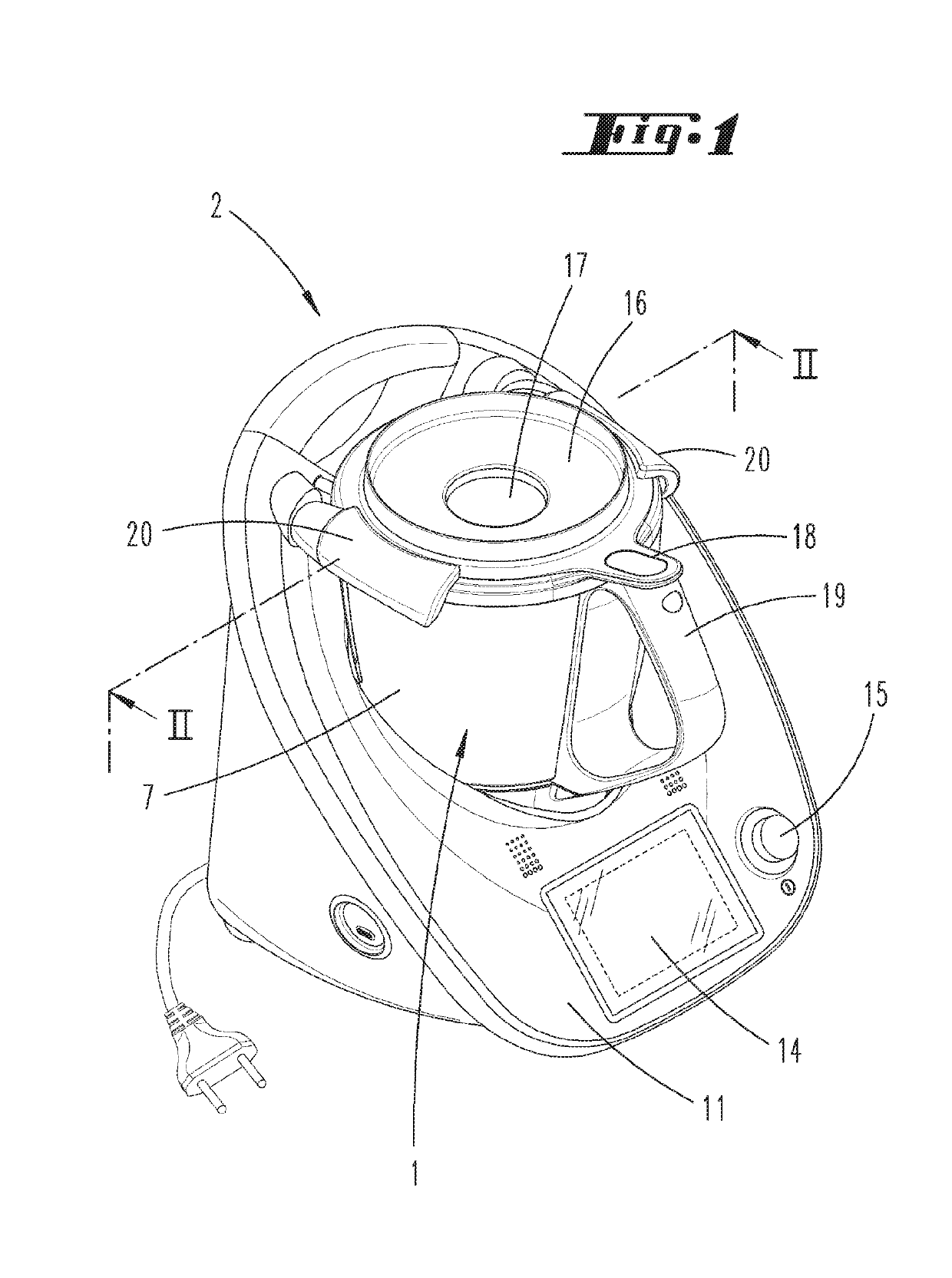
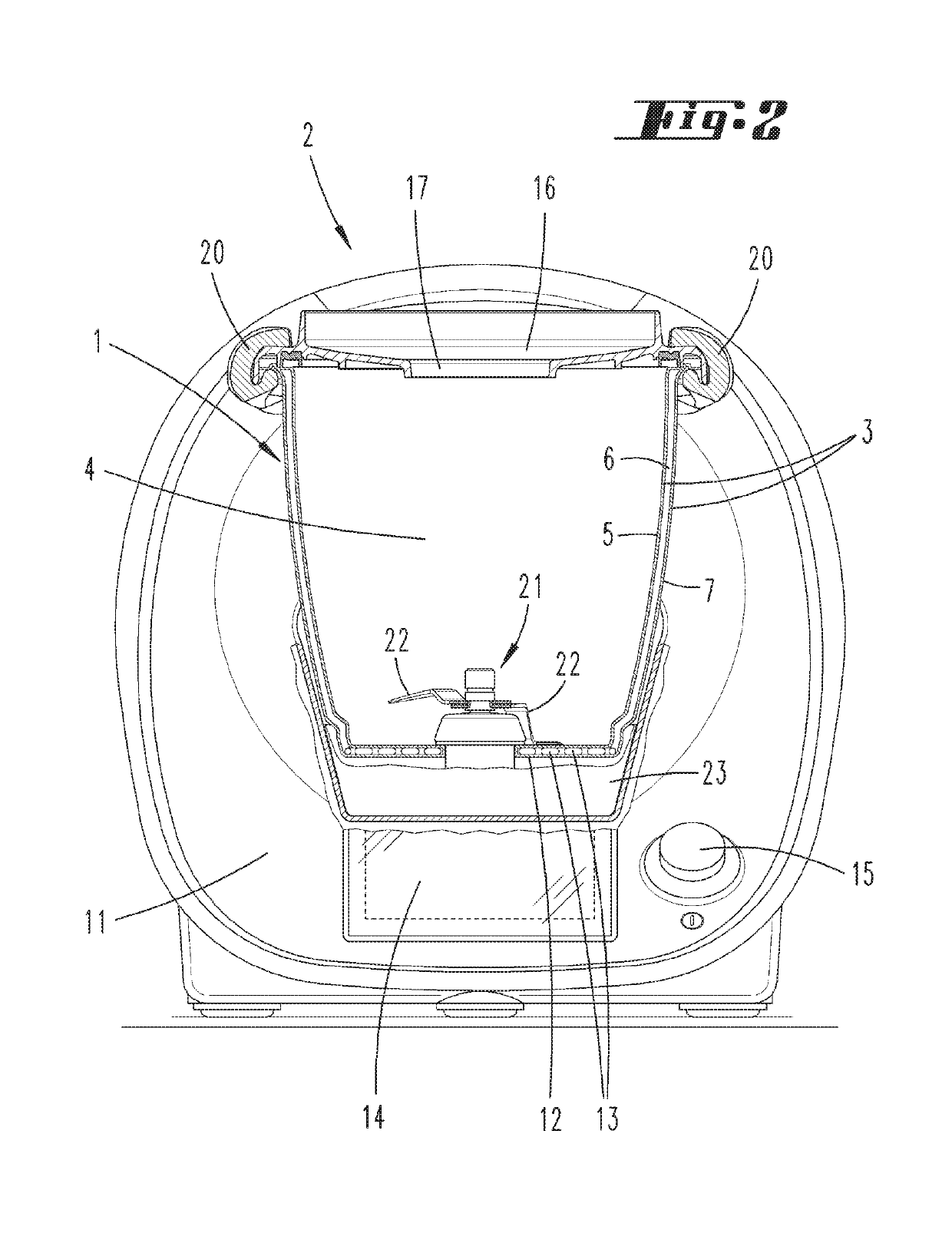
Preparation vessel for a food processor
InactiveUS20190174945A1Improve insulation performanceIncrease insulation functionSteam cooking vesselsVessels with intergral heatingDouble wallEngineering
A preparation vessel for a food processor has a vessel wall, at least a partial area of which has a double-wall configuration, with an inner wall that borders a preparation chamber of the preparation vessel and an outer wall designed separately from the latter and spaced apart from the inner wall by an intermediate space. In order to optimize such a preparation vessel with respect to both heating and / or heat retention as well as outward insulation, the intermediate space has a vacuum with a gas pressure of less than 300 mbar and / or an insulating material with a thermal conductivity of less than 0.02 W / (mK).
Owner:VORWERK & CO INTERHOLDING GMBH
Bio-battery with enhanced yield
ActiveUS20110135967A1Easily implanted in human bodyPrevents orIndirect fuel cellsBiochemical fuel cellsHydronium ionElectron exchange
A novel cell including first and second chambers containing a solvent and separated by a wall permeable to the solvent and impermeable to hydronium and / or hydroxyl ions; a first electrode in the first chamber; a second electrode in the second chamber; a first redox couple in the first chamber comprising a first oxidizer and a first reducer taking part in first oxidation-reduction reactions resulting in an electron exchange with the first electrode; a second redox couple in the second chamber comprising a second oxidizer and a second reducer taking part in second oxidation-reduction reactions resulting in an electron exchange with the second electrode, the wall being impermeable to the first and second redox couples; and first enzymes or first microorganisms placed in the first or second chamber and promoting a third oxidation-reduction reaction resulting transforming a first substance to a second substance comprising acid or alkaline species.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Printing blanket including a non-extensible backing layer and a relief area which may be mounted in a variety of lockup mechanisms
InactiveUS20070101884A1Gauge of the blanket to be reducedReduce the gap widthRotary pressesPrinting blanketsEngineeringPrinting press
A printing blanket is provided which may include a printable surface ply, a compressible ply, one or more reinforcing fabric plies, and a non-extensible backing layer comprising a polymeric material. The printing blanket has first and seconds ends which are adapted to be inserted into the gap of a printing blanket cylinder, where each of the first and second ends may include a relief area formed by removing or molding a portion of the blanket or by removing or molding a portion of the non-extensible backing layer such that the blanket may be mounted in a wide variety of printing presses using a number of different lock-up mechanisms.
