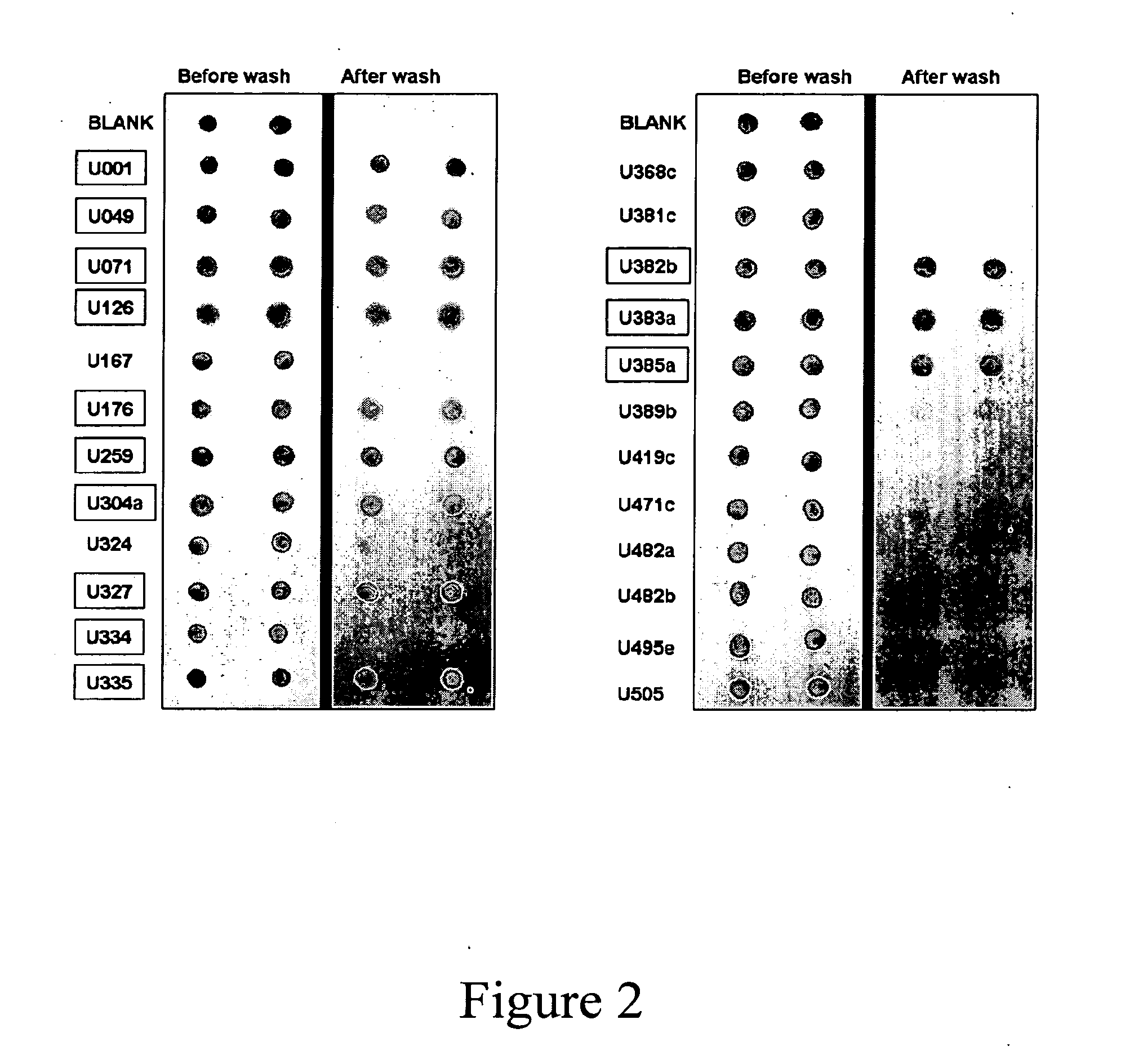
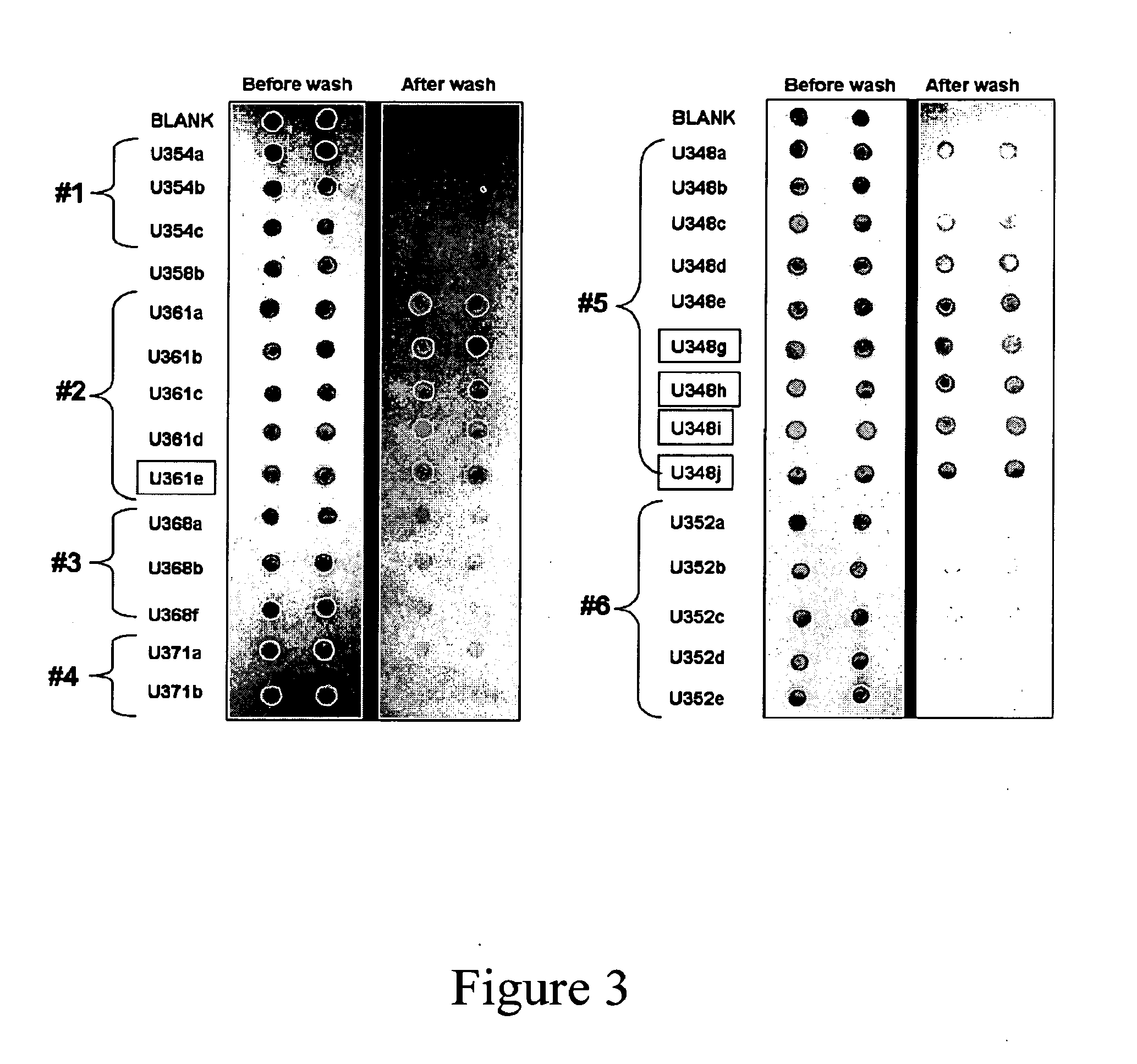
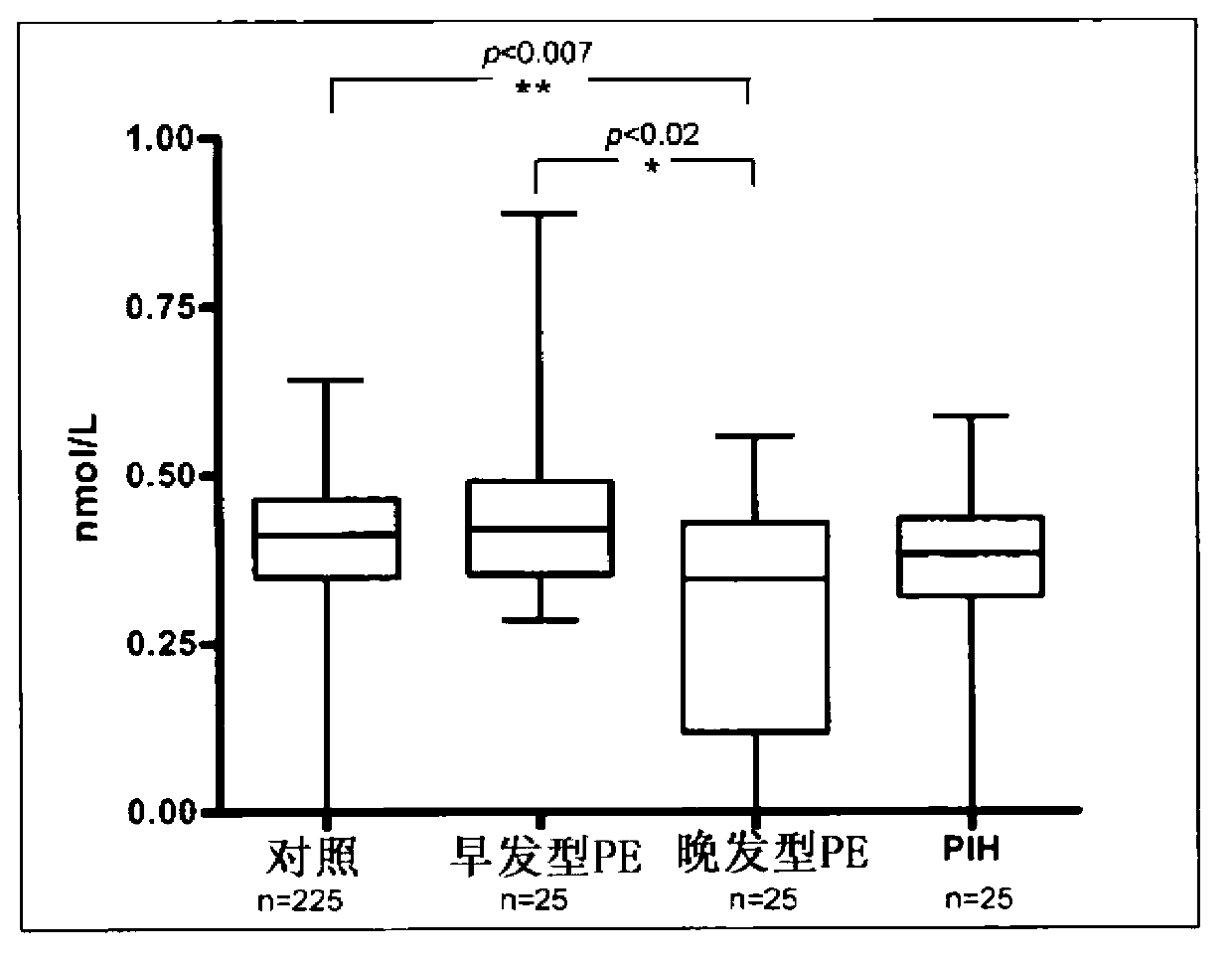
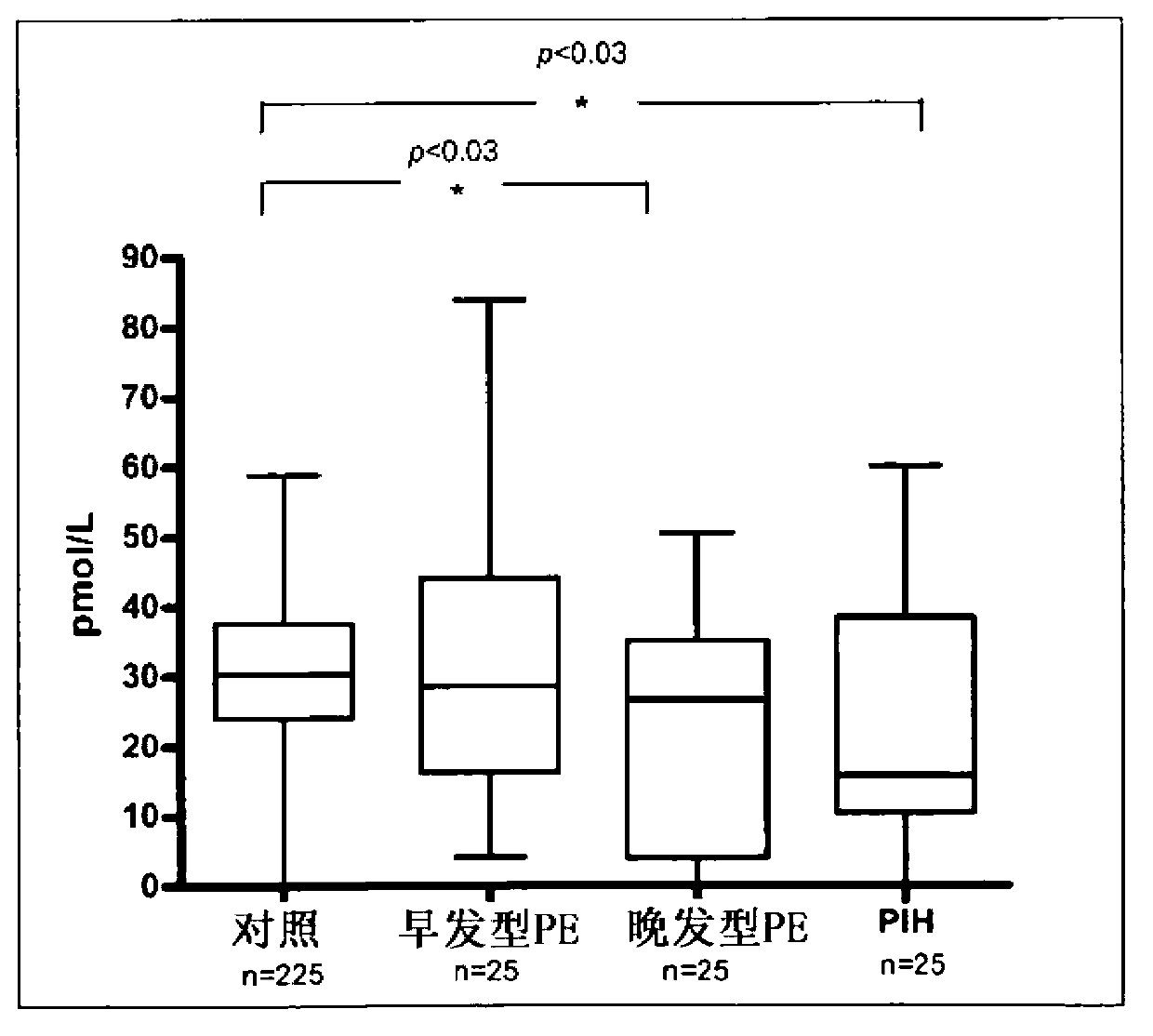
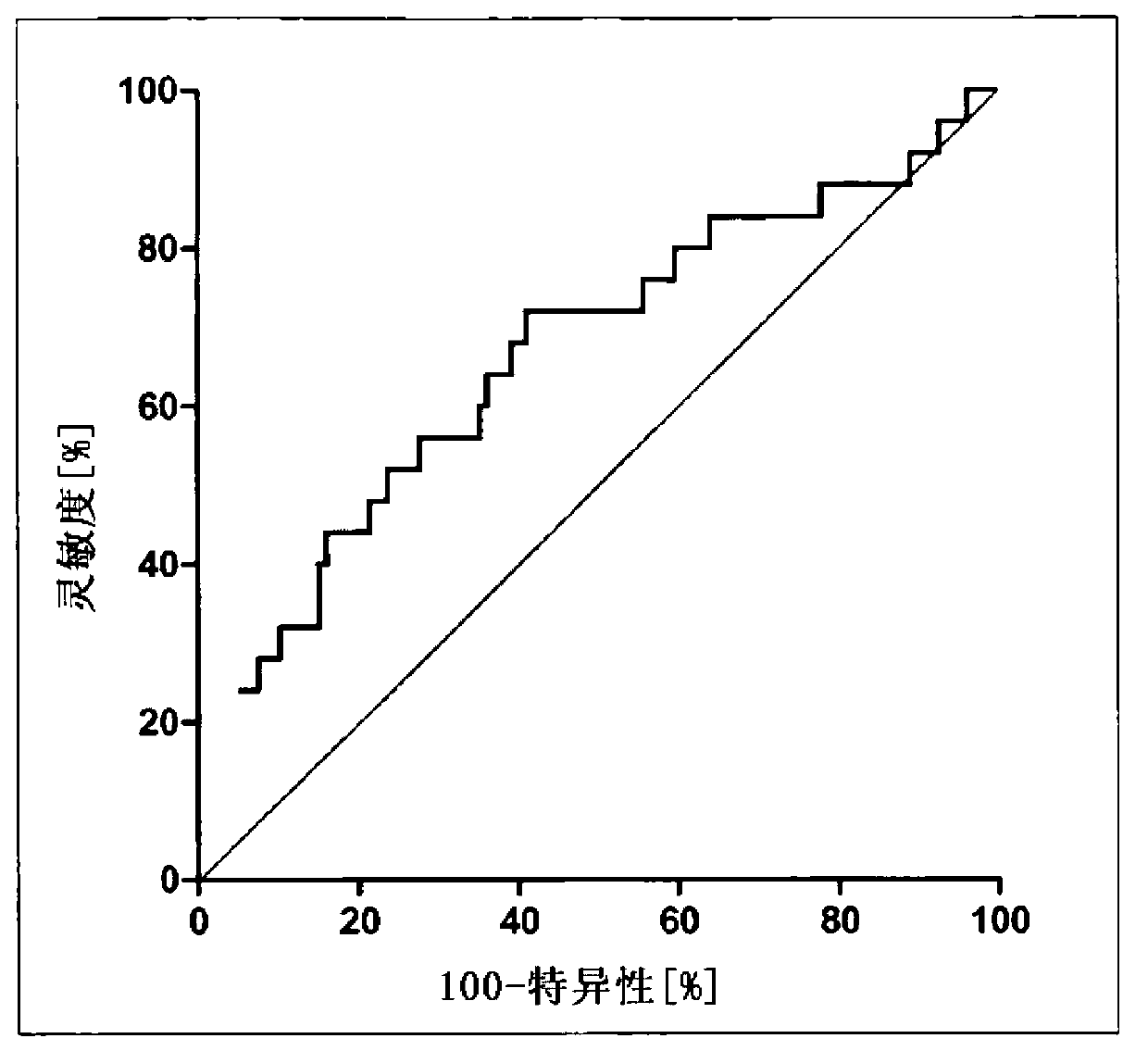
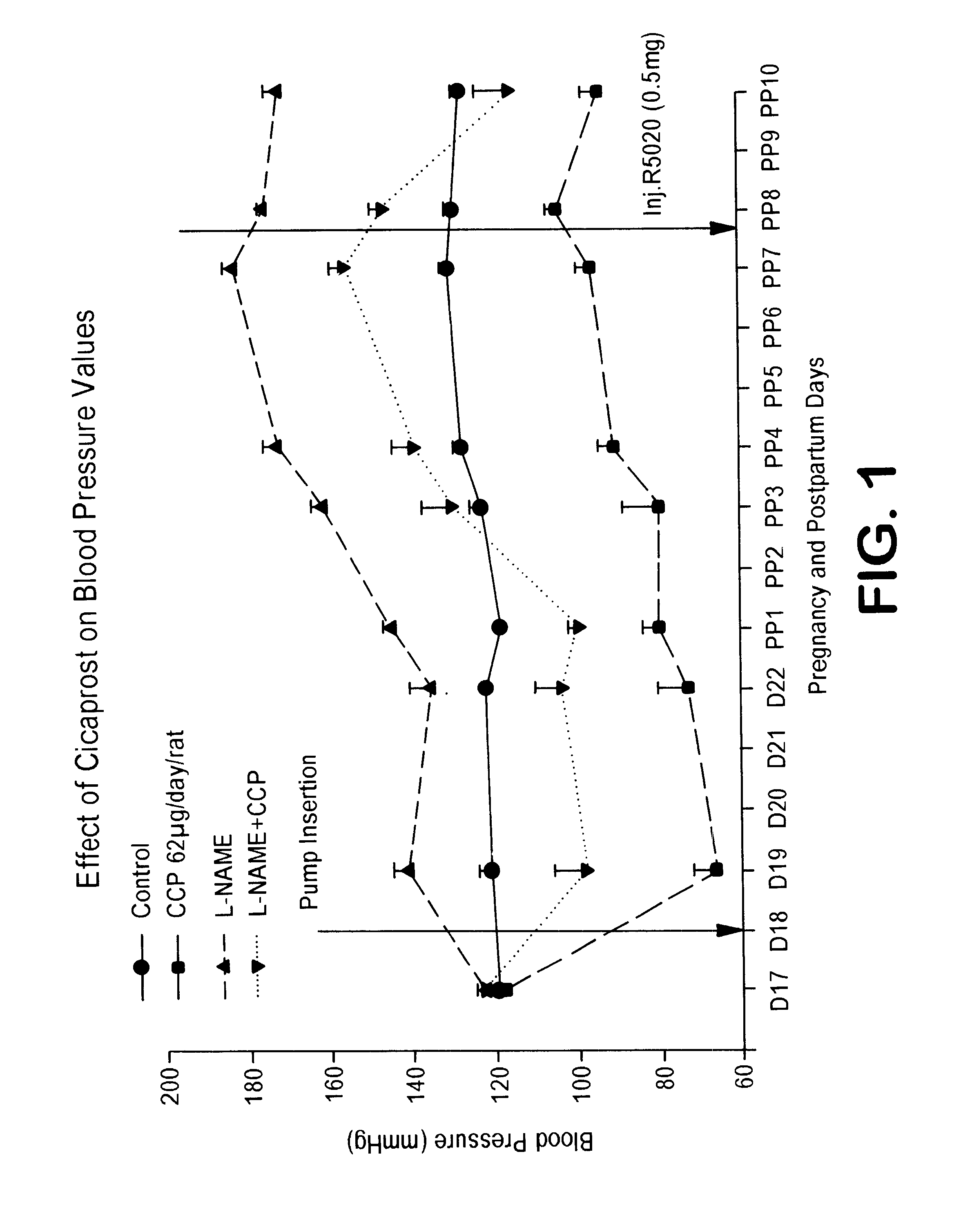
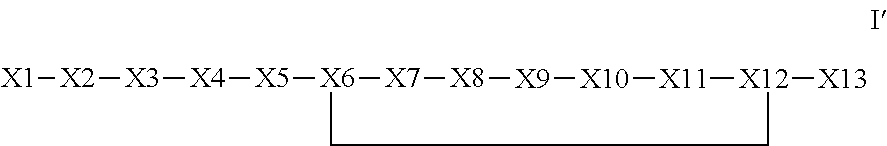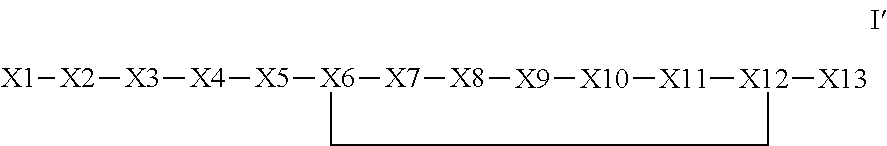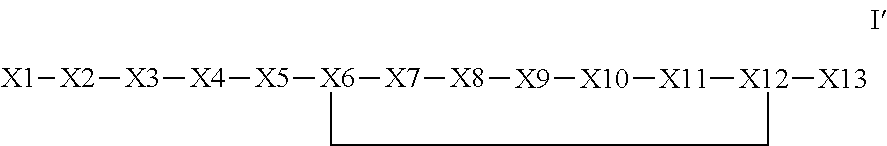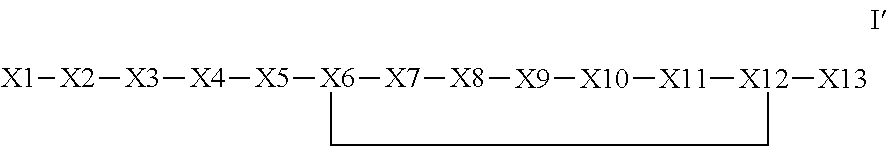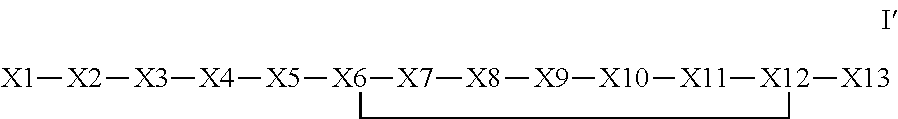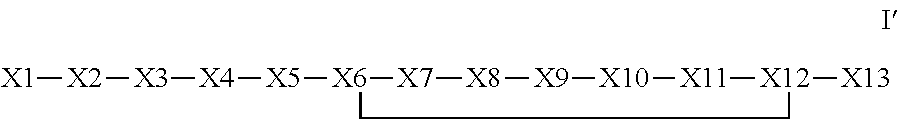Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
216 results about "Preeclampsia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition that develops in pregnant women, it is marked by high blood pressure and presence of proteins in urine.
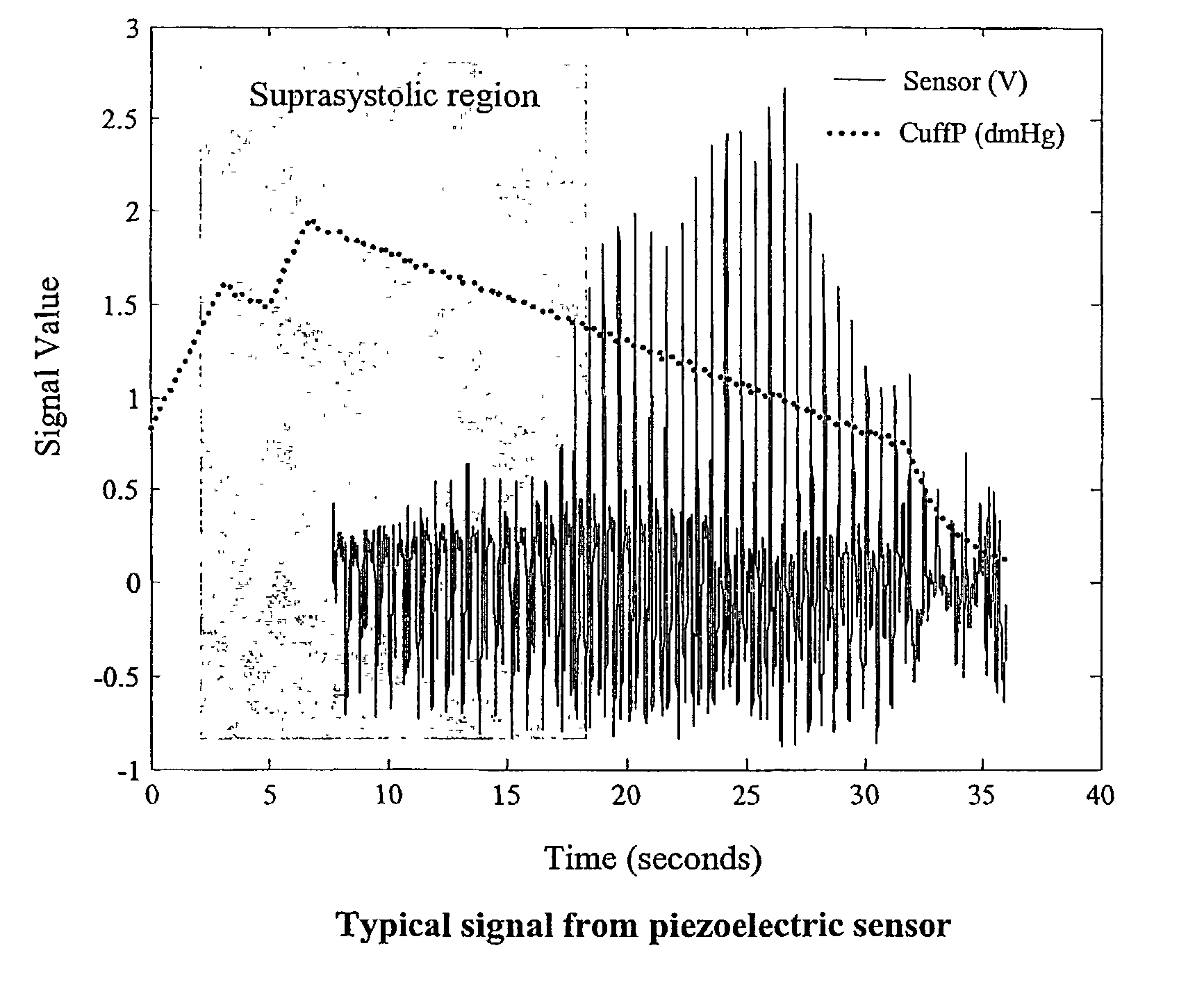
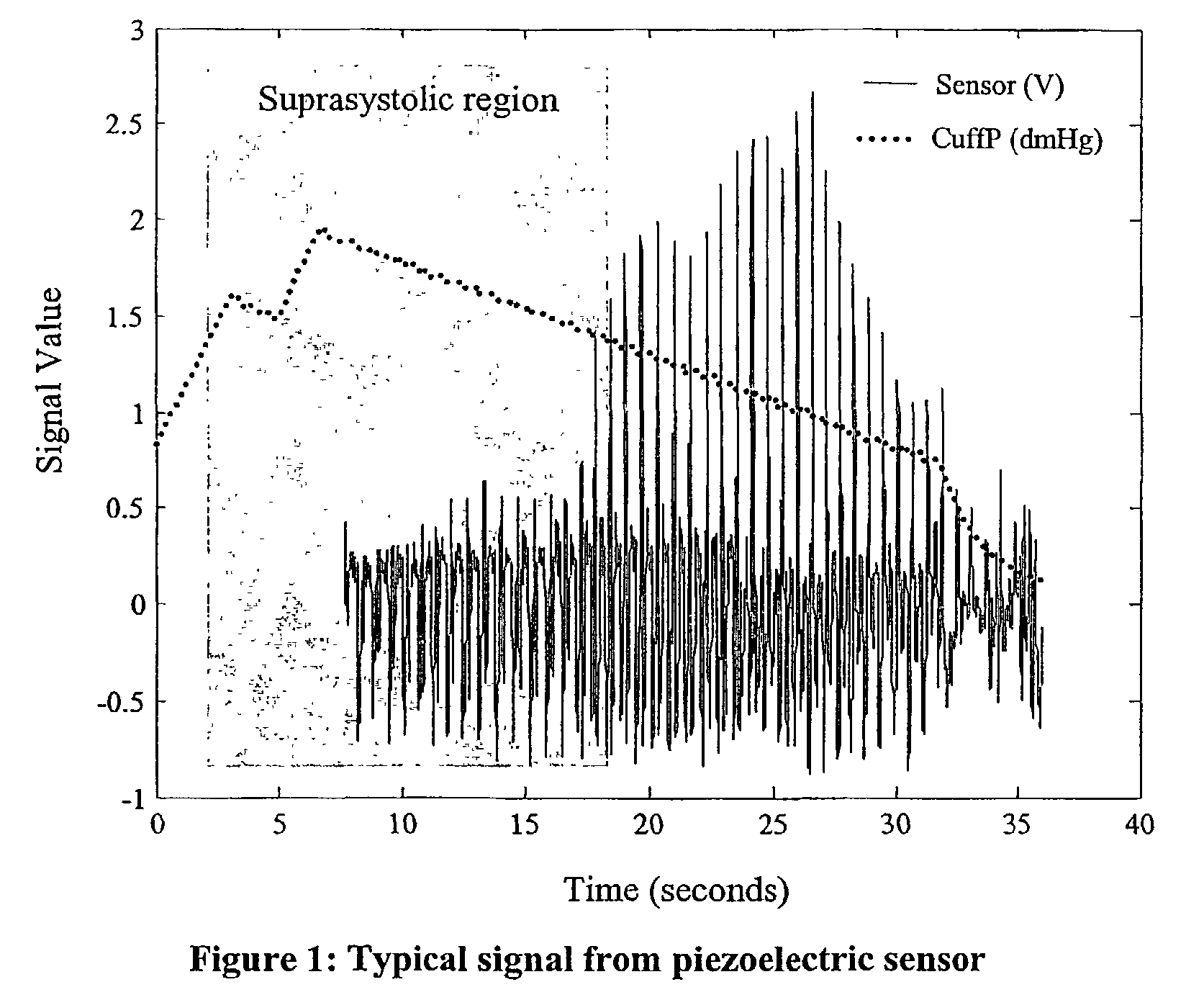
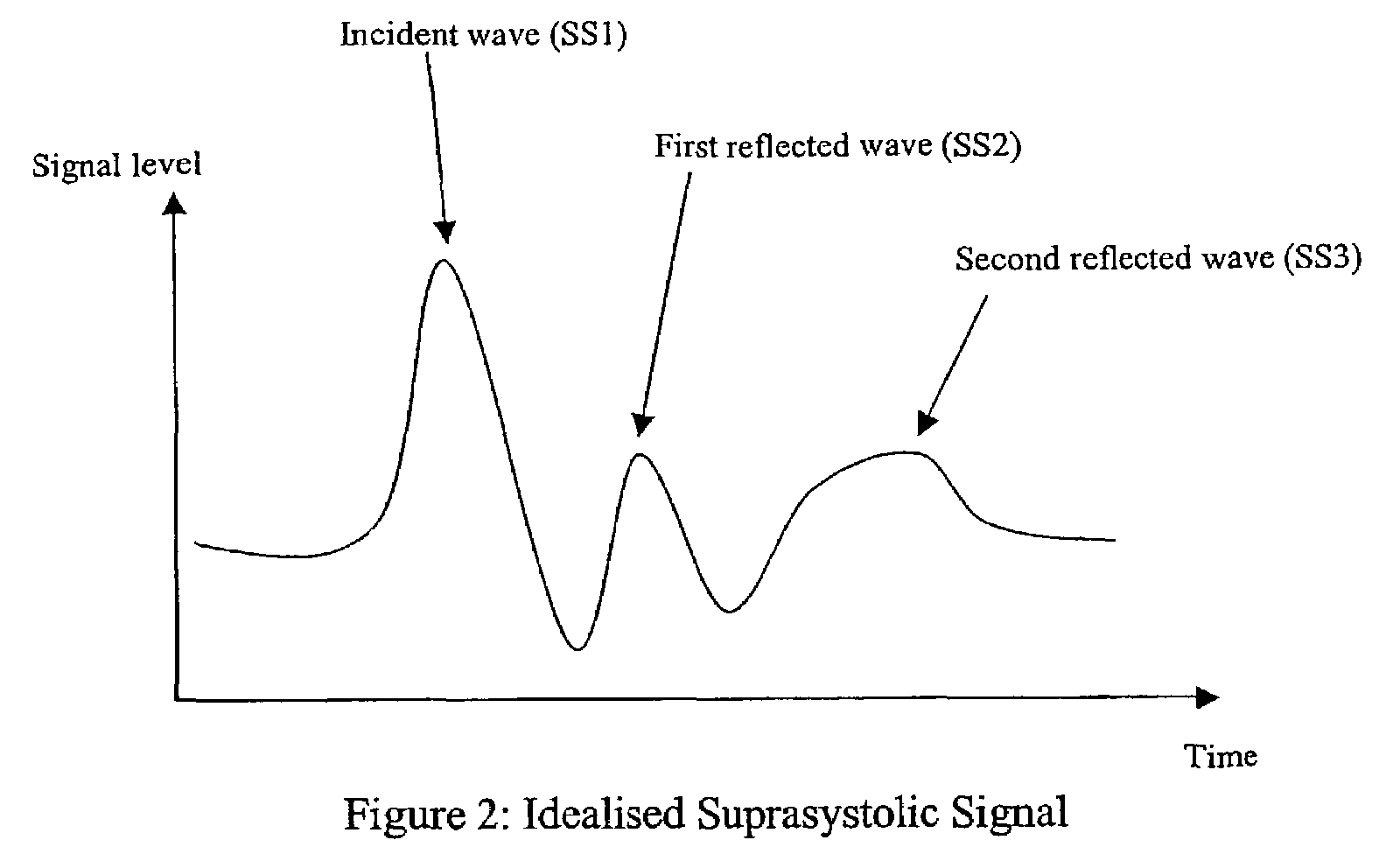
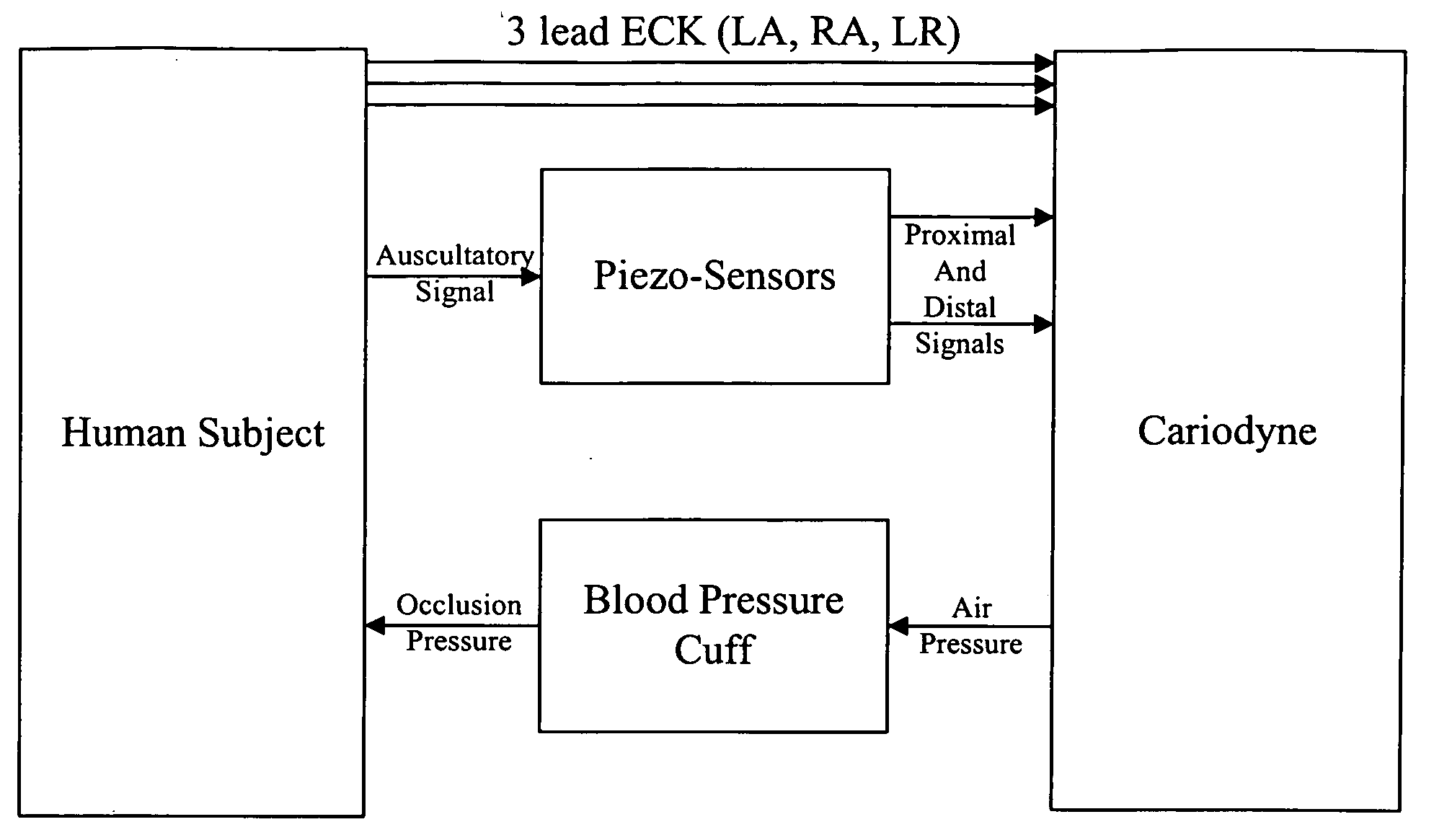
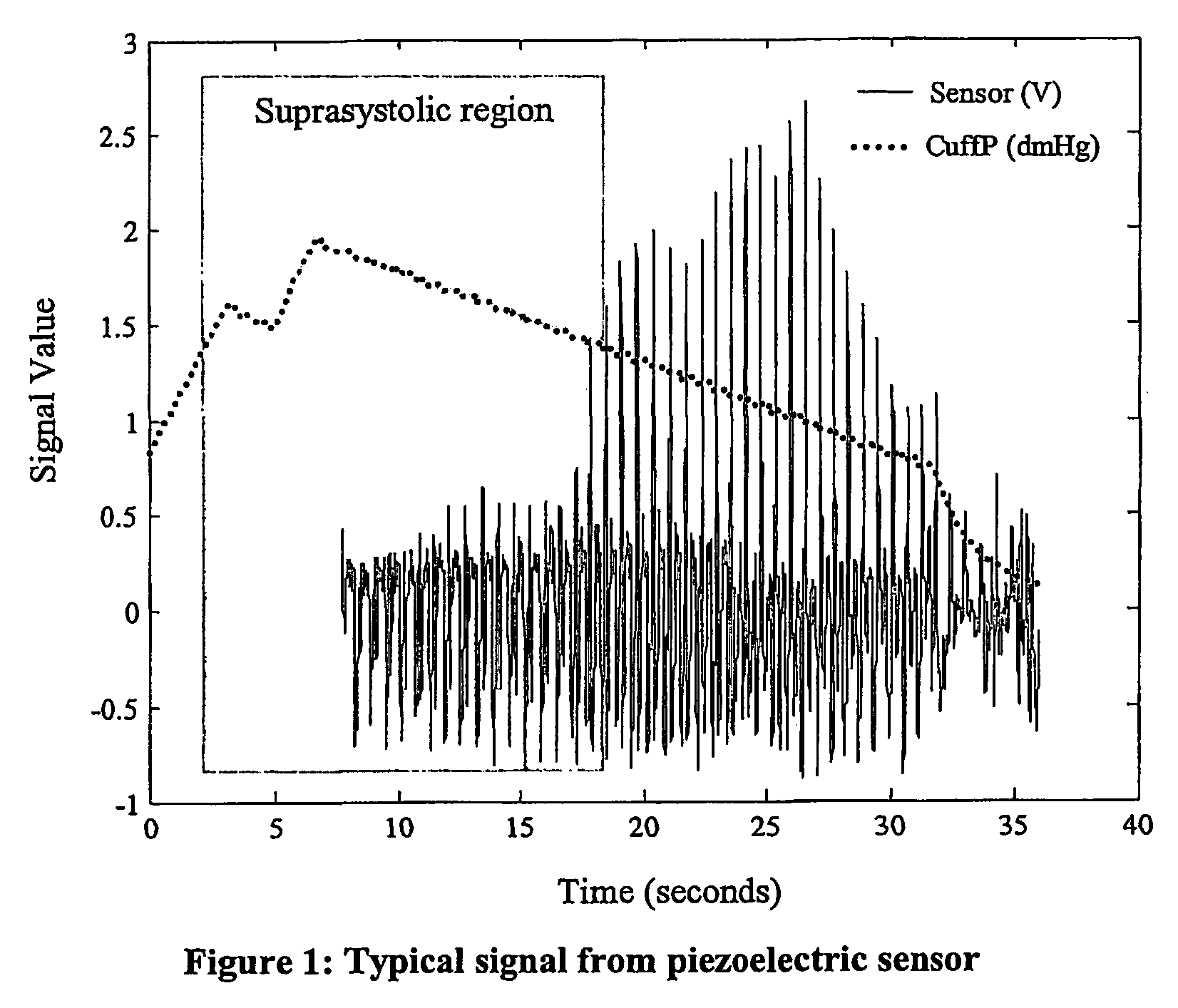
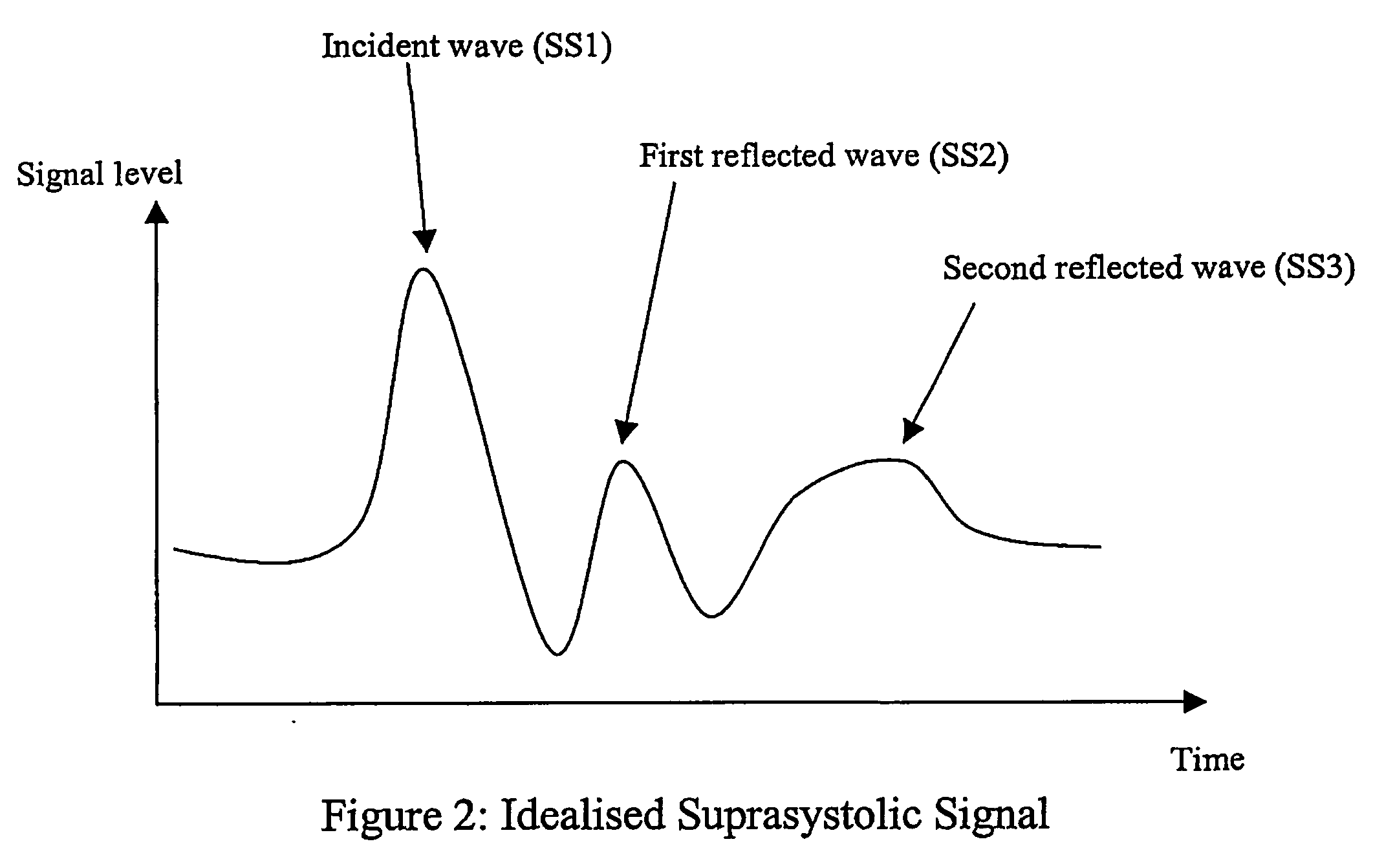
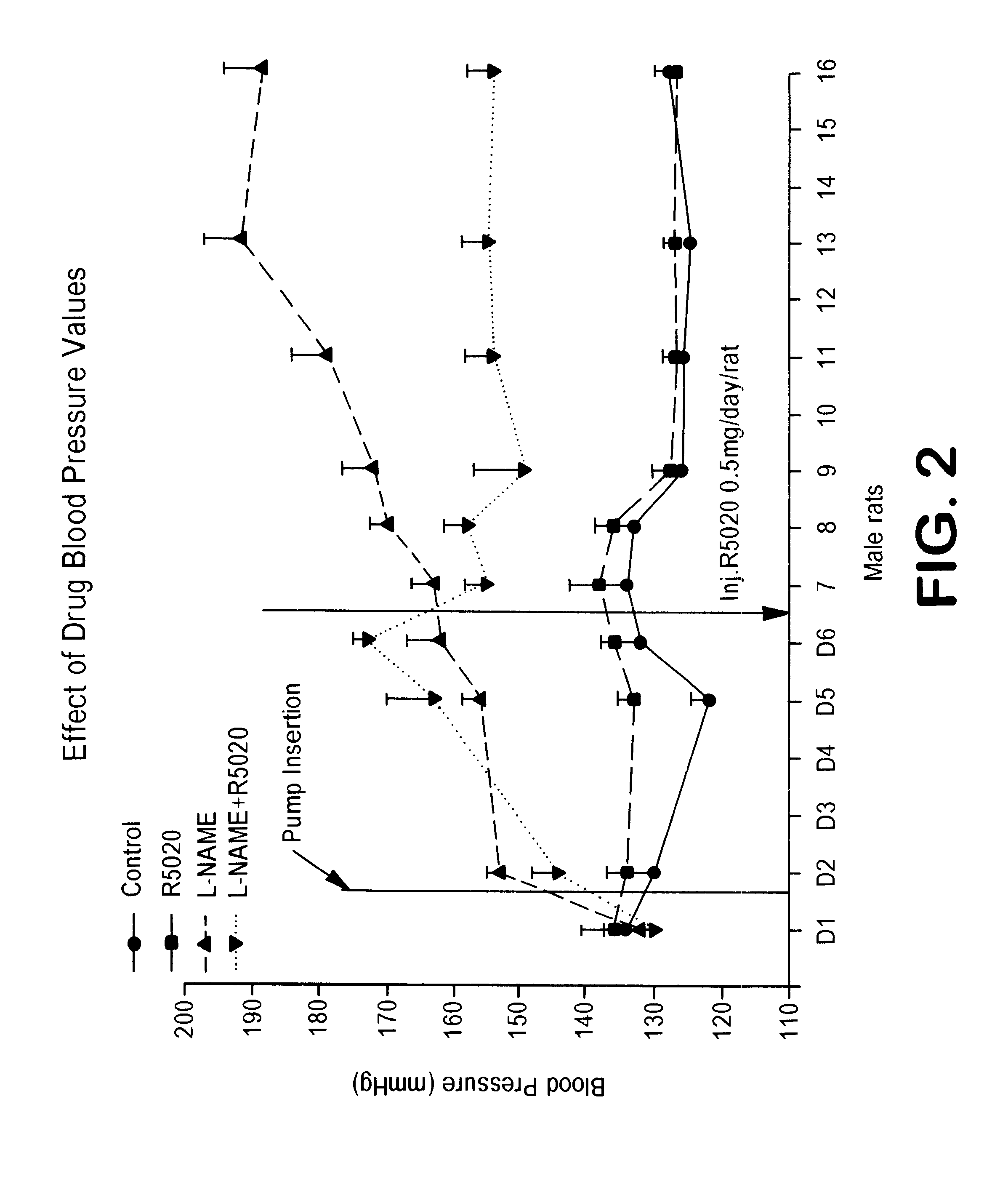
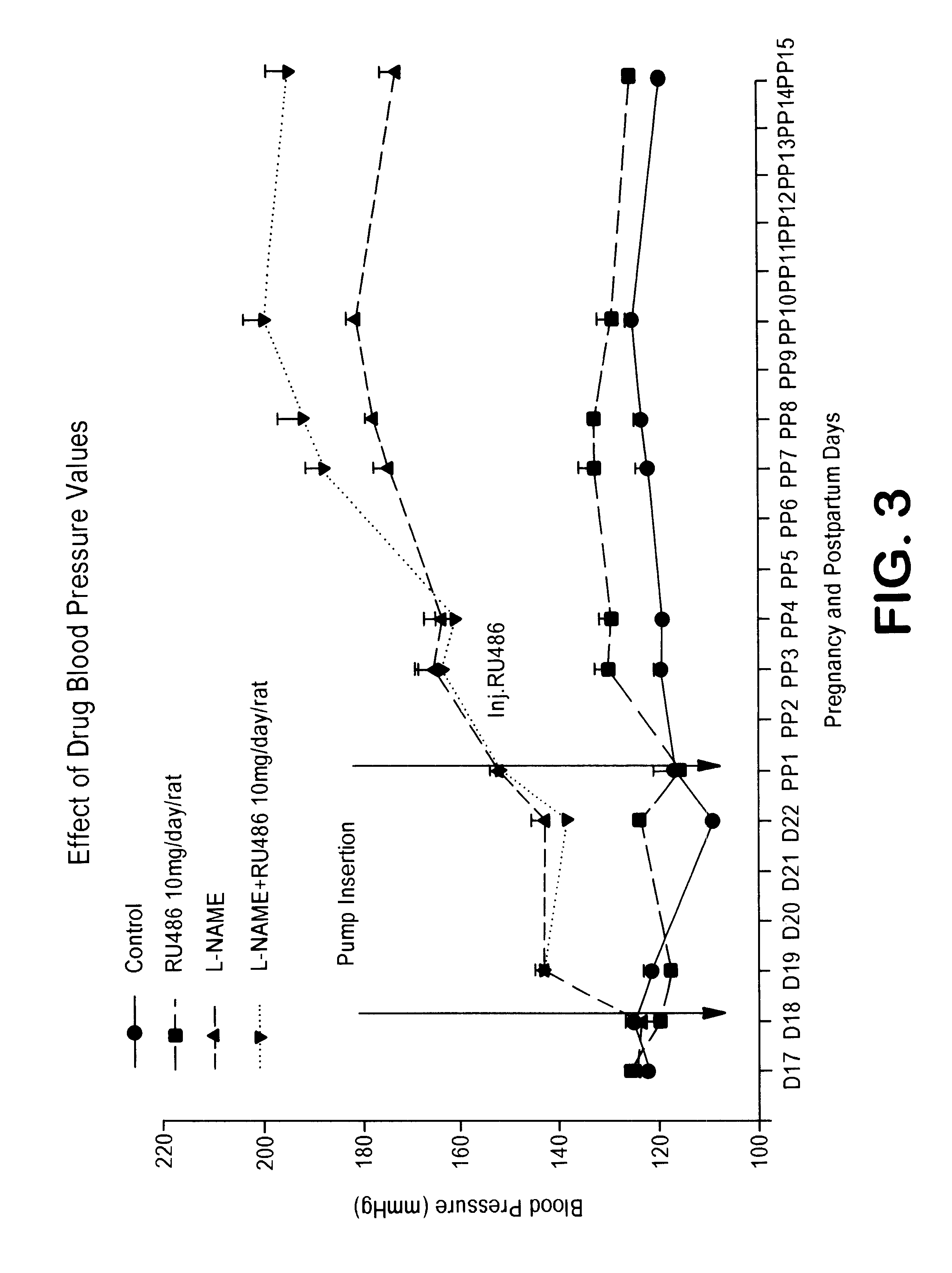
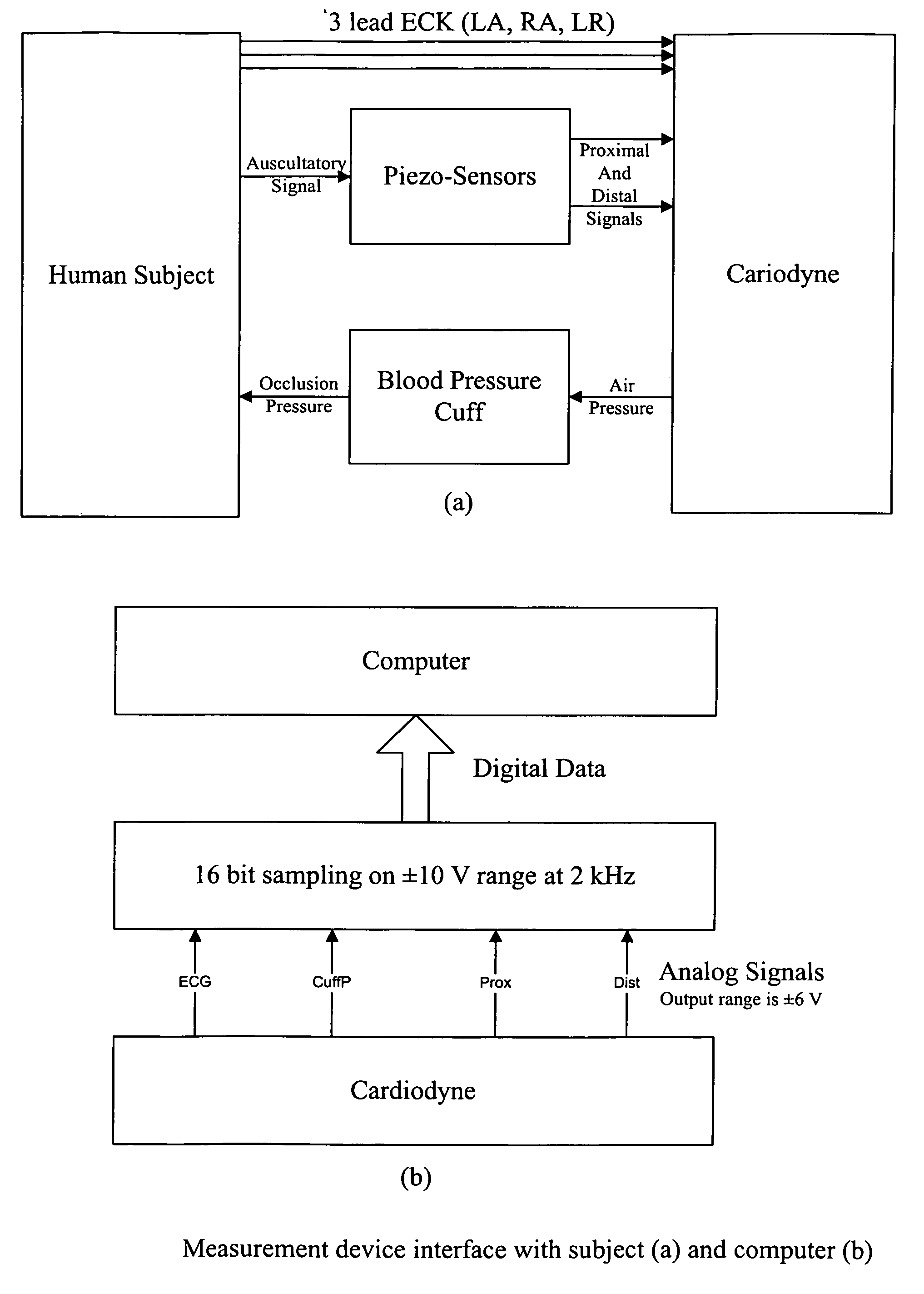
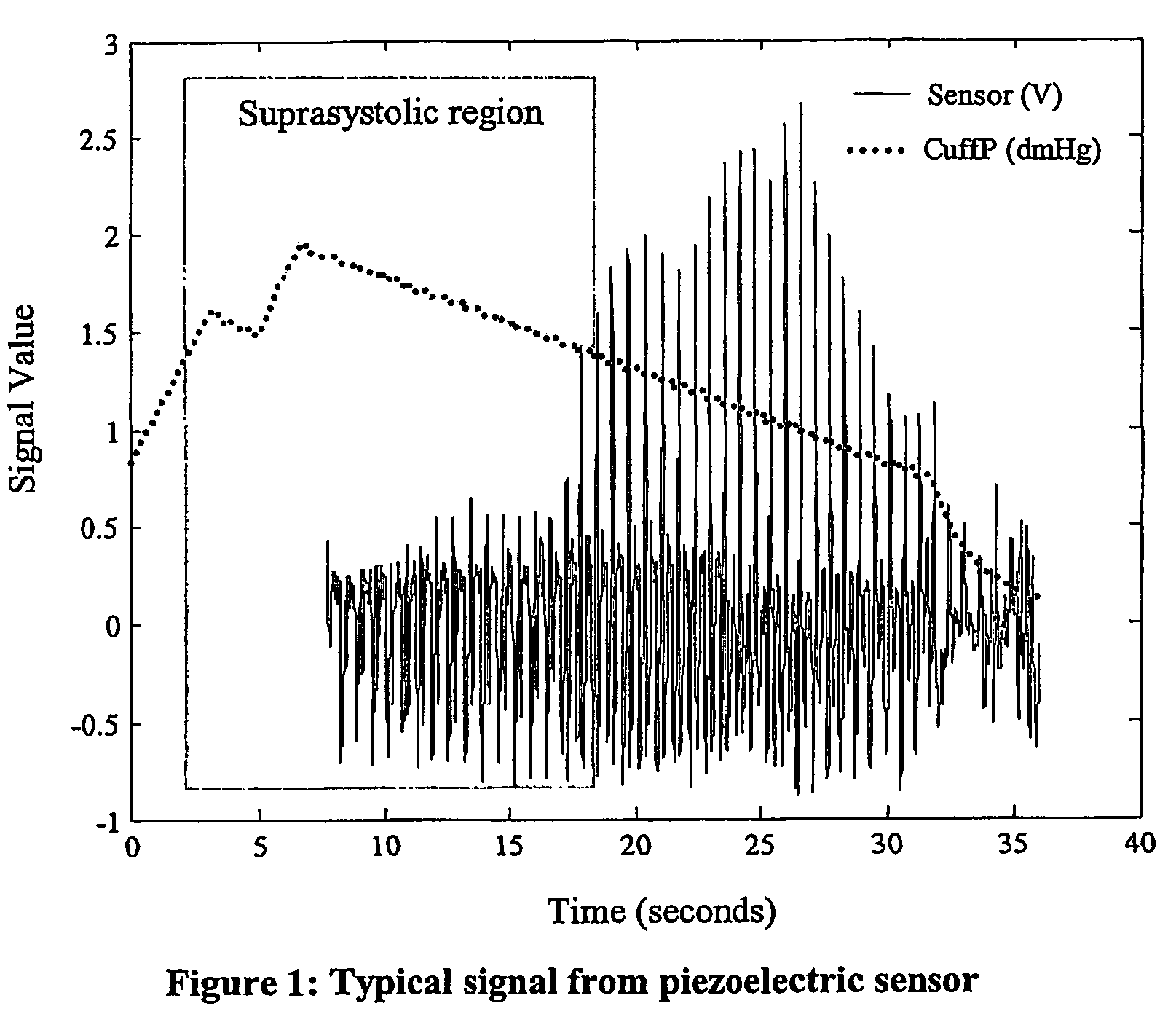
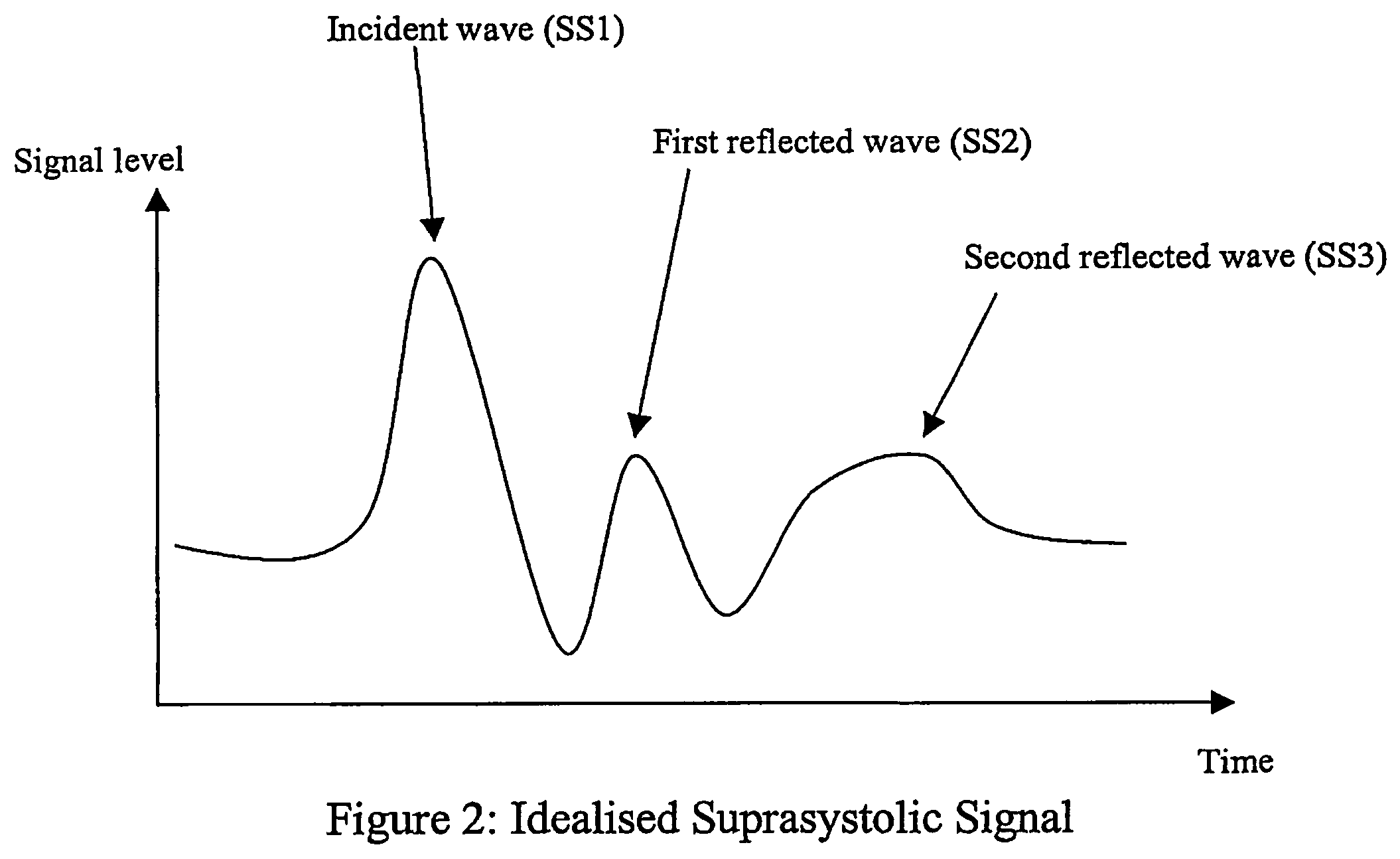
Non-invasive measurement of suprasystolic signals
InactiveUS6994675B2Reduced arterial complianceReduced endothelial dysfunctionElectrocardiographyEvaluation of blood vesselsTransducerParasystole
An apparatus for assessing cardiovascular status of a mammal comprises a system for locally applying a pressure to an artery capable of restricting blood flow through said artery, a wideband external pulse transducer having an output and situated to measure suprasystolic signals proximate to said artery, and a computing device receiving said output for calculating vascular compliance values. The method described is particularly useful for determining cardiac output, assessing whether a pregnant female has preeclampsia or a patient has cardiac insufficiency, or assessing cardiac arrhythmias.