Owner:DAY INT
Stabilized filament drawing device for a meltspinning apparatus and meltspinning apparatus including such stabilized filament drawing devices
InactiveUS20060172024A1Hinders its propagationReduce randomnessCeramic shaping apparatusMelt spinning methodsEngineeringHigh velocity
A stabilized filament drawing device for a meltspinning apparatus and a meltspinning apparatus including the stabilized filament drawing device. The stabilized filament drawing device applies a high-velocity flow of air to attenuate the filaments, which are discharged from a device outlet in a discharge direction. The filament drawing device includes multiple inclined guides adjacent to the outlet that cause the filaments and high-velocity flow of air to deviate from the discharge direction. Each of the guides has a major surface that is angled relative to a common plane containing the discharge direction and a cross-machine direction of the device outlet of the filament drawing device. The guides are oriented such that the major surface is also inclined relative to the discharge direction.
Owner:AKTIENGES ADOLPH SAURER
Stabilized filament drawing device for a meltspinning apparatus
InactiveUS20050104261A1Reduce turbulencePrevent propagationCeramic shaping apparatusMelt spinning methodsAirflowEngineering
A stabilized filament drawing device for a meltspinning apparatus. The stabilized filament drawing device applies a high-velocity flow of air to attenuate the filaments, which are discharged from a device outlet in a discharge direction. The filament drawing device includes multiple inclined guides adjacent to the outlet that cause the high-velocity flow of air to deviate from the discharge direction by the Coanda effect. The filaments, which are entrained in the high-velocity process air, likewise deviate from the discharge direction. The guides are arranged such that spaces are absent between adjacent guides.
Owner:OERLIKON TEXTILE GMBH & CO KG
Hem flange joint
ActiveUS9034135B2Prevents orInhibits bubble formationLaminationAdhesive processes with adhesive heatingAdhesiveEngineering
The present invention relates to a method of producing an edging-fold bond. For this purpose use is made more particularly of high-viscosity adhesives. The method features reduced bubble formation within the edging fold and also within the sealant (where present) that seals the edging-fold seam. More particularly the adhesive comprises spacers which further reinforce this effect.
Owner:SIKA TECH AG
Nozzle assembly with fuel tube deflector
ActiveUS20050067506A1Prevents and at least reduces thermal stressOvercomes drawbackLiquid surface applicatorsContinuous combustion chamberFuel lineInjector
A deflector at the downstream end of a fuel tube deflects incoming fuel into an annular fuel channel in a fuel swirler of an injector. The deflector prevents direct impact of the cooler fuel on the exposed walls of the swirler body. The deflector can be formed by a tab or other integral portion of the heatshield, or as a separate piece fixed to the heatshield or swirler body. The deflector minimizes disruption of flow through any flow slots that are covered by the deflector, and may have i) a relatively small circumferential extent, such that the deflector covers only a few flow slots, ii) flow openings or slots that allow a portion of the fuel to flow inwardly of the deflector into the otherwise covered flow slot(s), or iii) a space or gap between the side(s) of the deflector and the inner walls of the annular channel.
Owner:PARKER INTANGIBLES LLC
Needle catheter with an angled distal tip lumen
ActiveUS20100004627A1Improve retention timeImprove stabilityInfusion syringesMedical devicesNeedle catheterInjection site
A needle catheter configured for injecting an agent into a wall of a patient's body cavity, which directs a needle from the distal tip of the catheter into the wall of the body cavity at an angle relative to the axis of the shaft. The resulting angled injection pathway improves the retention of the agent in the body cavity wall, while keeping a distal section of the catheter substantially perpendicular to the body cavity wall for optimal push against the tissue at the injection site.
Owner:ABBOTT CARDIOVASCULAR
Stabilized filament drawing device for a meltspinning apparatus
InactiveUS7320581B2Hinders its propagationReduce randomnessCeramic shaping apparatusMelt spinning methodsHigh velocityWaste management
A stabilized filament drawing device for a meltspinning apparatus. The stabilized filament drawing device applies a high-velocity flow of air to attenuate the filaments, which are discharged from a device outlet in a discharge direction. The filament drawing device includes multiple inclined guides adjacent to the outlet that cause the high-velocity flow of air to deviate from the discharge direction by the Coanda effect. The filaments, which are entrained in the high-velocity process air, likewise deviate from the discharge direction. The guides are arranged such that spaces are absent between adjacent guides.