Owner:USCOM
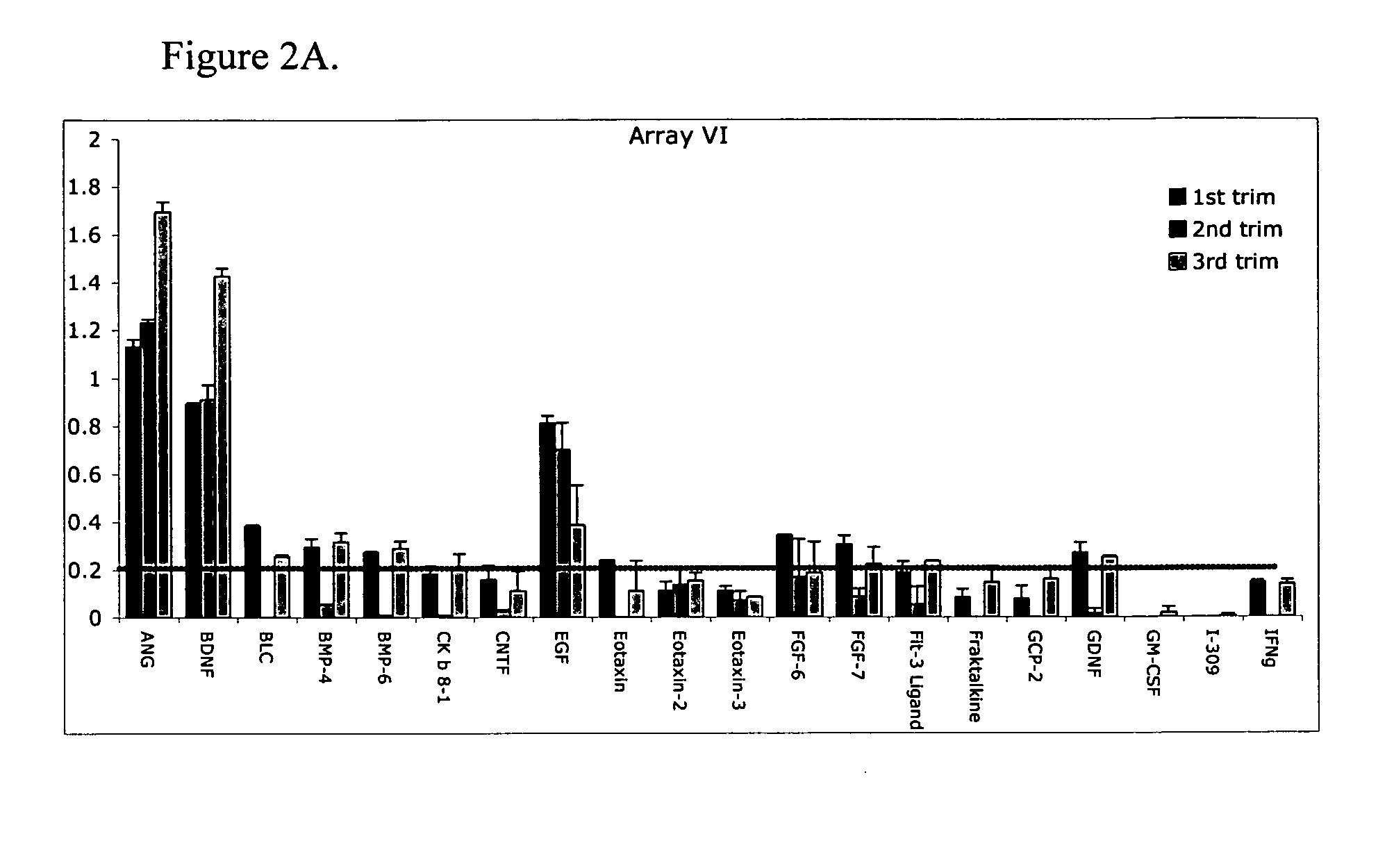
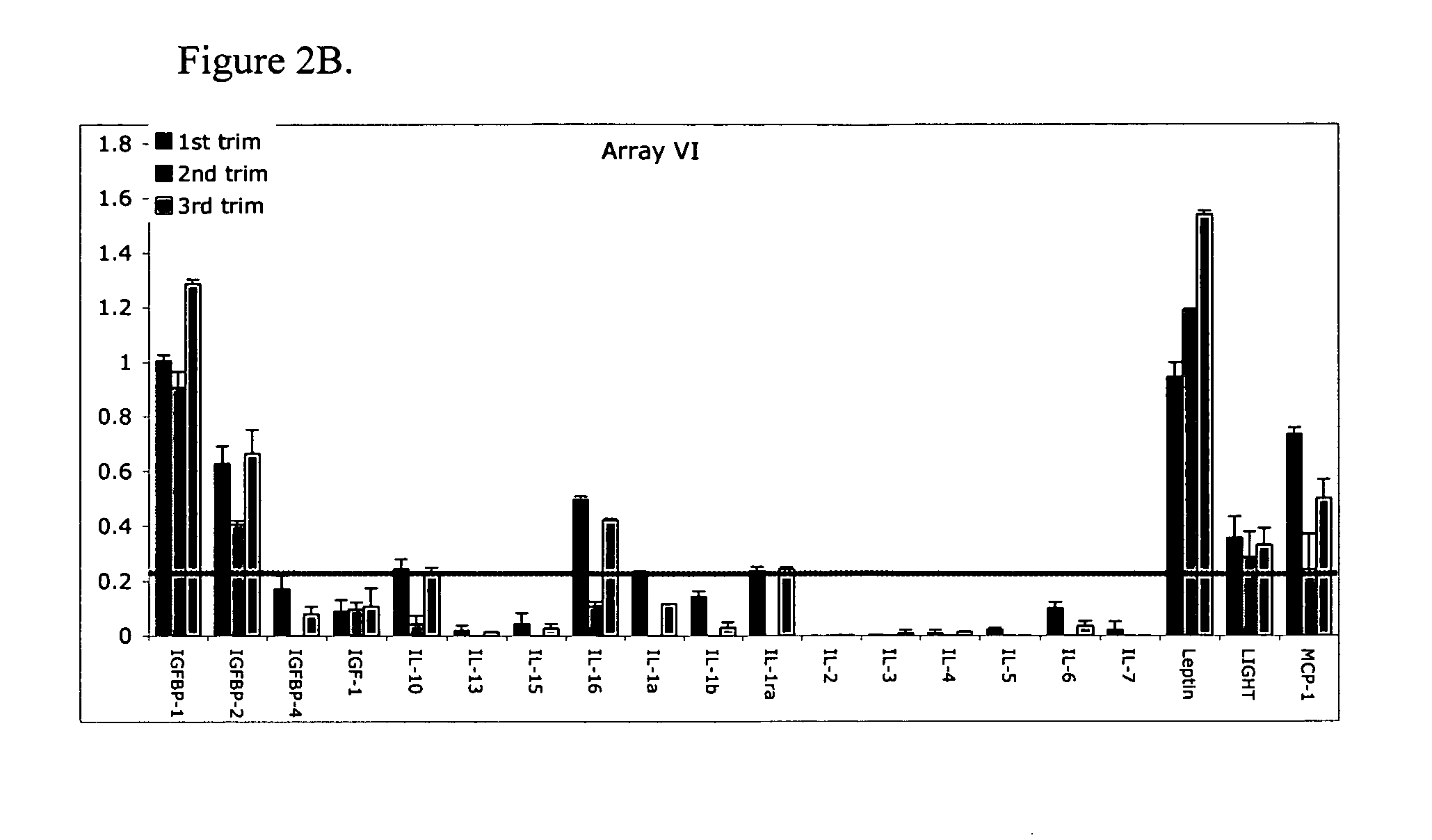
Pregnancy biomarker profiles, methods and compositions related thereto
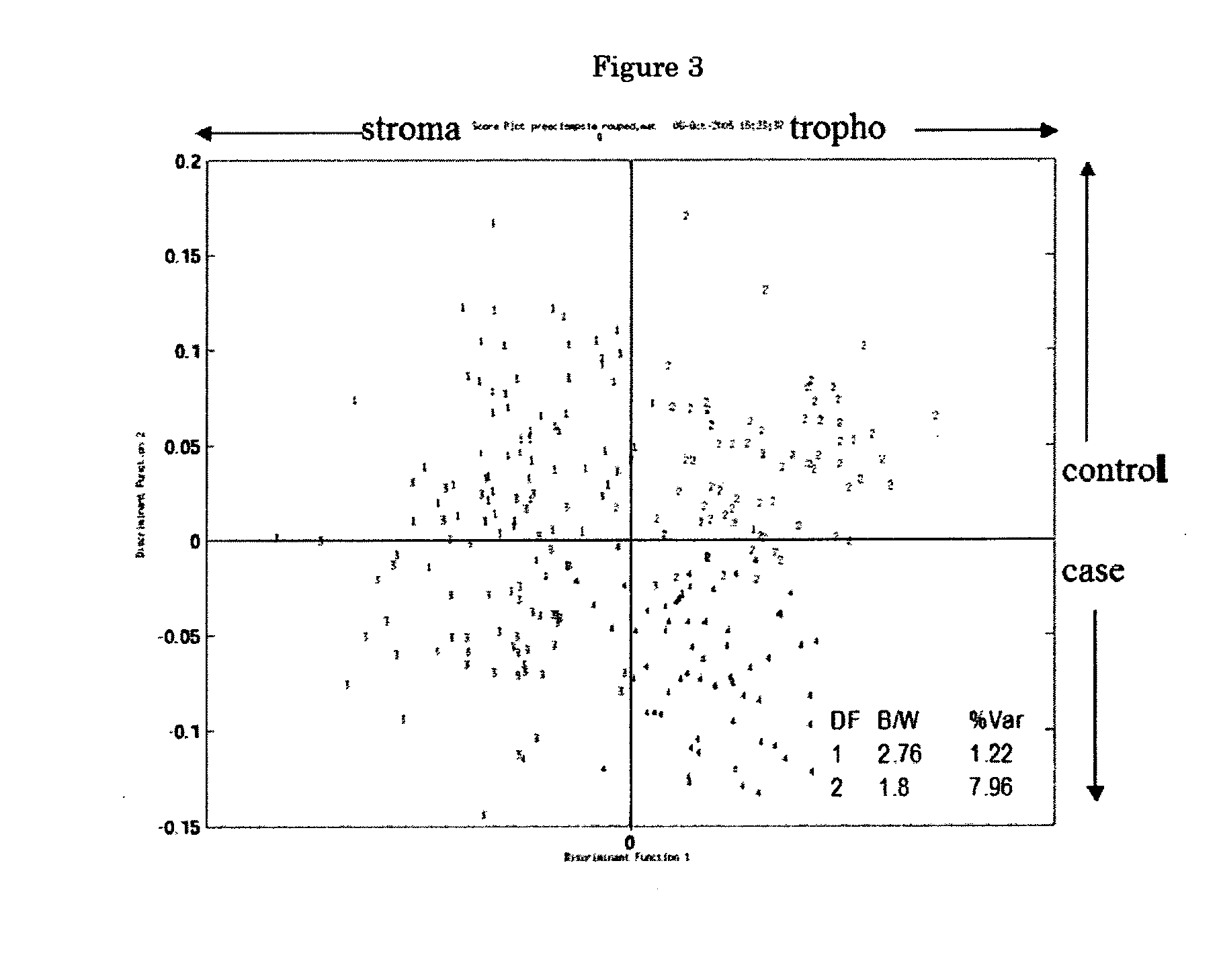
ActiveUS20070178605A1Reduction in trophoblast viabilityFunctional impairmentCompound screeningApoptosis detectionComplicated pregnancyObstetrics
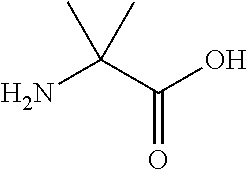
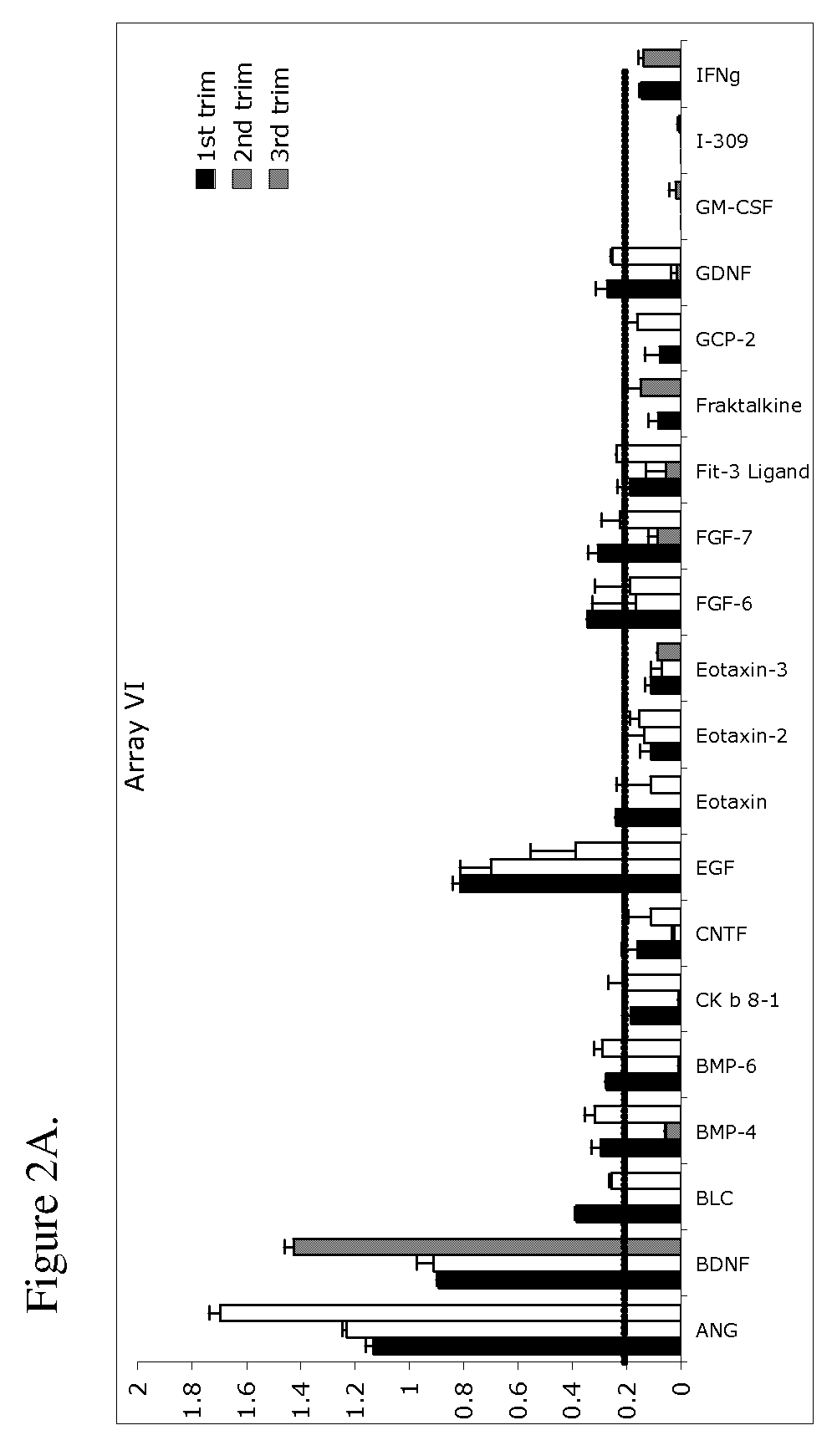
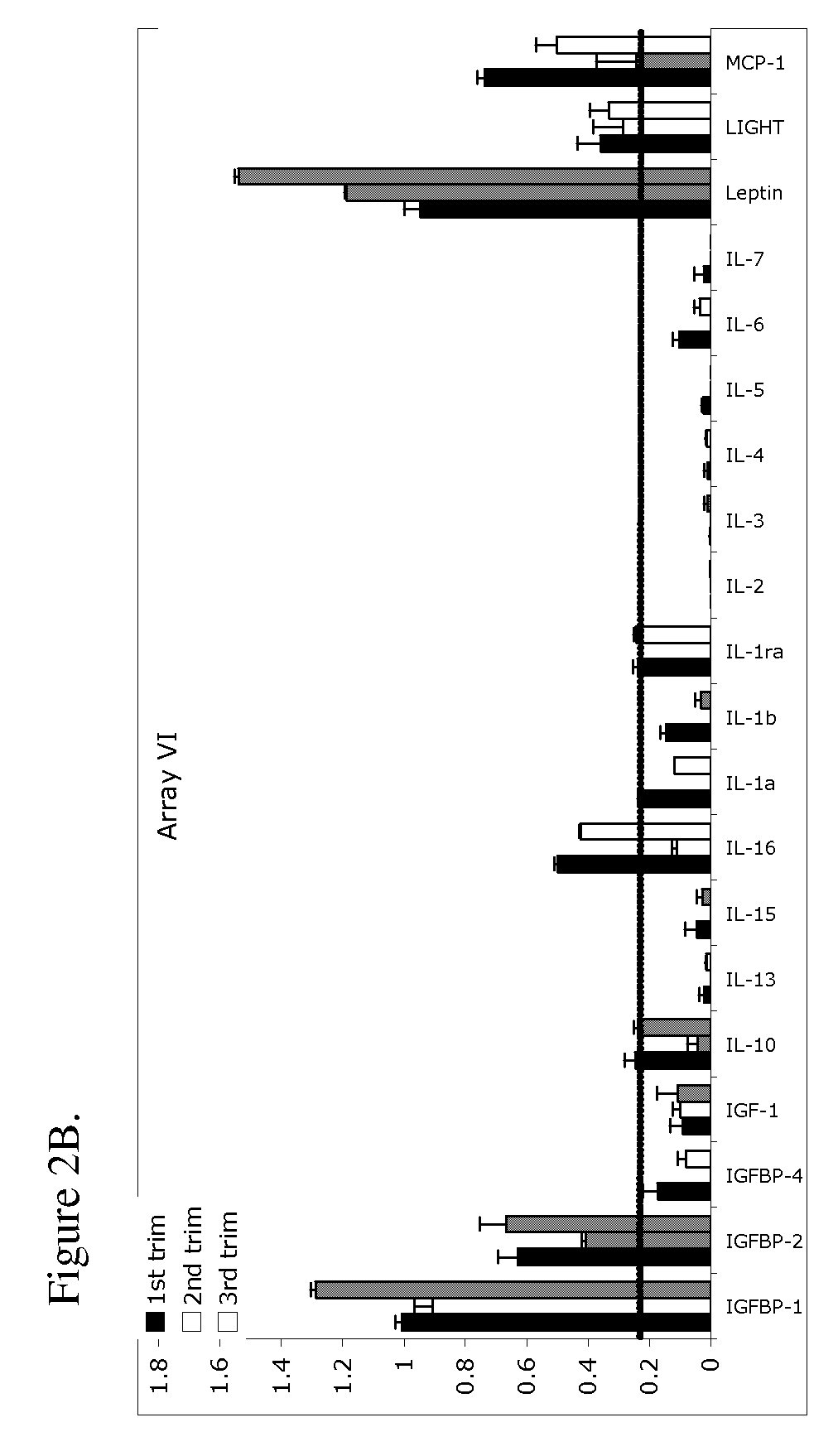
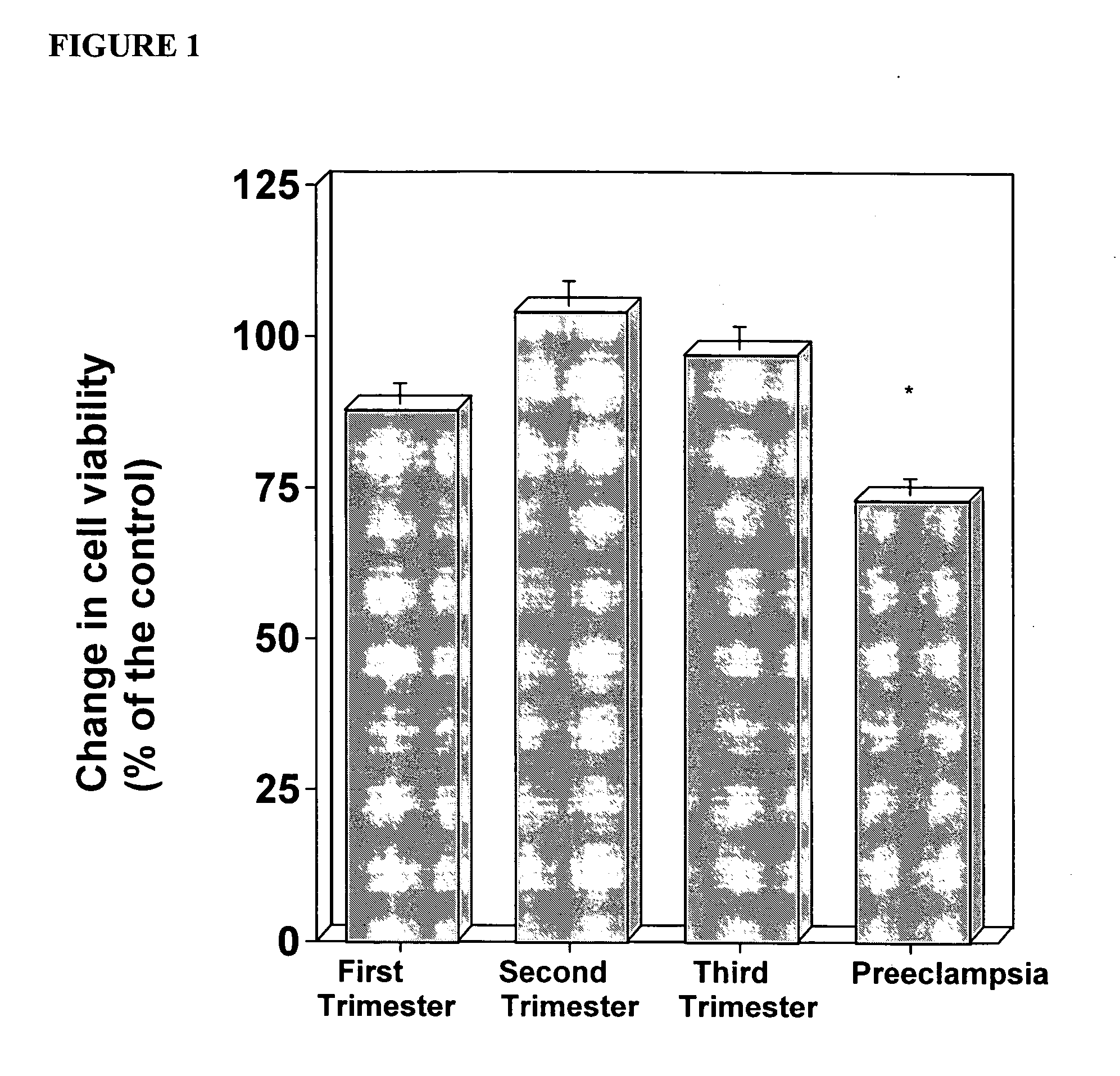
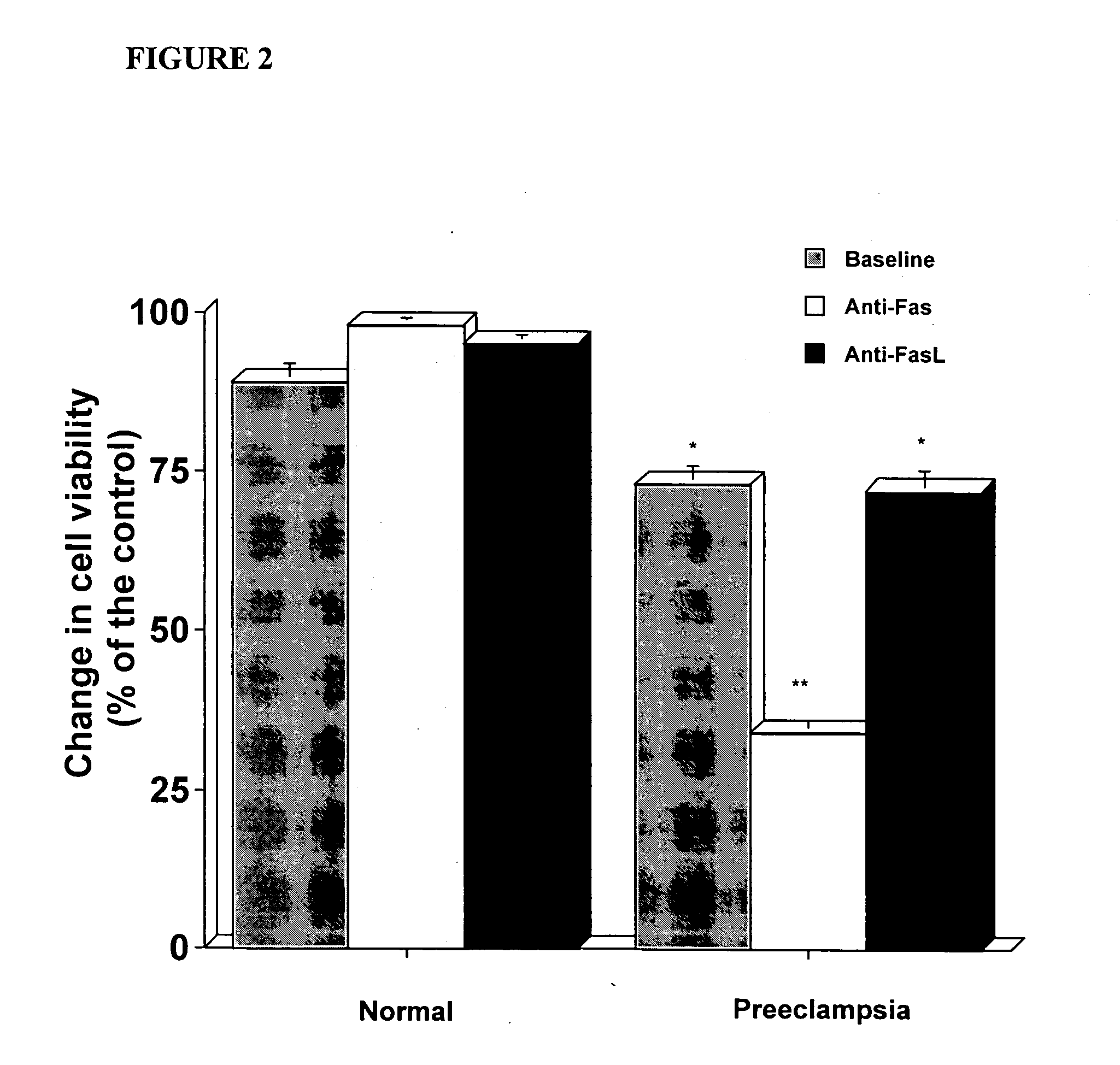
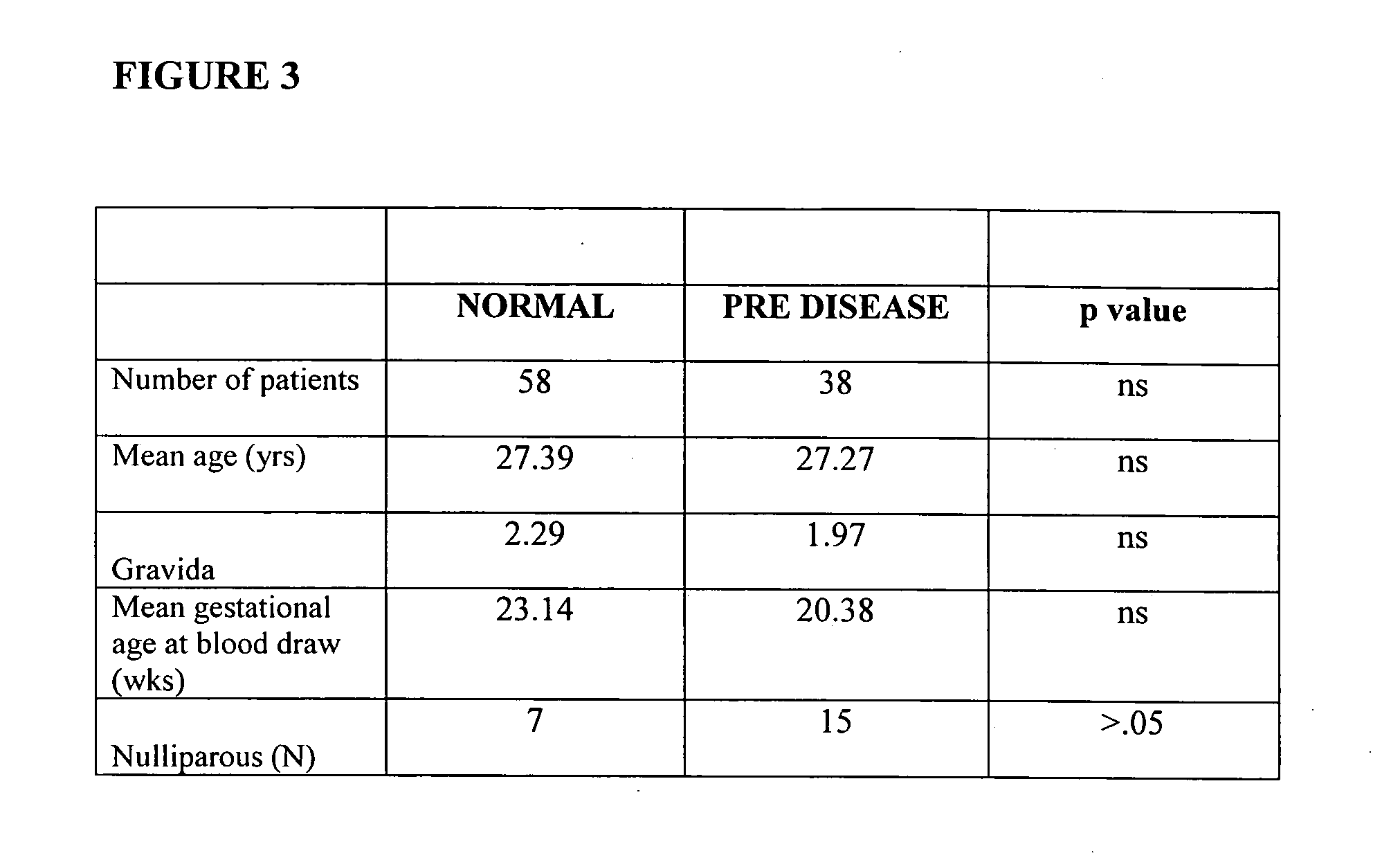
The present invention provides methods and compositions related to biomarker profiles for each trimester of pregnancy. The present invention also provides methods for identifying patients at risk of developing a complication of pregnancy, such as preeclampsia. In further embodiments, the present invention relates to methods for the diagnosis of patients with preeclampsia.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS8673848B2Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Pharmaceutical composition and method for the transdermal delivery of magnesium
InactiveUS20050196434A1Reduce disadvantagesBiocideAerosol deliveryAutonomic bladder dysfunctionMagnesium salt
The present invention relates to a method and transdermal pharmaceutical composition for preventing magnesium deficiency or imbalances associated with magnesium deficiency including diabetes, hypertension, high cholesterol, cardiac arrhythmias, acute myocardial infarction, arteriosclerosis, atherosclerosis, preeclampsia, dysautonomia, mitral valve prolapse, asthma, constipation, irritable bowel syndrome, migraines, muscle spasms and cramping, premenstrual syndrome, osteoporosis, kidney stones, chronic fatigue syndrome, and fibromyalgia. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of magnesium and a pharmaceutically acceptable carrier. A therapeutically effective amount of a pharmaceutically acceptable salt of zinc a vitamin such as B-complex vitamin, a carotenoid, a mineral, or a combination thereof may also be included in the transdermal pharmaceutical composition. A therapeutically effective amount of progesterone may also be included in the transdermal pharmaceutical composition. The transdermal pharmaceutical composition may be topically administered to prevent magnesium deficiency or imbalances caused by magnesium deficiency.
Owner:BRIERRE BARBARA T
Methods of determining whether a pregnant woman is at risk of developing preeclampsia
ActiveUS7790463B2Reduced viabilityFunctional impairmentCompound screeningApoptosis detectionComplicated pregnancyGestational period
The present invention provides methods and compositions related to biomarker profiles for each trimester of pregnancy. The present invention also provides methods for identifying patients at risk of developing a complication of pregnancy, such as preeclampsia. In further embodiments, the present invention relates to methods for the diagnosis of patients with preeclampsia.
Owner:UNITED STATES OF AMERICA +1
Non-invasive measurement of suprasystolic signals
ActiveUS20060178585A1Reduced arterial complianceReduced endothelial dysfunctionEvaluation of blood vesselsCatheterSystoleBlood flow
An apparatus for assessing cardiovascular status of a mammal comprises a system for locally applying a pressure to an artery capable of restricting blood flow through said artery, a wideband external pulse transducer having an output and situated to measure suprasystolic signals proximate to said artery, and a computing device receiving said output for calculating vascular compliance values. The method described is particularly useful for determining cardiac output, assessing whether a pregnant female has preeclampsia or a patient has cardiac insufficiency, or assessing cardiac arrhythmia.
Owner:USCOM
Methods and compositions for the detection and treatment of preeclampsia