Owner:OERLIKON TEXTILE GMBH & CO KG
Enriched platelet wound healant
InactiveUS7112342B2Improved enriched platelet compositionDelay and prolong activated gelation periodBiocideHydroxy compound active ingredientsVitamin A AlcoholAntibiotic Y
An improved platelet gel wound healant, and methods of preparation and use thereof for healing wounds are disclosed. The improved wound healant comprises a therapeutically effective amount of activated growth factors and ascorbic acid with optional one or more additional anti-oxidant such as vitamin A and / or E, and optional one or more antibiotics.
Owner:NUO THERAPEUTICS
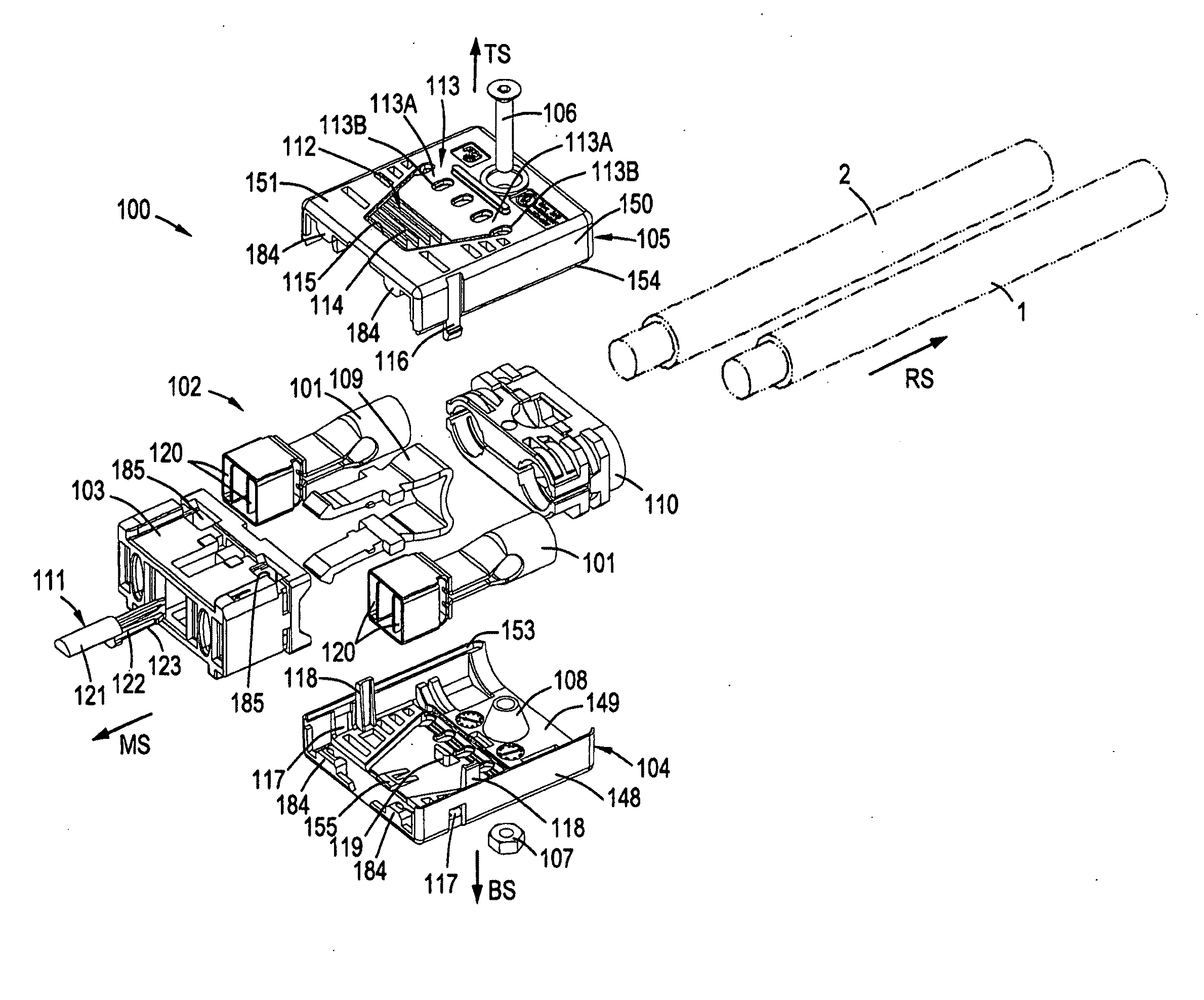
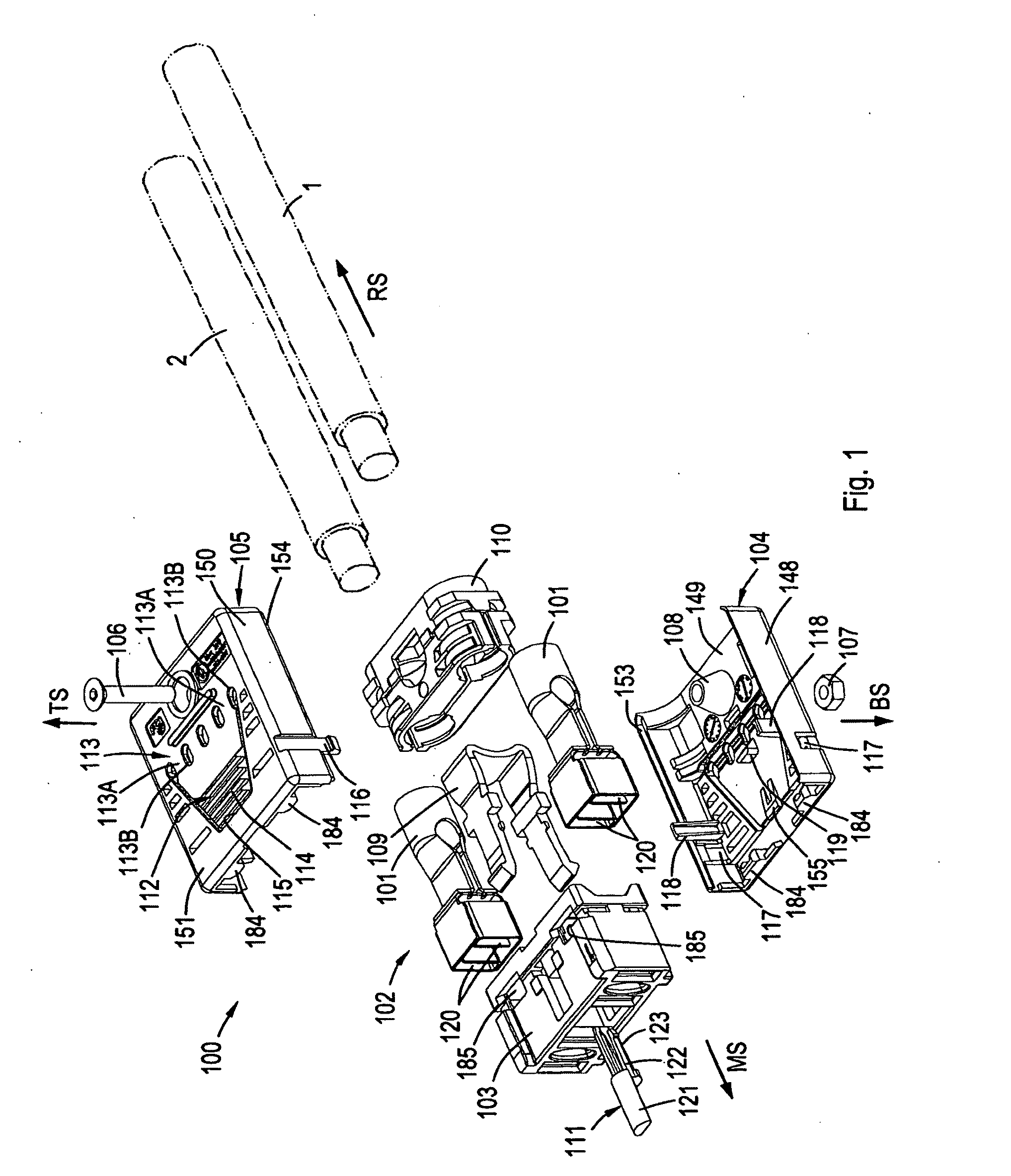
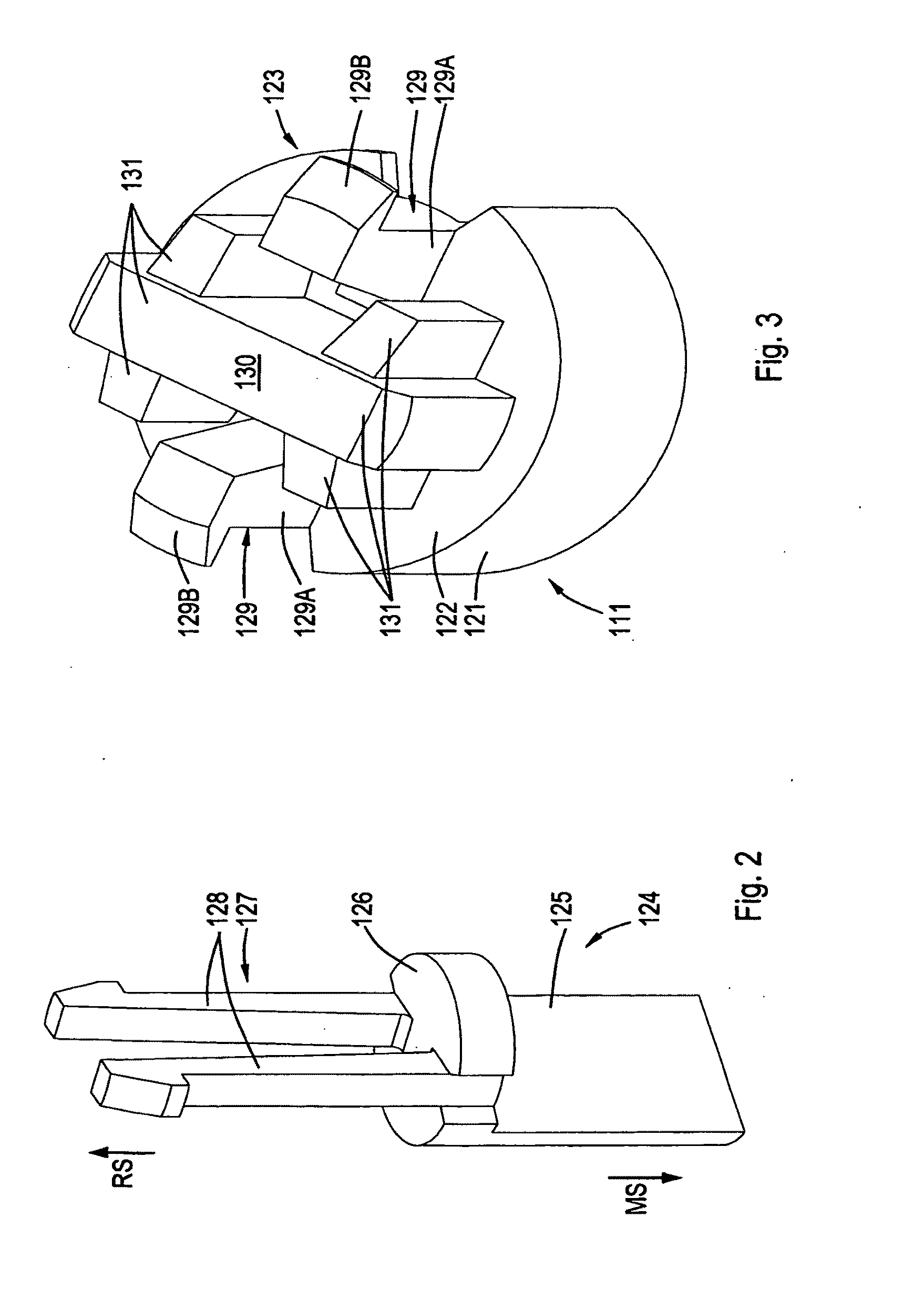
Electrical connector
ActiveUS20100087100A1Accurate guidePrevents orContact member manufacturingElectric discharge tubesMating connectionEngineering
The present invention pertains to a connector, having a rear side and a mating side and including a housing having at least one contact receiving space and at least one non-contact receiving space, the spaces extending in a direction from the mating side towards the rear side. The non-contact receiving space is adapted for receiving a portion of a mating connector housing and has a substantially rounded cross sectional shape substantially perpendicular to the direction from the mating side towards the rear side.
Owner:FCI ASIA PTE LTD
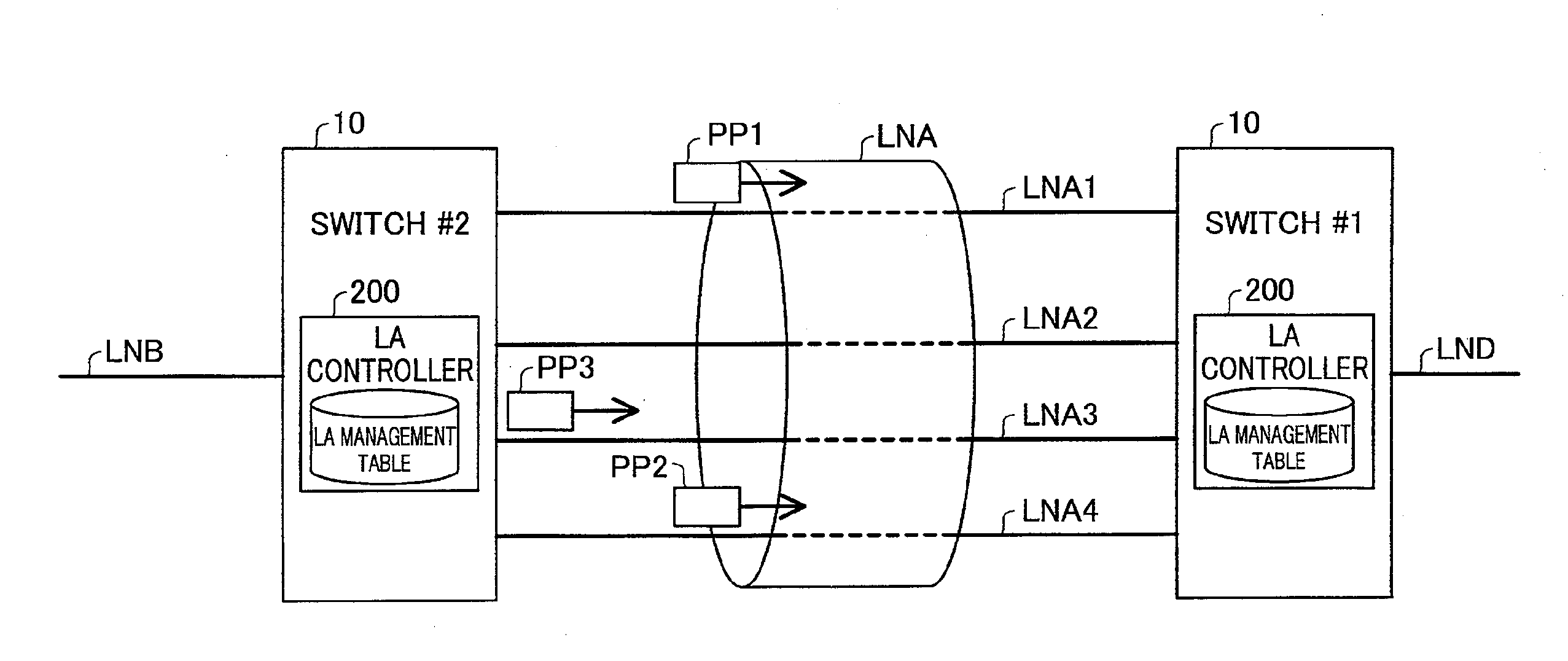
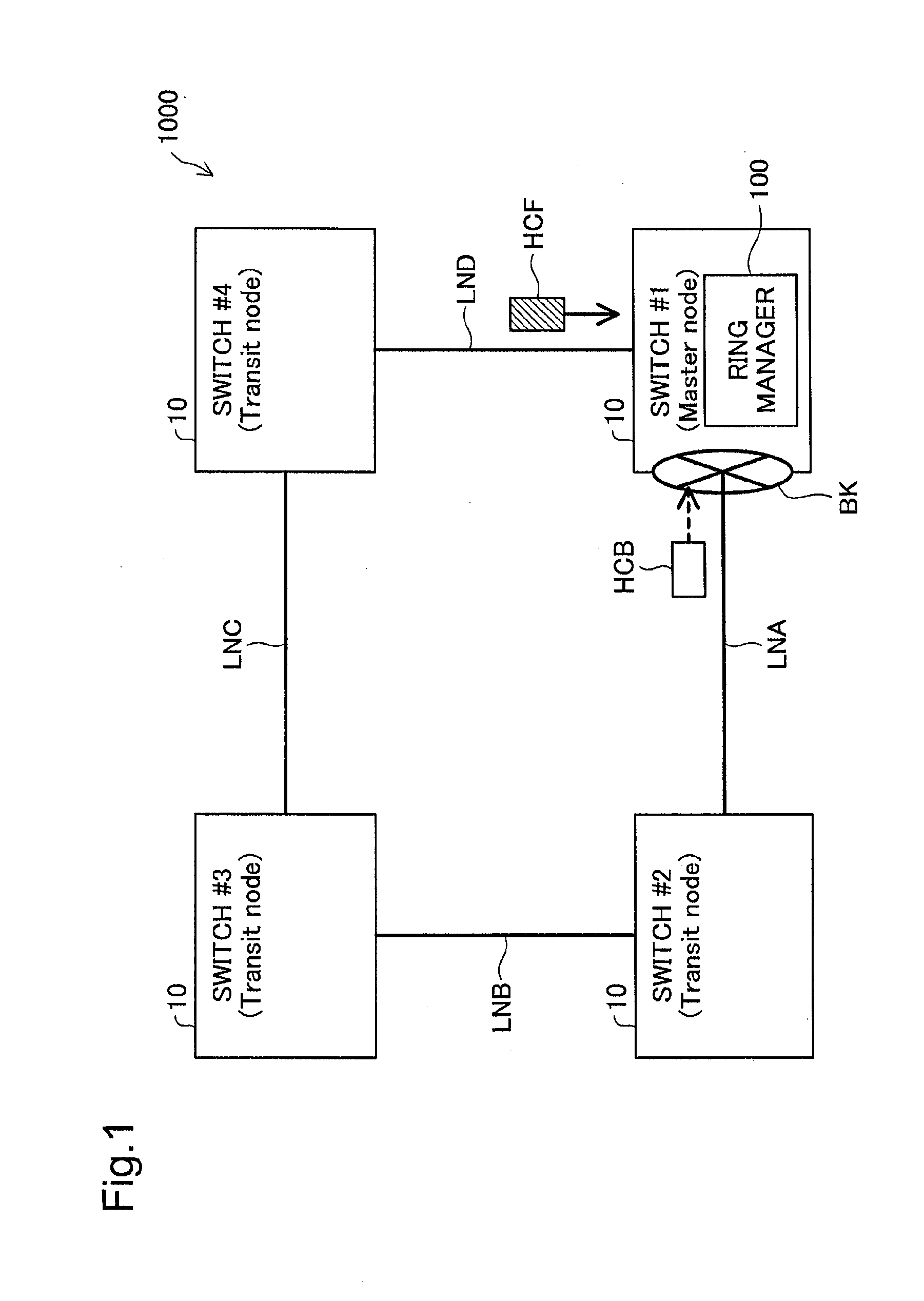
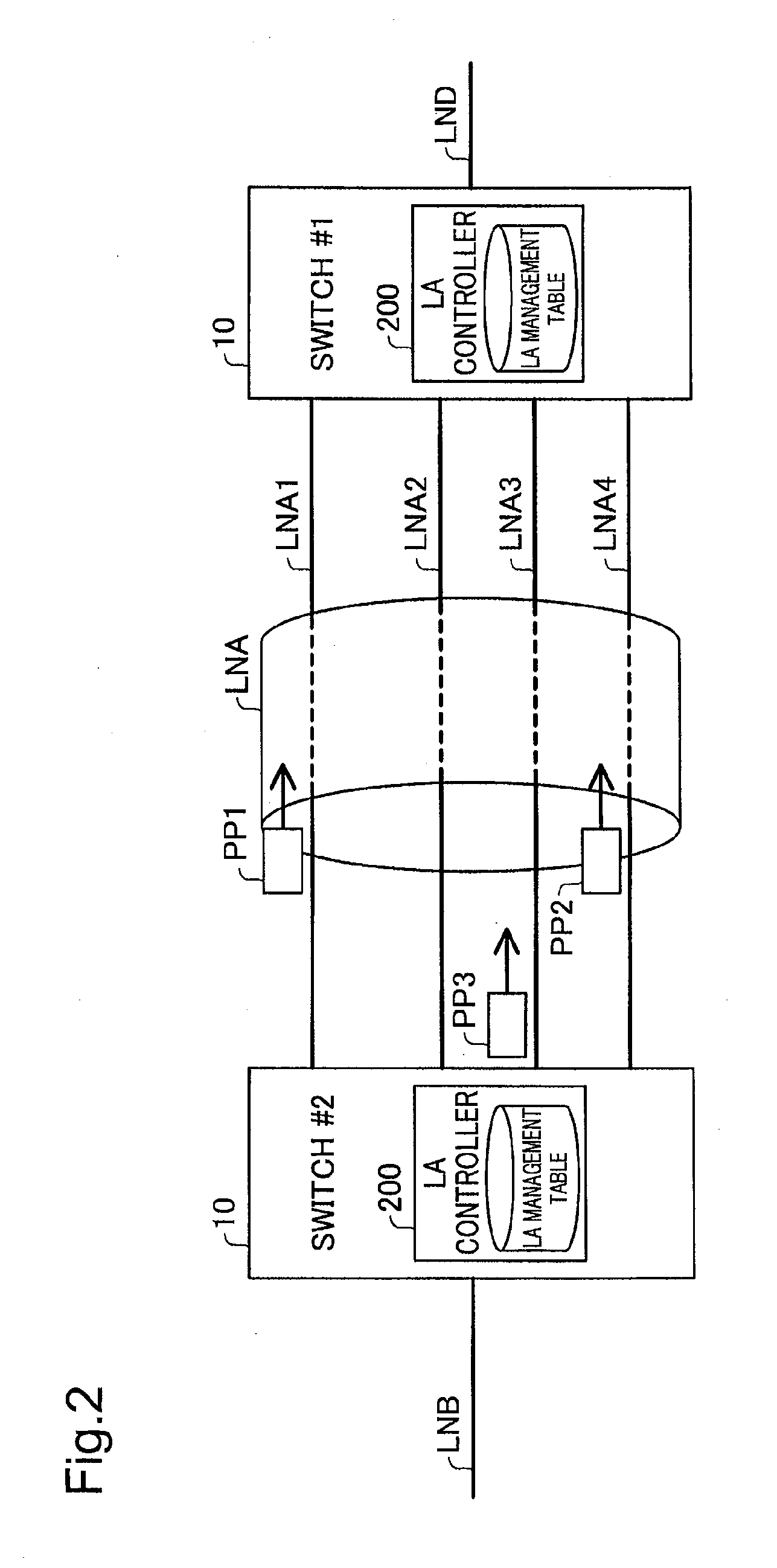
Network relay device and control method thereof
InactiveUS20130016617A1False detection of failureFalse detectionError preventionTransmission systemsContinuous useEmbedded system
The network relay device is provided. The network relay device includes: a plurality of ports, each being configured to be connectable with one physical line; a virtual line controller configured to treat a plurality of physical lines, which are respectively connected with the plurality of ports, to constitute a virtual line; and a status check frame controller configured to send via the virtual line a status check frame for use in checking status of a network, which the network relay device is connected with via the virtual line, wherein the status check frame controller changes a frame-sending port to be used to send a next status check frame, in order to avoid continuously using an identical port as both a frame-sending port to send the status check frame and a frame-receiving port to receive the status check frame from another network relay device.
Owner:ALAXALA NETWORKS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com