Methods, kits and compounds are provided that relate to the diagnosis, treatment, and / or prevention of preeclampsia.
Owner:RGT UNIV OF CALIFORNIA +1
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS20130196899A1Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
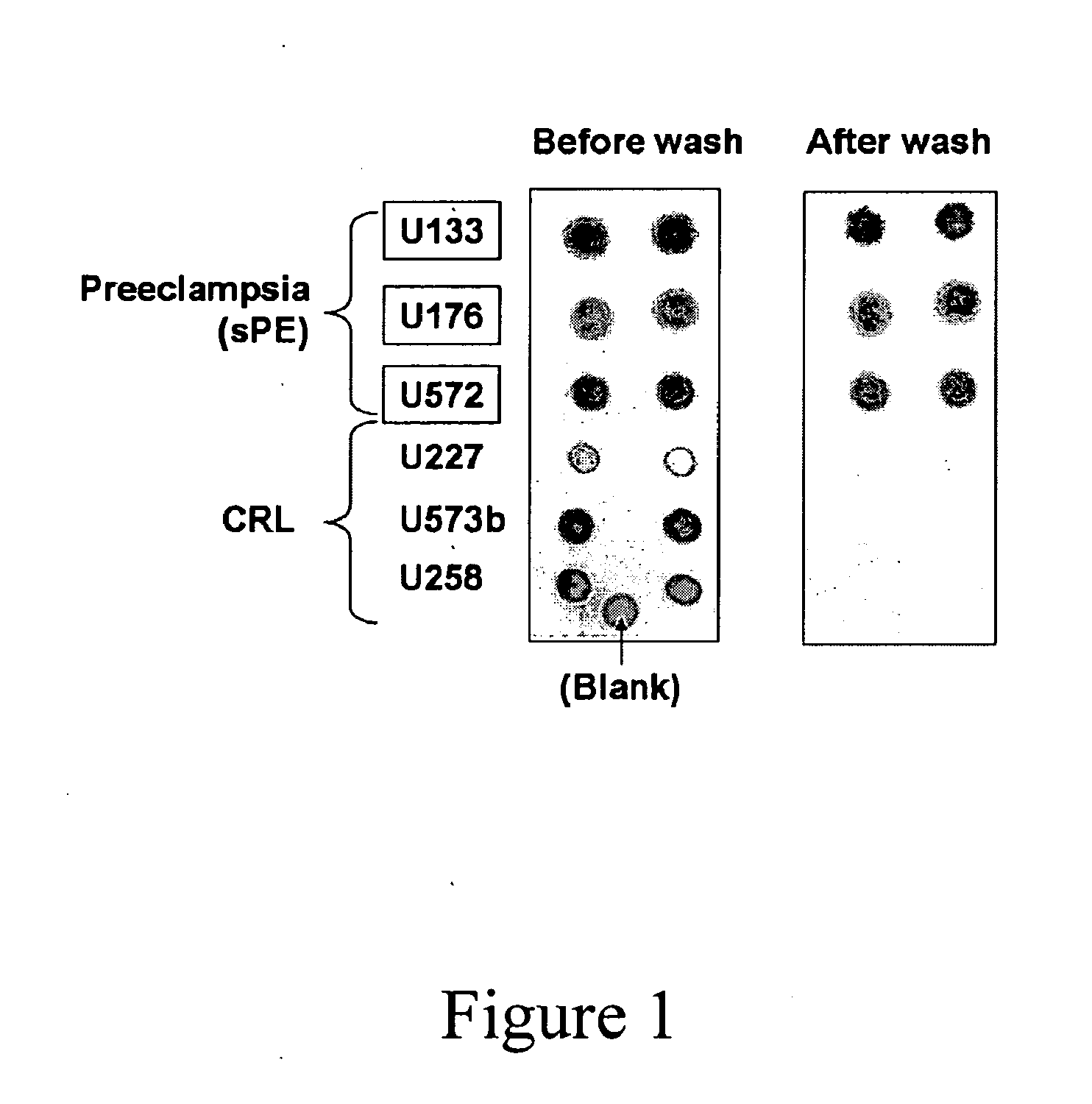
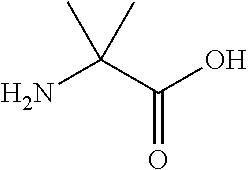
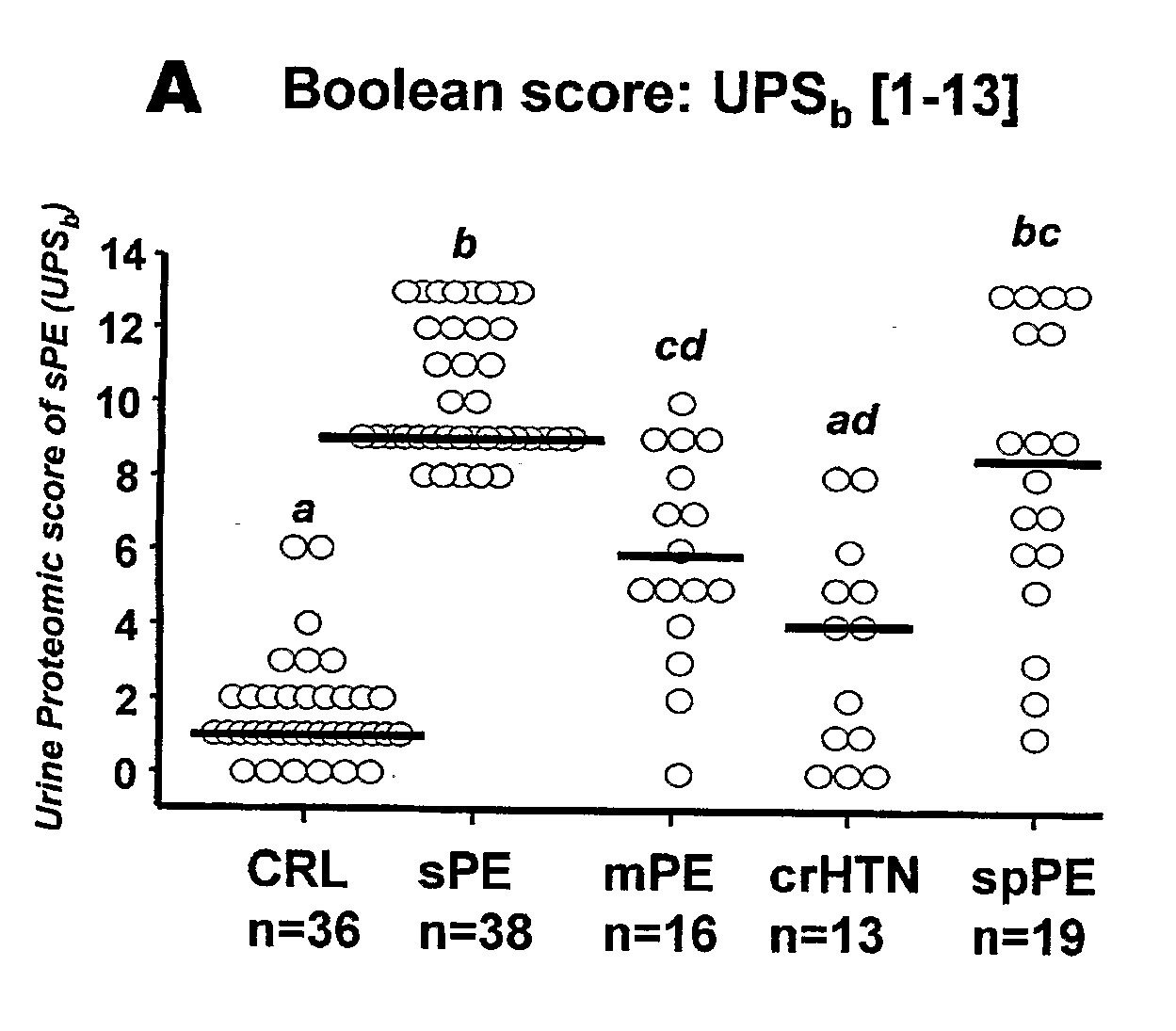
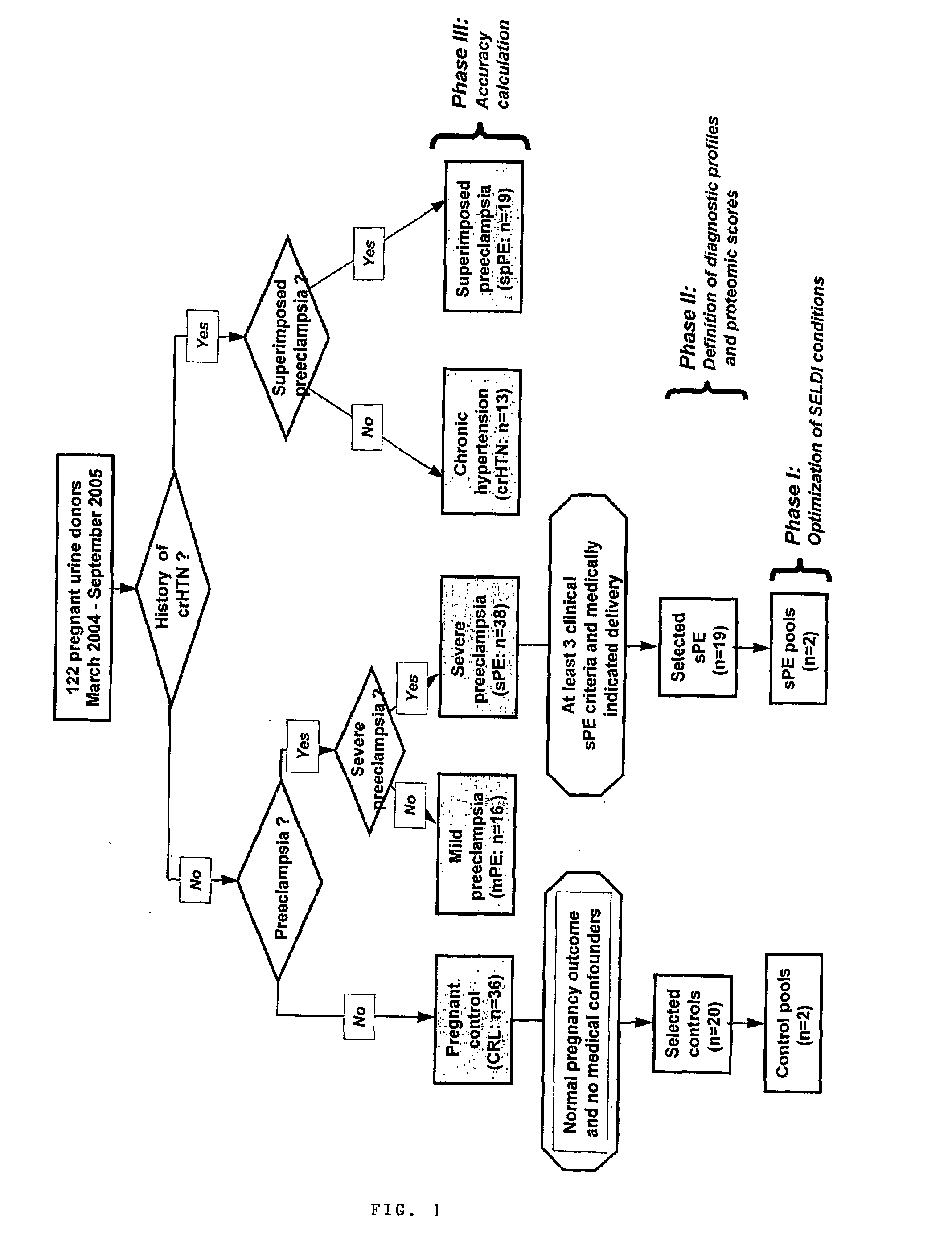
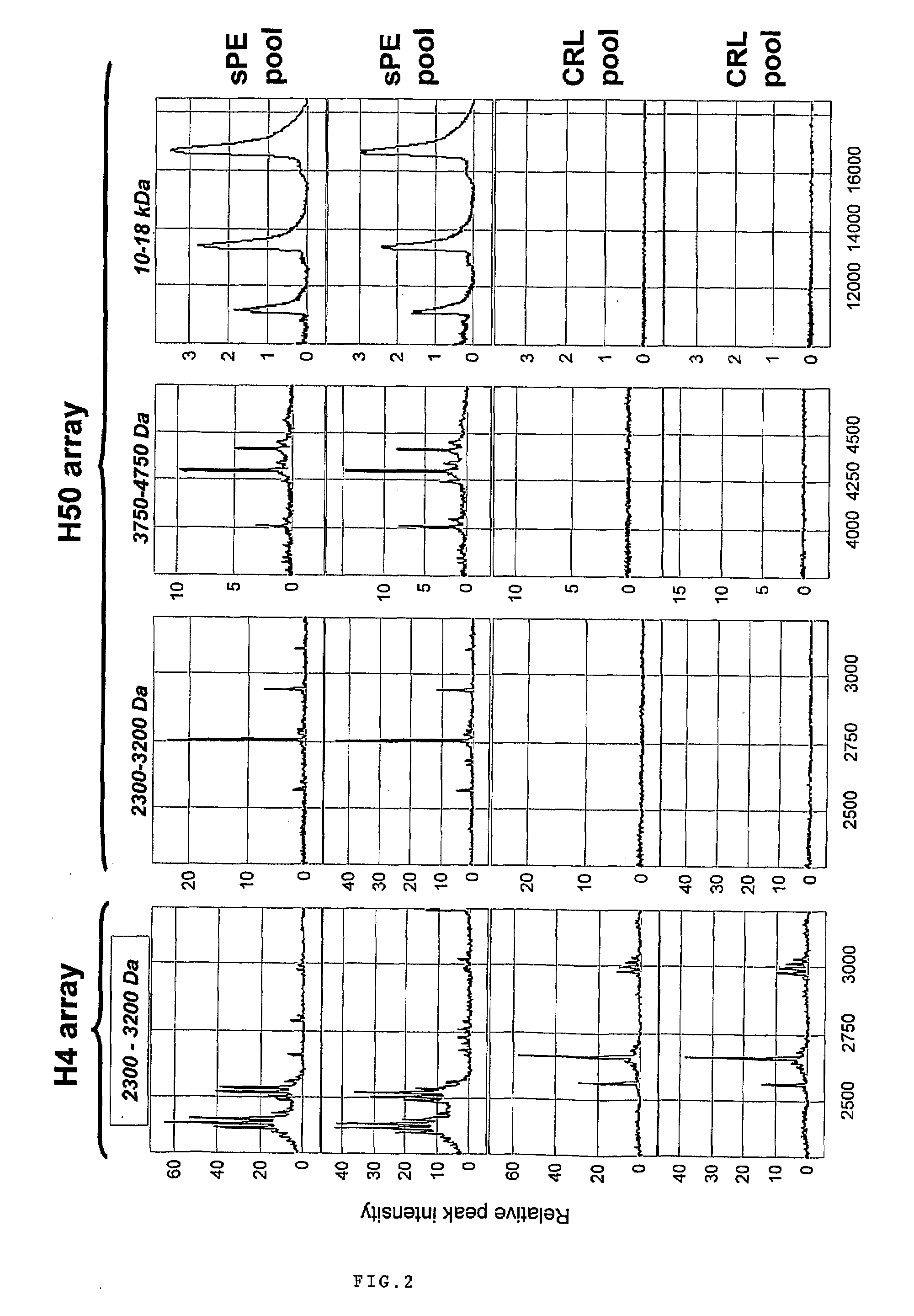
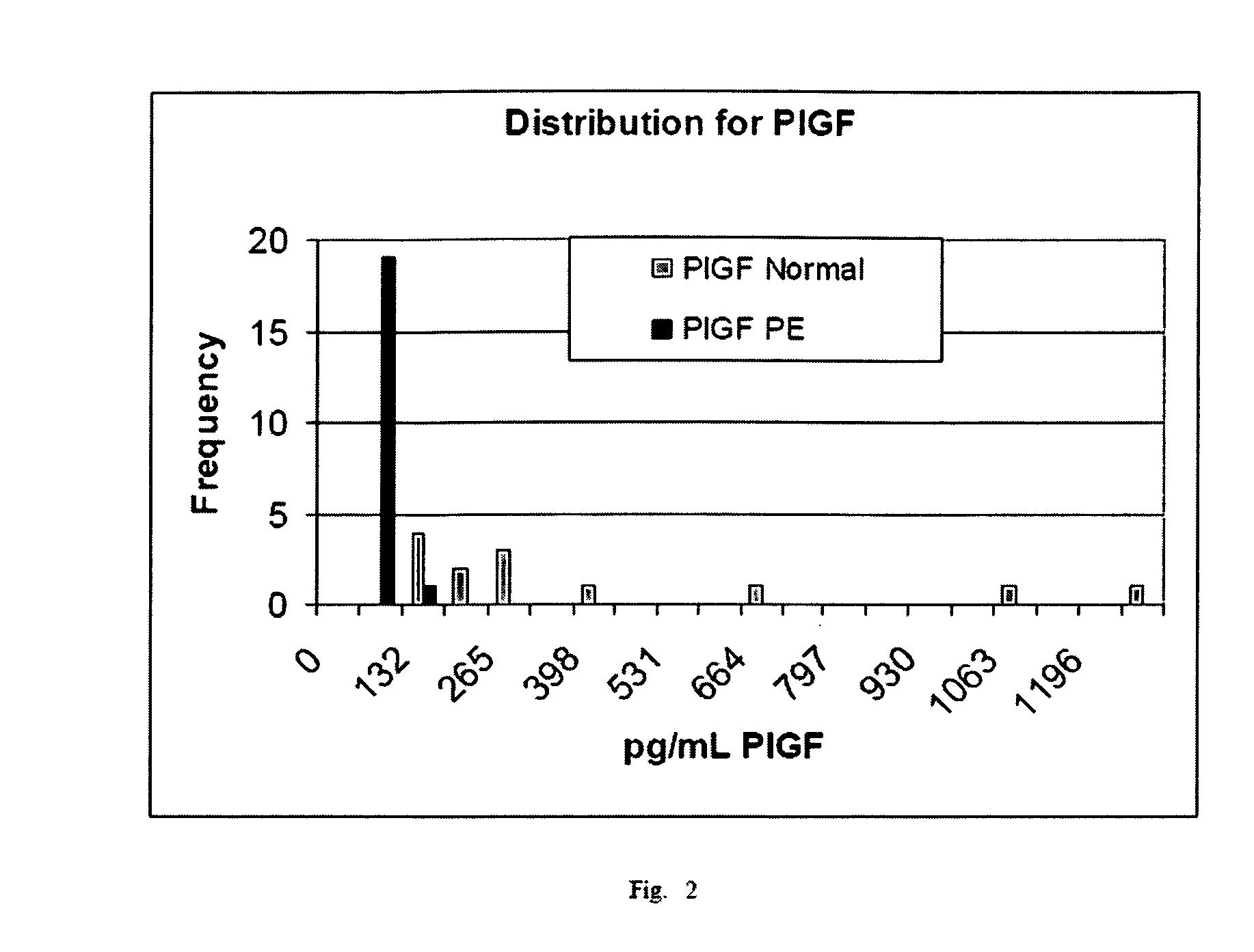
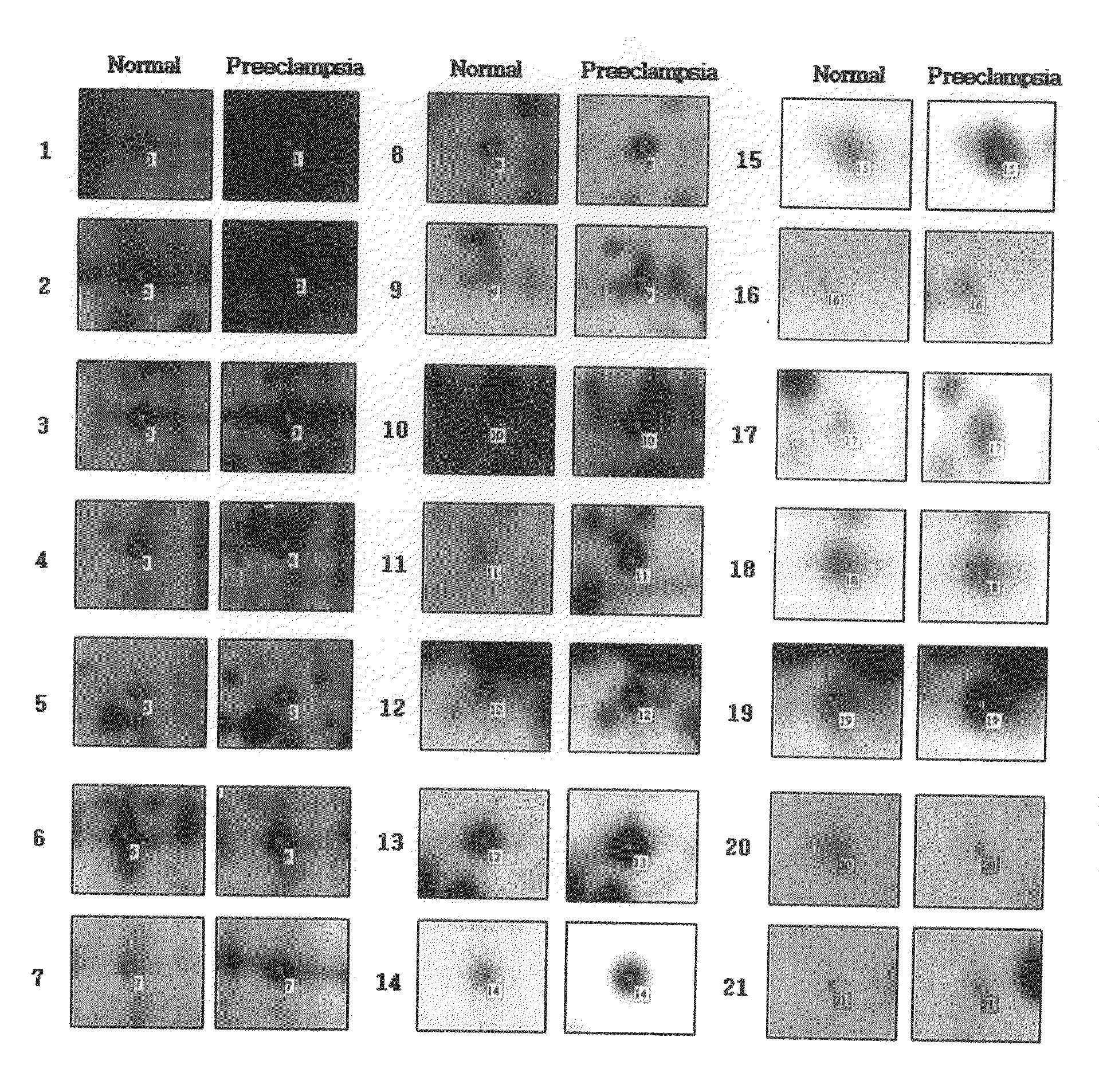
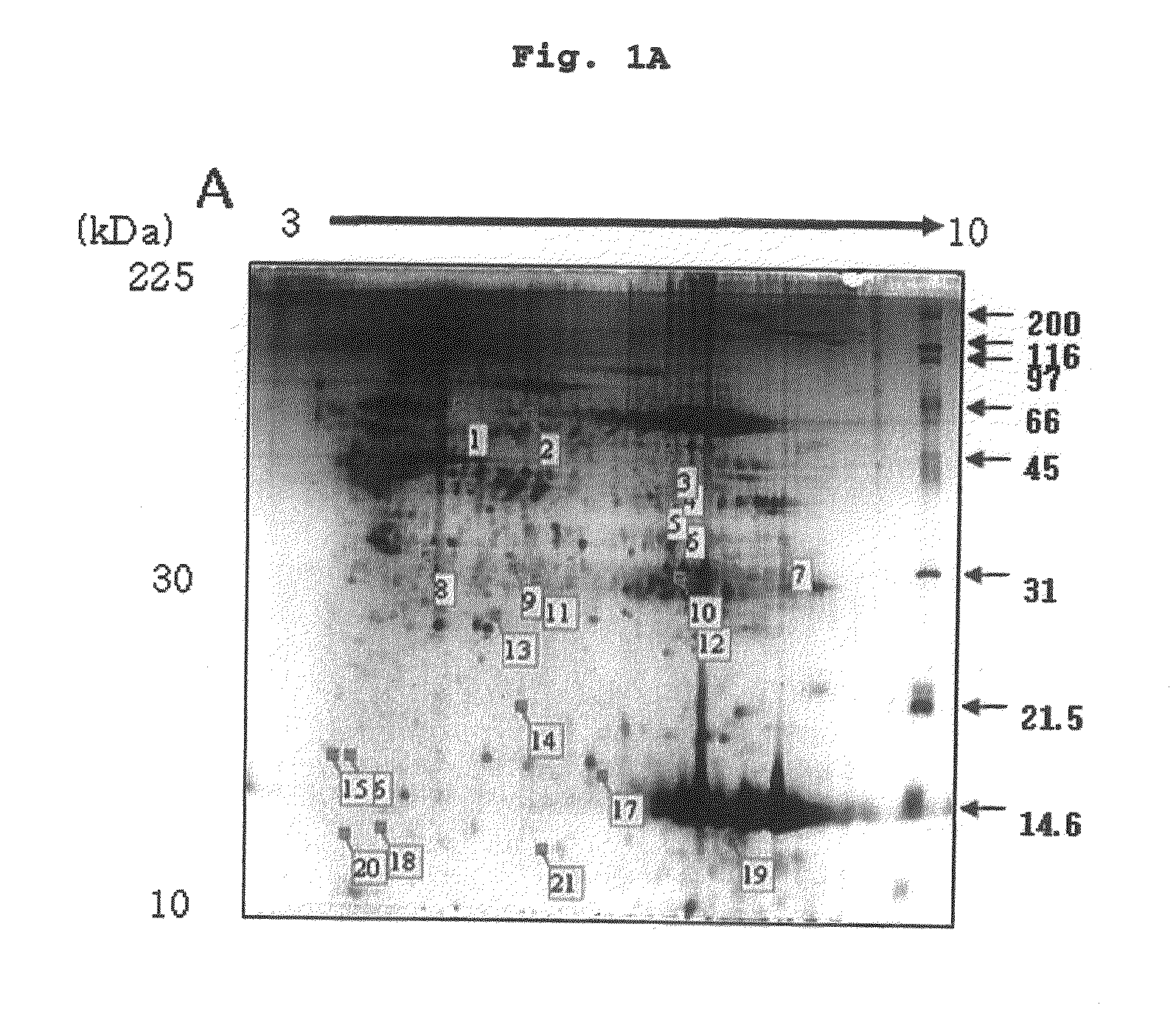
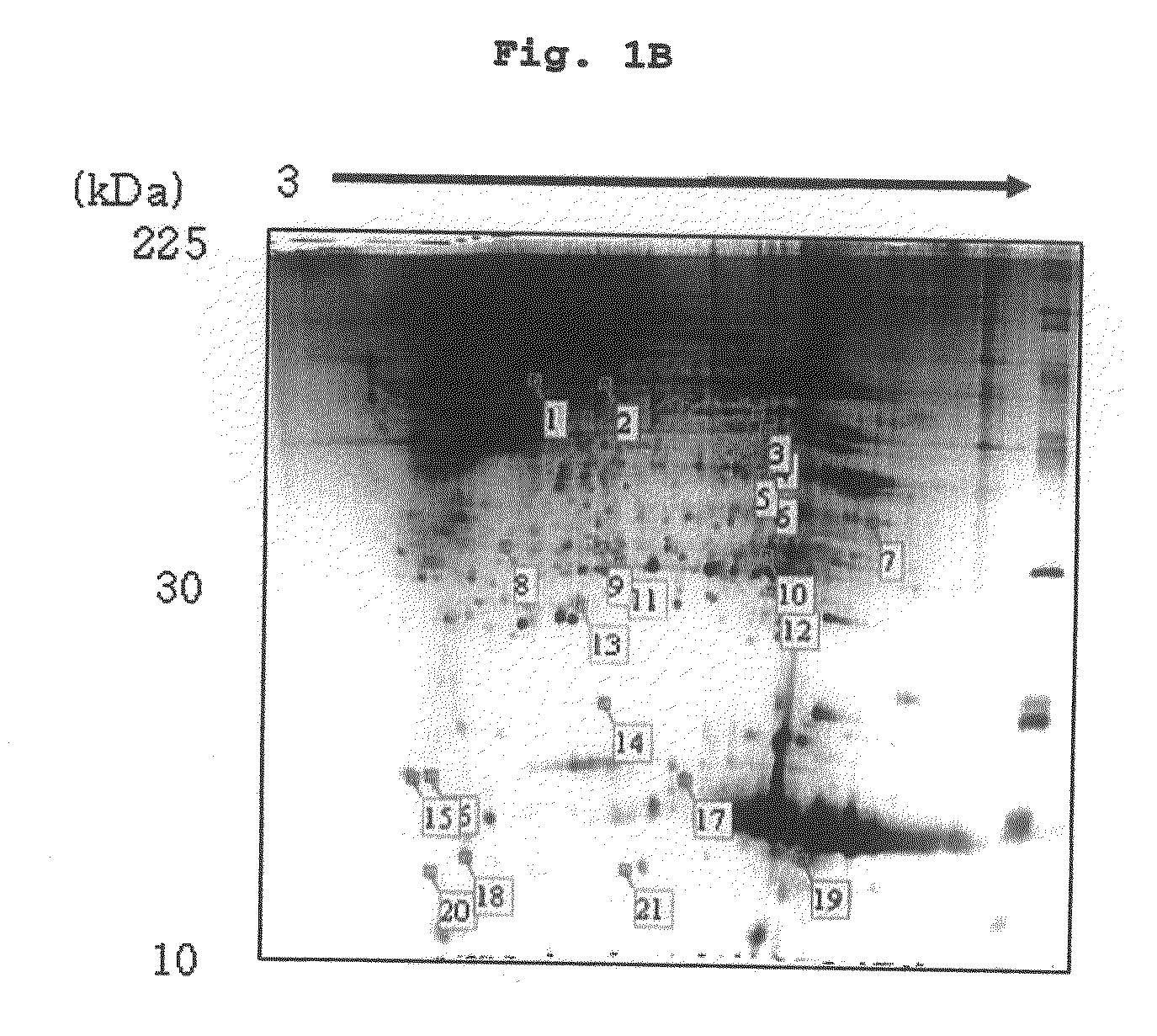
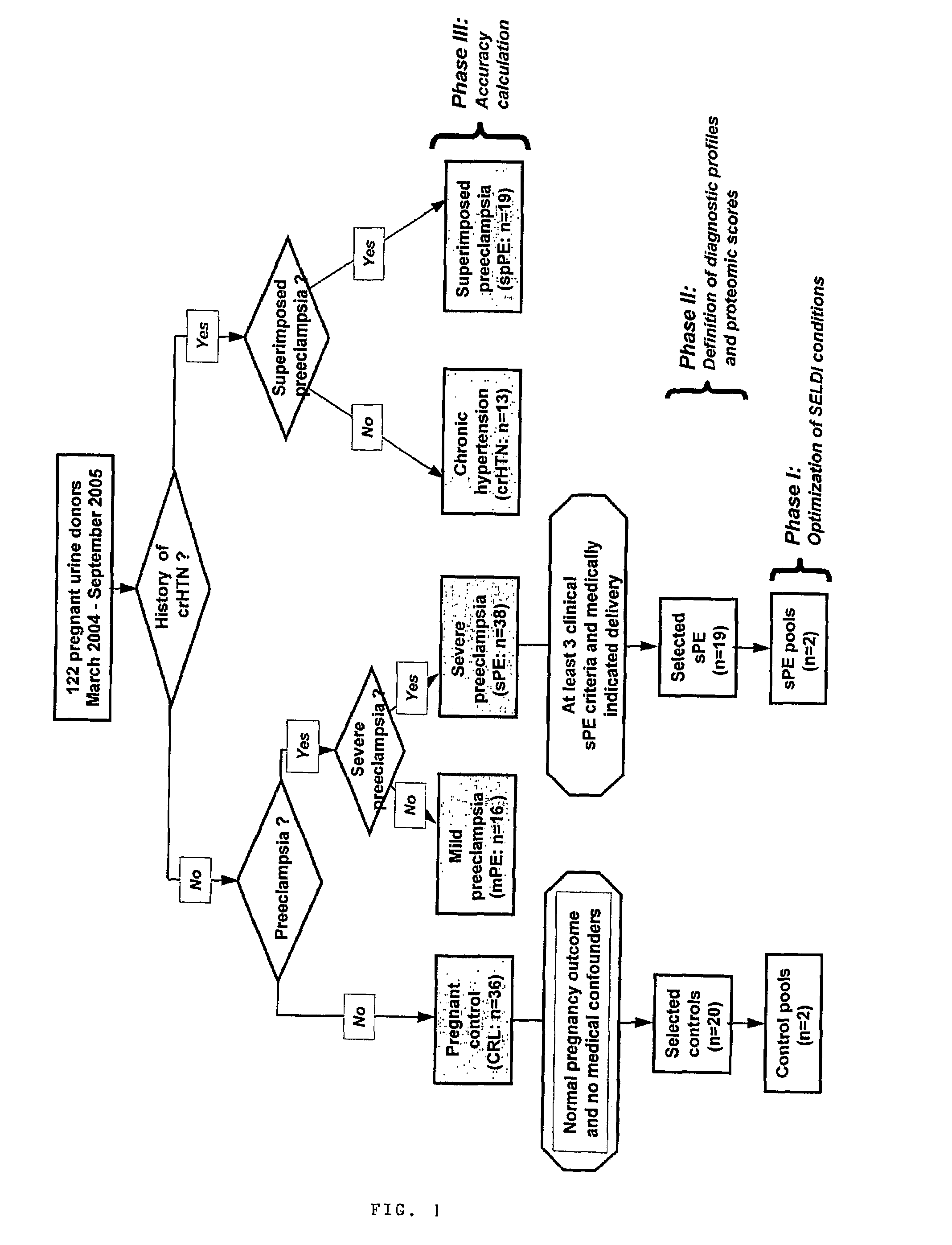
Urinary Proteomic Biomarker Patterns in Preeclampsia
The invention relates, in part, to methods of using proteomic biomarkers to diagnose preeclampsia. In some aspects the invention, in part, relates to the detection of serpina-1 polypeptide and / or albumin polypeptide in samples from pregnant subjects. Samples from subjects may be compared to control samples to diagnose preeclampsia and / or to determine the onset, progression, or regression of preeclampsia in a subject. The invention also relates, in part, to screening methods to identify agents that can be used to treat preeclampsia and to determine the efficacy of a preeclampsia treatment. The invention, in part, also includes kits that are useful to diagnose and assess preeclampsia in a subject.
Owner:YALE UNIV
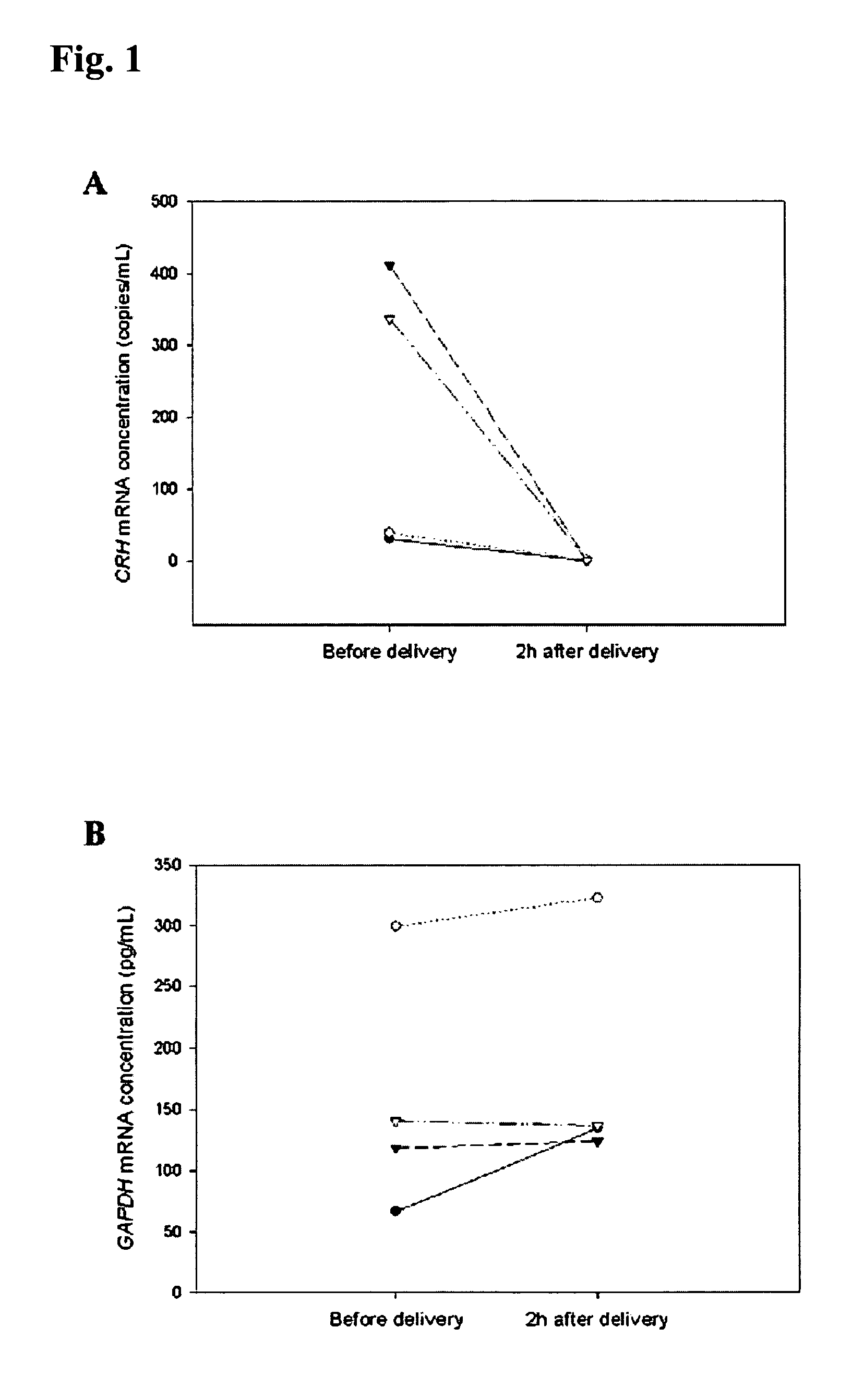
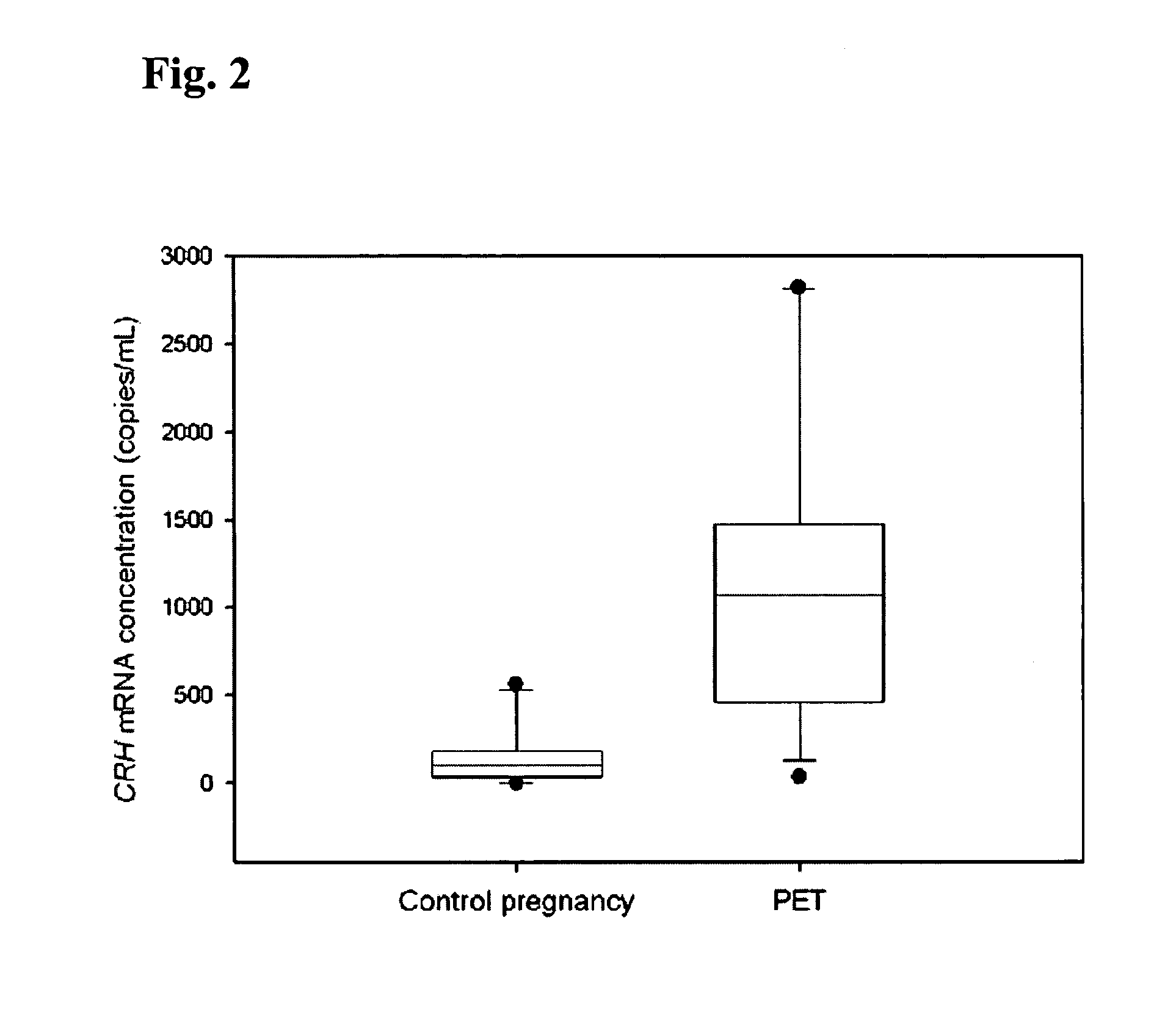
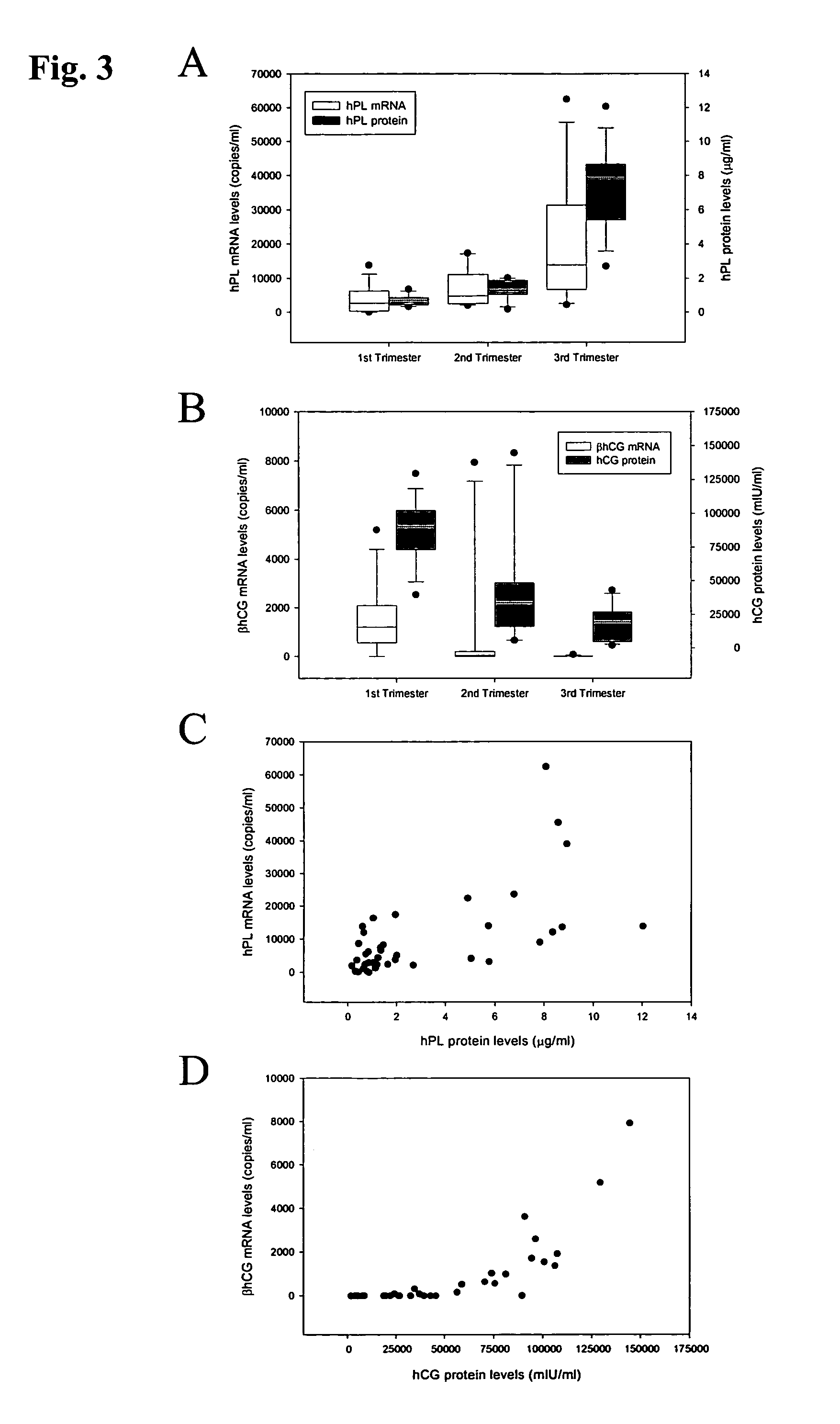
Method for diagnosing preeclampsia by detecting hCRH mRNA
ActiveUS7235359B2Increased risk of developingIncrease volumeSugar derivativesMicrobiological testing/measurementGlyceraldehydeMetastasis suppressor
Methods and kits are provided for diagnosing, monitoring, or predicting the conditions of pre-eclaimpsia, fetal chromosomal aneuploidy, and pre-term labor in a pregnant woman, as well as for detecting pregnancy in a woman, by quantitatively measuring in the maternal blood the amount of one or more mRNA species encoding human chorionic gonadotropin β subunit (hCG-β), human placental lactogen (hPL), human corticotropin releasing hormone (hCRH), KiSS-1 metastasis-suppressor (KISS1), tissue factor pathway inhibitor 2 (TPFI2), placenta-specific 1 (PLAC1), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and comparing the amount of the mRNA species with a standard control.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
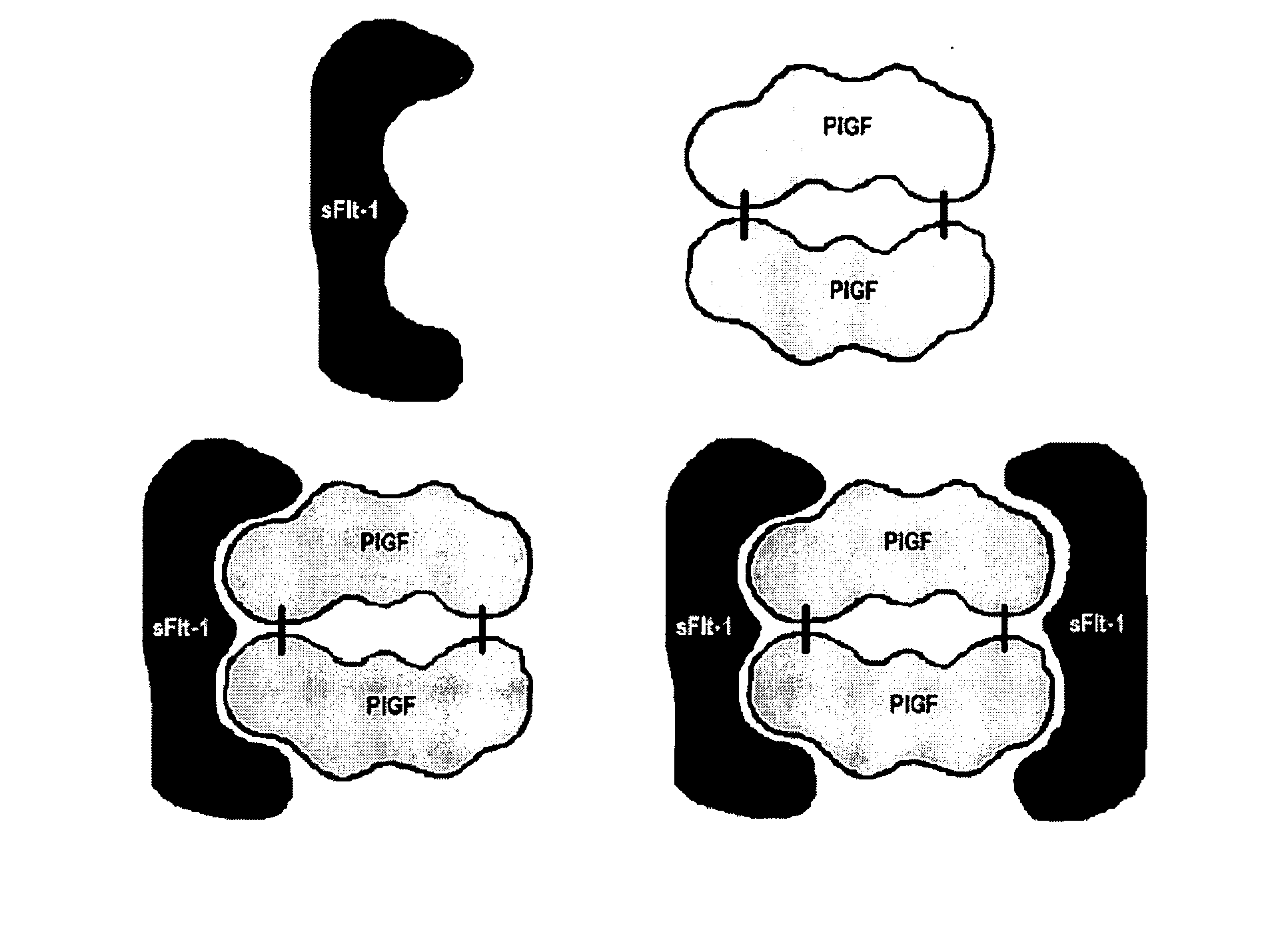
Diagnostic method for proteinaceous binding pairs, cardiovascular conditions and preeclampsia
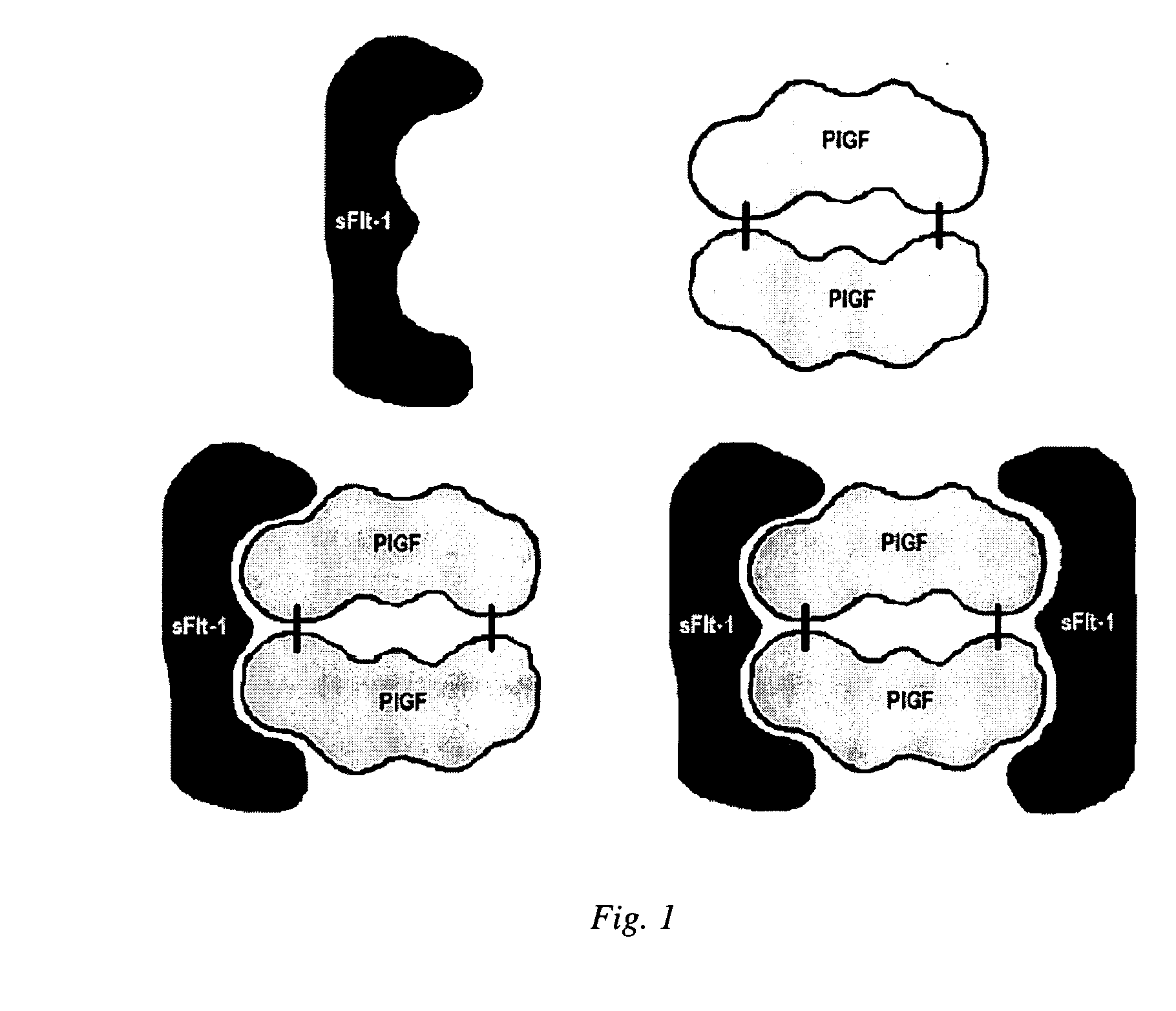
Method of measuring the quantity of a first proteinaceous specific binding partner (sbp) in a biological sample comprising detecting the binding of the first proteinaceous sbp with a labeled second proteinaceous sbp, wherein neither the first or second sbp is an antibody or fragment thereof, which is preferably a method of determining the amount of sFlt-1, particularly free sFlt-1, and the amount of PlGF, particularly free PlGF, in a sample, which is preferably used in a method of predicting risk of preeclampsia comprising comparing free PlGF to free sFlt-1.
Owner:ABBOTT LAB INC
Biomarkers for preeclampsia
InactiveUS20080233583A1Avoiding maternal immune rejectionMicrobiological testing/measurementDisease diagnosisBiomarker (petroleum)Cancer research
The present invention provides methods for predicting the development of and diagnosing preeclampsia, providing a prognosis, and predicting recurrence of the disease using molecular markers that are overexpressed or underexpressed in preeclampia. Also provided are methods to identify compounds that are useful for the treatment or prevention of preeclampsia.
Owner:RGT UNIV OF CALIFORNIA
System and method for detecting preeclampsia
InactiveUS20150164404A1Minimizes mortalityAvoid problemsElectrocardiographyEvaluation of blood vesselsObstetricsSAPS II
A system and method for detecting preeclampsia in a patient is provided. Also provided is a system and method for diagnosing preeclampsia in a patient prior to the detection of conventional symptoms and / or clinical signs associated with preeclampsia. The preeclampsia detection system of the invention comprises at least one sensor and a processor comprising a preeclampsia recognizer. In certain embodiments, the system farther comprises a user interface.
Owner:CONVERGENT ENG +1
Markers for the prognosis and risk assessment of pregnancy-induced hypertension and preeclampsia
InactiveCN103109192AHeart/pulse rate measurement devicesDisease diagnosisRisk evaluationPregnancy induced
The present invention relates to the prognosis and risk assessment in pregnant women to develop pregnancy-induced hypertension and / or preeclampsia by the determination of marker levels.
Owner:CEZANNE
Combination of prostacyclin with an estrogen or progestin for the prevention and treatment of atherosclerotic vascular disease including preeclampsia and for the treatment of hypertension, and for hormone replacement therapy
Cardiovascular disease, including preeclampsia in pregnant women and hypertension in both women and men, are prevented or treated by administering thereto prostacyclin or a prostacyclin analog in combination with one or both of an estrogen and a progestin, which combination is also useful for HRT in peri- and post-menopausal women.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Non-invasive measurement of suprasystolic signals
ActiveUS7727157B2Increase strainReduce complianceEvaluation of blood vesselsCatheterCardiac dysfunctionCardiac arrhythmia
An apparatus for assessing cardiovascular status of a mammal comprises a system for locally applying a pressure to an artery capable of restricting blood flow through said artery, a wideband external pulse transducer having an output and situated to measure suprasystolic signals proximate to said artery, and a computing device receiving said output for calculating vascular compliance values. The method described is particularly useful for determining cardiac output, assessing whether a pregnant female has preeclampsia or a patient has cardiac insufficiency, or assessing cardiac arrhythmia.
Owner:USCOM LTD
In vitro test to detect risk of preeclampsia
ActiveUS20050074746A1Reduction in trophoblast viabilityFunctional impairmentMicrobiological testing/measurementArtificial cell constructsObstetricsIn vitro test
The present invention provides methods for identifying patients at risk of developing preeclampsia. In further embodiments, the present invention relates to methods for the diagnosis of patients with preeclampsia.
Owner:YALE UNIV +1
Methods and compositions for diagnosing, prognosing, and confirming preeclampsia
Methods, compositions, kits, algorithms, systems, specialized computer, software, business methods and reagents are provided for diagnosing, prognosing, monitoring, characterizing, determining the severity of preeclampsia, confirming the presence of preeclampsia or confirming the absence of preeclampsia in a female subject. The methods and compositions find use in a number of applications, including, for example, predicting if an individual will develop preeclampsia, diagnosing whether the individual has preeclampsia, characterizing preeclampsia, monitoring an individual with preeclampsia, determining the severity of preeclampsia, confirming the presence of preeclampsia or confirming the absence of preeclampsia in a subject.
Owner:马修库珀 +4
Methods and compositions for diagnosing complications of pregnancy
ActiveUS20100291585A1Improve stabilitySugar derivativesMicrobiological testing/measurementComplicated pregnancyComplications of pregnancy
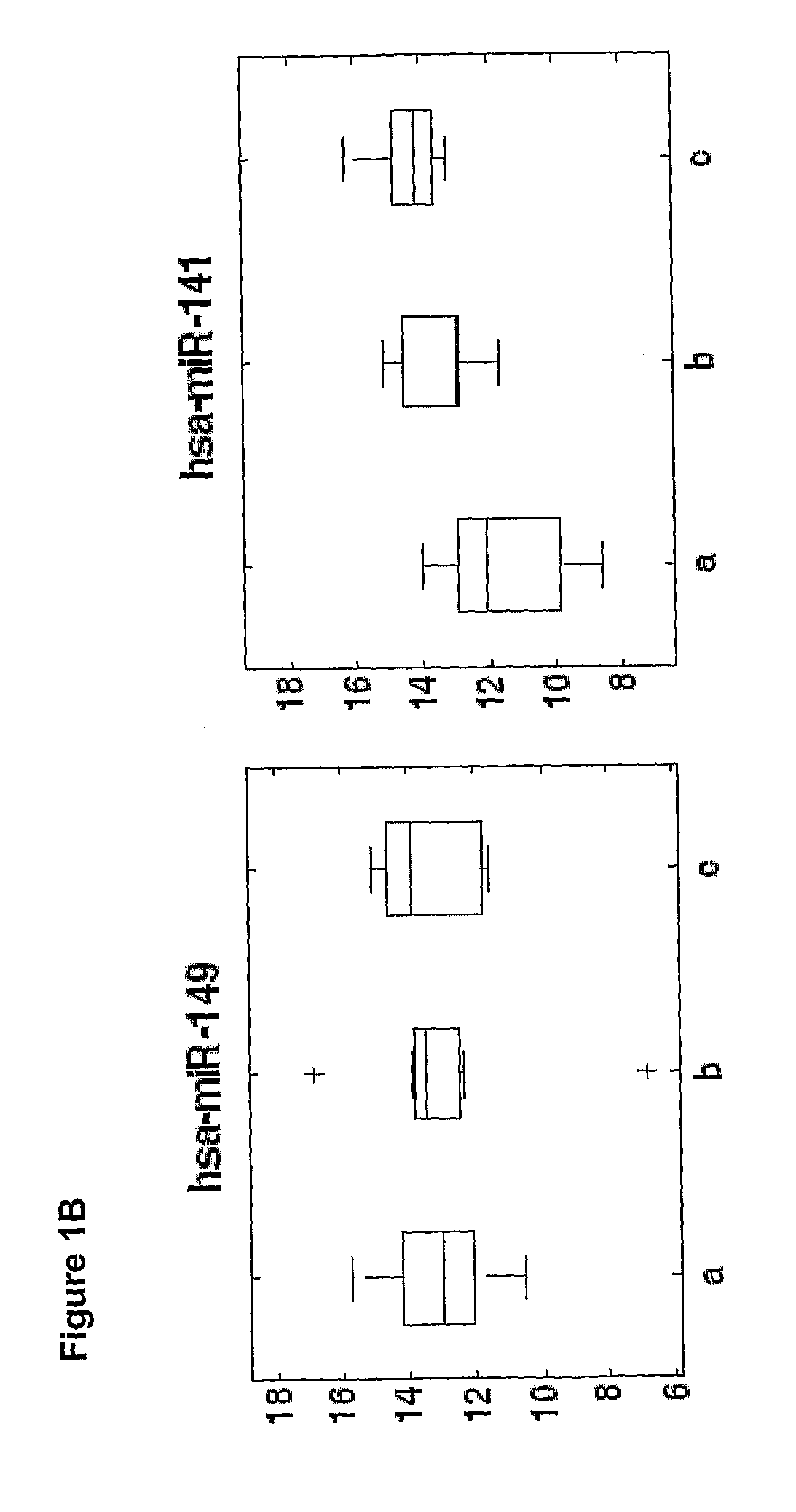
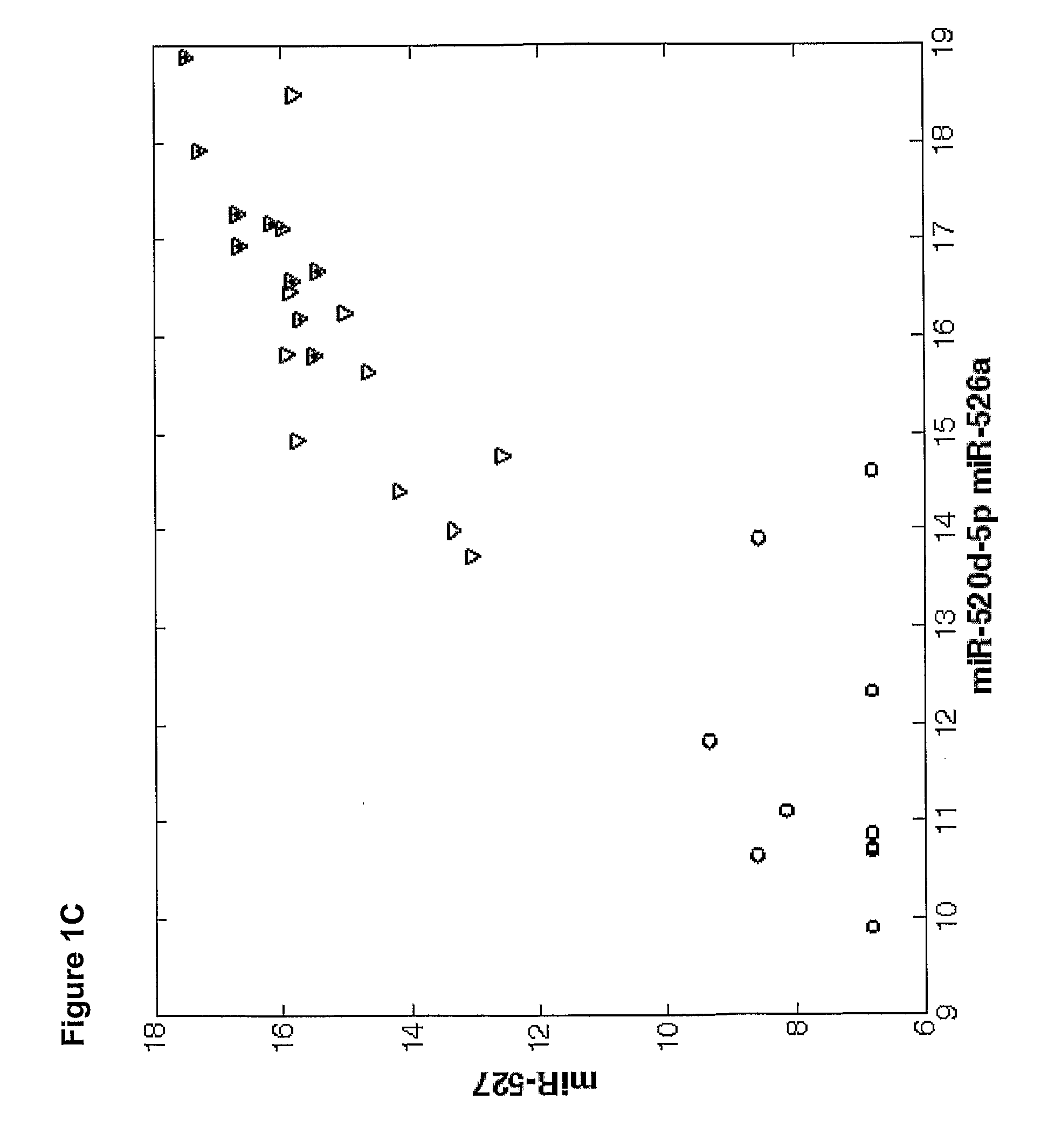
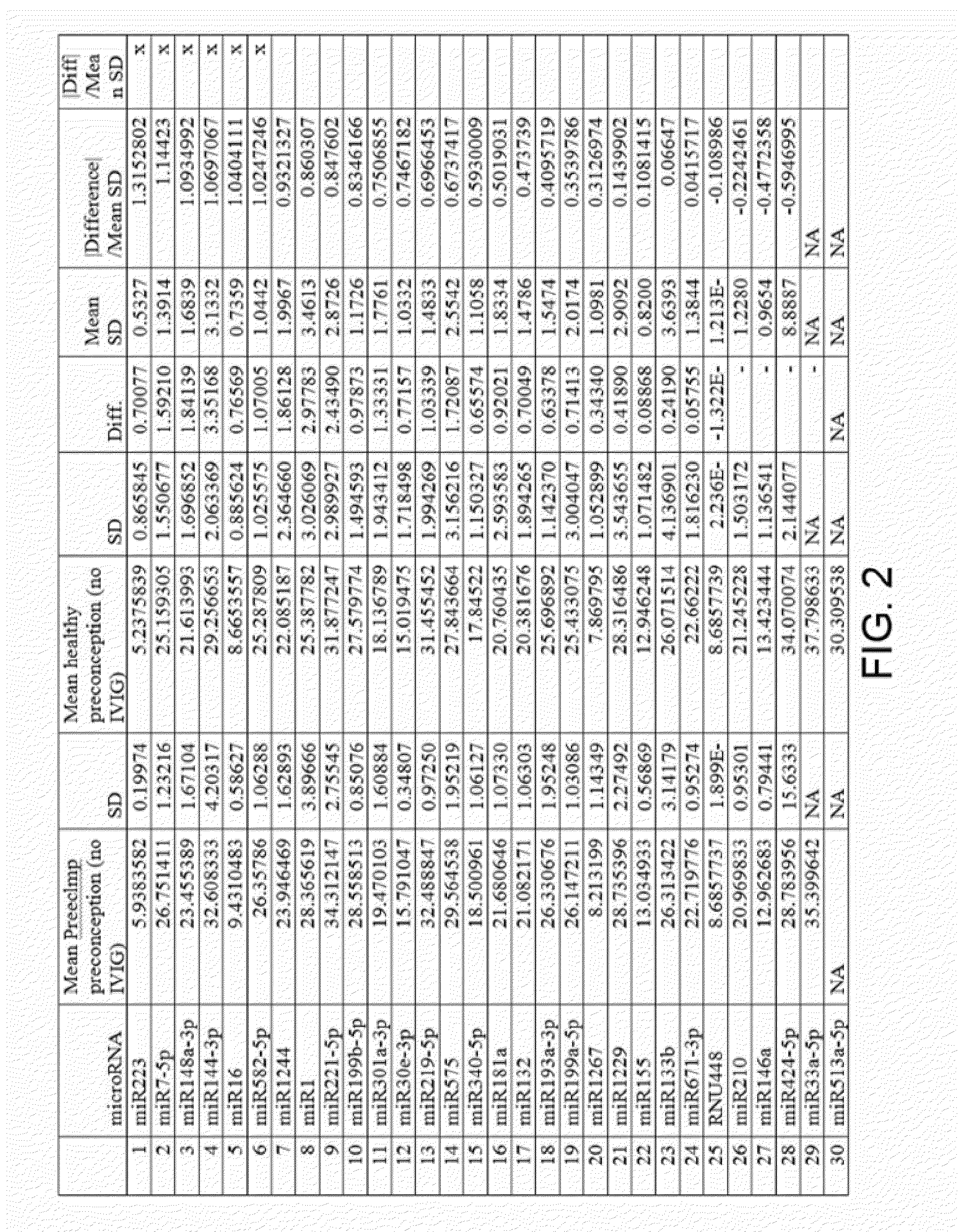
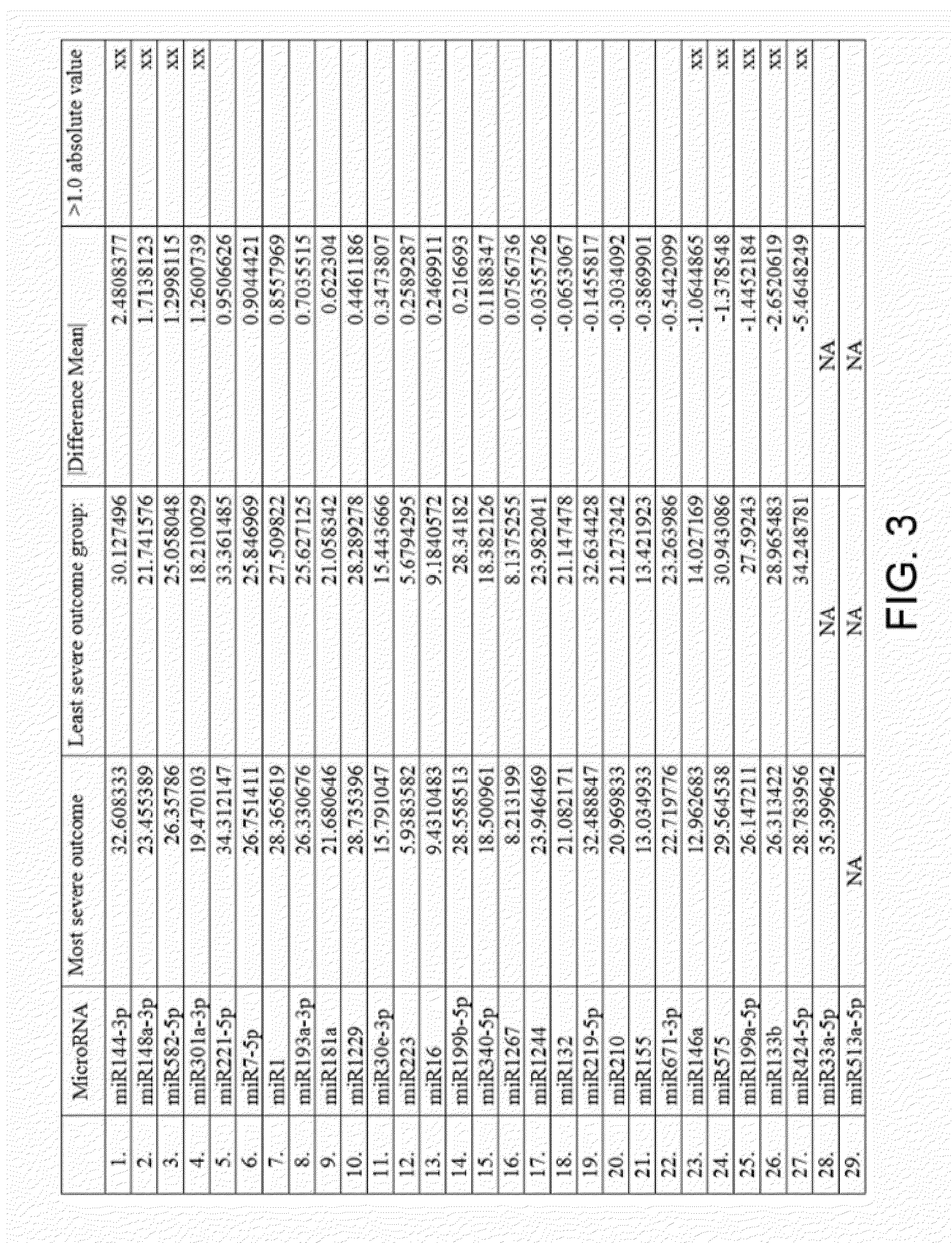
The present invention provides methods and compositions for identifying subjects at risk of developing a complication of pregnancy, such as preeclampsia or preterm labor. The compositions are microRNAs and associated nucleic acids.
Owner:ROSETTA GENOMICS +1
Methods and kits for detecting misfolded proteins
Methods, kits and compounds are provided that relate to the diagnosis, treatment, and / or prevention of preeclampsia.
Owner:RGT UNIV OF CALIFORNIA +1
Bioconjugates of synthetic apelin polypeptides
InactiveUS20150030594A1Extended half-lifeIncrease constraintsNervous disorderPeptide/protein ingredientsCardiac fibrosisVentricular tachycardia
The invention provides a bioconjugates comprising a synthetic polypeptide of Formula I′ (SEQ ID NO: 1):or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein and a half-life extending moiety wherein the peptide and the half-life extending moiety are covalently linked or fuse, optionally via a linker. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the bioconjugates of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Method of screening placental proteins responsible for pathophysiology of preeclampsia, and marker for early diagnosis and prediction of preeclampsia
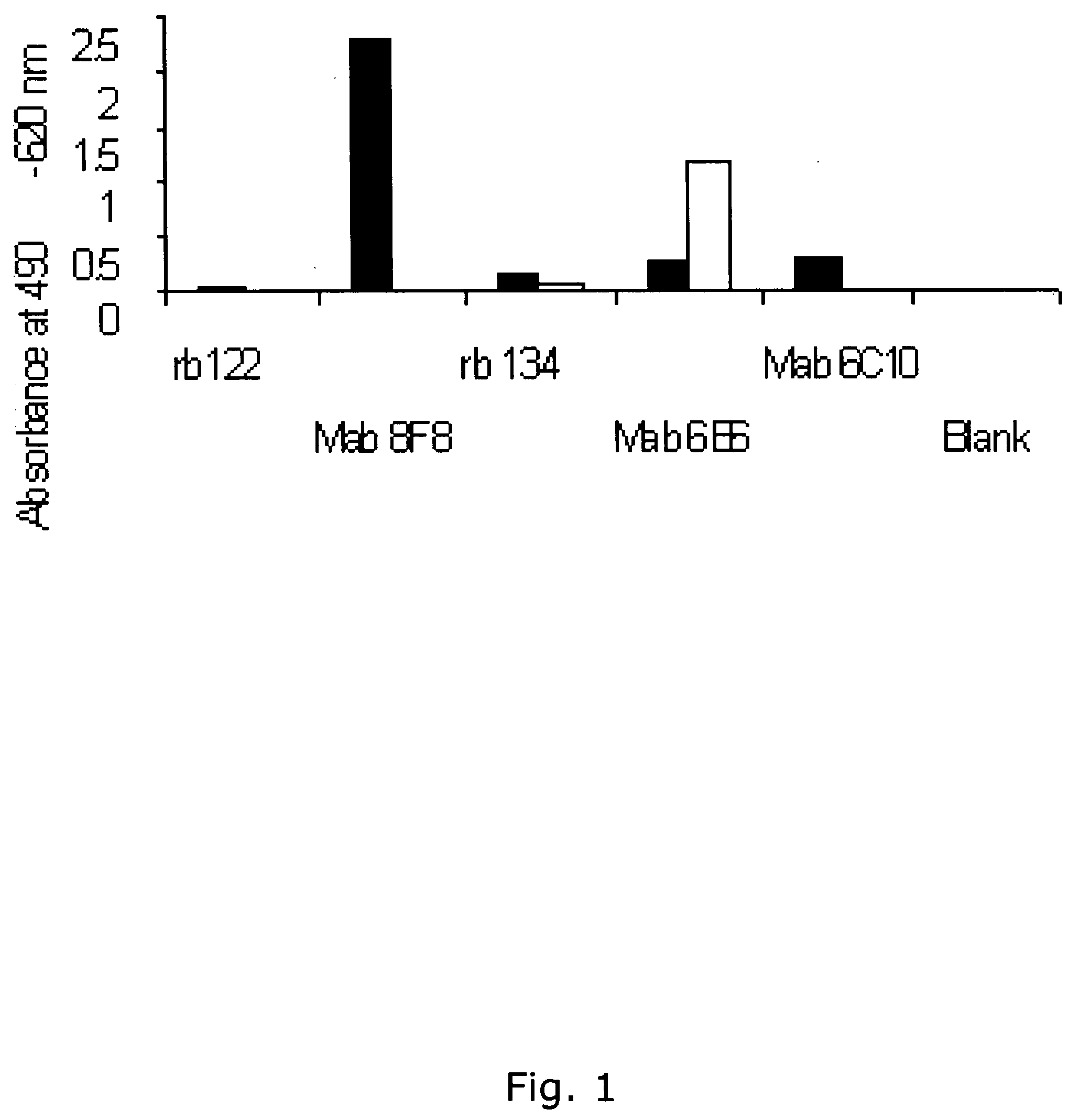
The present invention relates to a method of screening placental proteins responsible for pathophysiology of preeclampsia, and a marker for early diagnosis and prediction of preeclampsia. In accordance with one aspect of the present invention, there is provided a method of screening placental proteins responsible for pathophysiology of preeclampsia by 2D E-proteomics analysis, comprising: isolating placental proteins from a placental tissue; separating the isolated proteins two-dimensionally through 2D electrophoresis; and comparing and analyzing the separated proteins based on scanned gel images and differences in the images between normal placental proteins and preeclamptic placental proteins, wherein the comparison and analysis of the placental proteins based on the scanned gel images and differences in the images are accomplished by selecting proteins with differences of 140% or more between two placentas.
Owner:HAN OU JIN
Urinary proteomic biomarker patterns in preeclampsia
The invention relates, in part, to methods of using proteomic biomarkers to diagnose preeclampsia. In some aspects the invention, in part, relates to the detection of serpina-1 polypeptide and / or albumin polypeptide in samples from pregnant subjects. Samples from subjects may be compared to control samples to diagnose preeclampsia and / or to determine the onset, progression, or regression of preeclampsia in a subject. The invention also relates, in part, to screening methods to identify agents that can be used to treat preeclampsia and to determine the efficacy of a preeclampsia treatment. The invention, in part, also includes kits that are useful to diagnose and assess preeclampsia in a subject.
Owner:YALE UNIV
Papp-a2 as a marker for monitoring, predicting and diagnosing preeclampsia
PAPP-A2 is used as a marker for monitoring, predicting and diagnosing preeclampsia in pregnant women. PAPP-A2 levels in pregnant women with preeclampsia are higher than PAPP-A2 levels in normal pregnant women. This is especially true of PAPP-A2 levels that are measured later on in the pregnancy. PAPP-A2 levels may be measured early in pregnancy in order to predict the likelihood of the patient having preeclampsia. Preeclampsia may also be diagnosed at later gestational ages when the levels of PAPP-A2 are more pronounced than normal PAPP-A2 levels at the same gestational age. The present invention relates to methods of assessing, predicting and diagnosing preeclampsia as well as a kit-of-parts for assessing, predicting and diagnosing preeclampsia.
Owner:ANSH LABS
Nutritional supplement for pregnant females
Preeclampsia and intrauterine growth restriction in a pregnant female mammal are prevented or decreased in severity by administering thereto a combination of a vitamin compound containing B1, Folic Acid (or active form 5-Methyl-Tetrahydrofolate i.e.; Metafolin®), B 6 (Pyridoxine or active form Pyridoxine 5-Phosphate P5P), B 12, Ascorbic Acid, Selenium, Zinc, Co-enzyme Q 10 and N-Acytyl Cysteine, Lycopene, optionally in further combination with Melatonin and / or Vitamin E or / and ASA 81 mg both of later, are cyclooxygenase inhibitors, a PGI.sub.2-mimetic, a thromboxane (TXA.sub.2) inhibitor, a compound possessing TXA.sub.2-agonistic and TXA.sub.2-inhibiting properties, a compound possessing TXA.sub.2-antagonistic and PGI.sub.2-mimetic activities, and a TXA.sub.2 antagonist.
Owner:MUENCH MICHAEL V +2
Methods and compositions for assessing patients with preeclampsia-related conditions using microrna
Owner:EDWARD E WINGER M D PROFESSIONAL
Peptide Markers for Diagnosis of Preeclampsia
InactiveUS20100291612A1Small volumeMicrobiological testing/measurementPeptide preparation methodsChorionic villiBiology
The present invention relates to a method for detecting preeclampsia, comprising determining the expression level of calcyclin in chorionic villi. The invention further relates to a marker for detecting preeclampsia wherein said marker is calcyclin.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Colloid selenium test paper for semi-quantitative determination of urinary trace albumin
The invention provides a test paper for semi-quantitatively detecting colloid selenium in microdose albumin in urine. The test paper can be applied in the fields of early diagnosis of diabetic nephropathy, hypertensive nephropathy and preeclampsia and injury of kidney caused by various poisonous substances. A plastic bottom plate of the test paper is orderly glued with a sampling liquid absorption part, a colloid selenium labeling part, a detection reaction part and a water absorption part, wherein the colloid selenium labeling part is a single-cloning antibody 1 which is coated by a glass fiber membrane, or acetic acid fiber membrane, or nylon membrane and labeled by the colloid selenium; the detection reaction part is a nitrocellulose membrane which is provided with two detection bands and quality control bands, T1 is a single-cloning antibody 2 which is precisely quantitative, and T2 is the single-cloning antibody 2 with a certain concentration; and the quality control bands are coated by antiplague IgG. The invention provides a method for semi-quantitatively detecting the microdose albumin in a urine sample conveniently, visually and quickly.
Owner:LANZHOU UNIVERSITY
ADAM12, a novel marker for abnormal cell function
InactiveUS20060134654A1Improve the detection rateReduce false alarm rateMicrobiological testing/measurementPeptide preparation methodsDiseaseADAM12
The present invention provides a method, an assay and a kit for providing an indication of abnormal cell function. It was surprisingly found that the change in the serum ADAM12 concentration in individuals was useful as a prognostic tool to predict the clinical outcome, complications and mortality following an abnormal cell function. The present inventors describes ADAM12 as a overall general marker for abnormal cell function, and the present inventor for the first time demonstrate that ADAM12 is an important indicator of fetal chromosomal disease and placenta function. Specifically ADAM12 is a good marker for e.g. Downs's syndrome, trisomy 18, preeclampsia, Turner syndrome in both first and second trimester. The present inventors developed an enzyme-linked immunosorbent assay (ELISA) and a time-resolved immunofluorometric assay for the quantification of ADAM12 in serum. The present application demonstrates in several examples the variation of the ADAM12 level in fetal abnormality and / or adverse pregnancy outcomes correlated gestational age when compared to normal controls. It is an object of the invention to provide an improvement of the existing marker tests that exhibits a decreased false positive rate.
Owner:STATENS SERUM INST +2
Gene expression related to preeclampsia
InactiveUS20110171650A1Microbiological testing/measurementBiological testingFirst trimesterBiomarker (petroleum)
Gene expression patterns contemporaneous with early placental development in the first trimester of preeclamptic versus unaffected pregnancies have been obtained. Observation of differences in these gene expression patterns has allowed the identification of biomarkers that are useful in predicting and monitoring preeclampsia. These biomarkers are also useful in screening potential therapeutics for efficacy in the prevention or treatment of preeclampsia.
Owner:UNIVERSITY OF PITTSBURGH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com