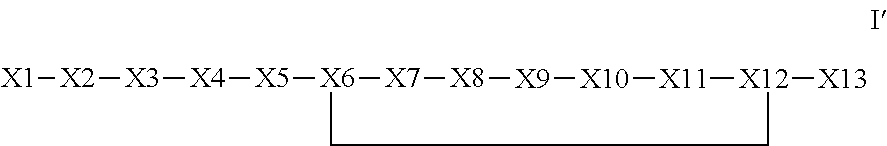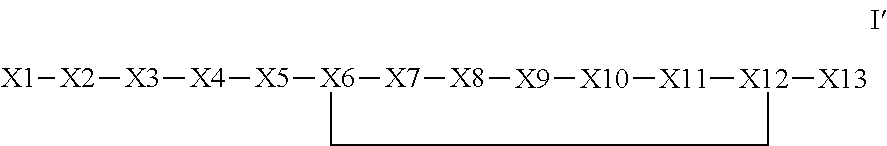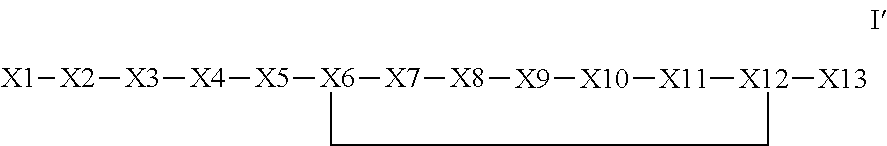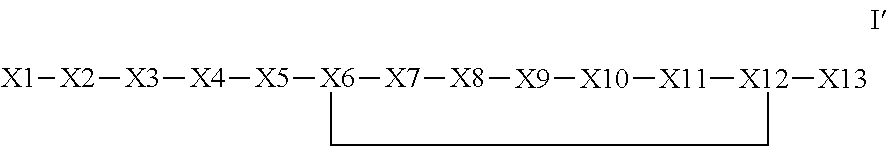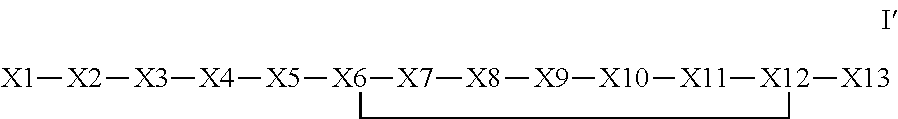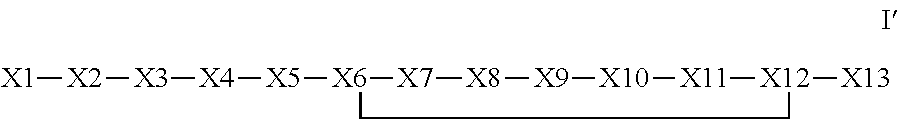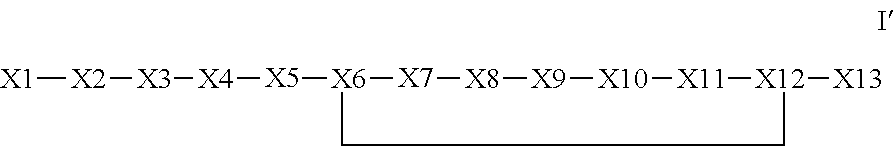Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Acute decompensated heart failure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
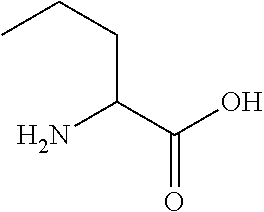
Acute decompensated heart failure (ADHF) is a sudden worsening of the signs and symptoms of heart failure, which typically includes difficulty breathing (dyspnea), leg or feet swelling, and fatigue. ADHF is a common and potentially serious cause of acute respiratory distress. The condition is caused by severe congestion of multiple organs by fluid that is inadequately circulated by the failing heart. An attack of decompensation can be caused by underlying medical illness, such as myocardial infarction, an abnormal heart rhythm, infection, or thyroid disease.
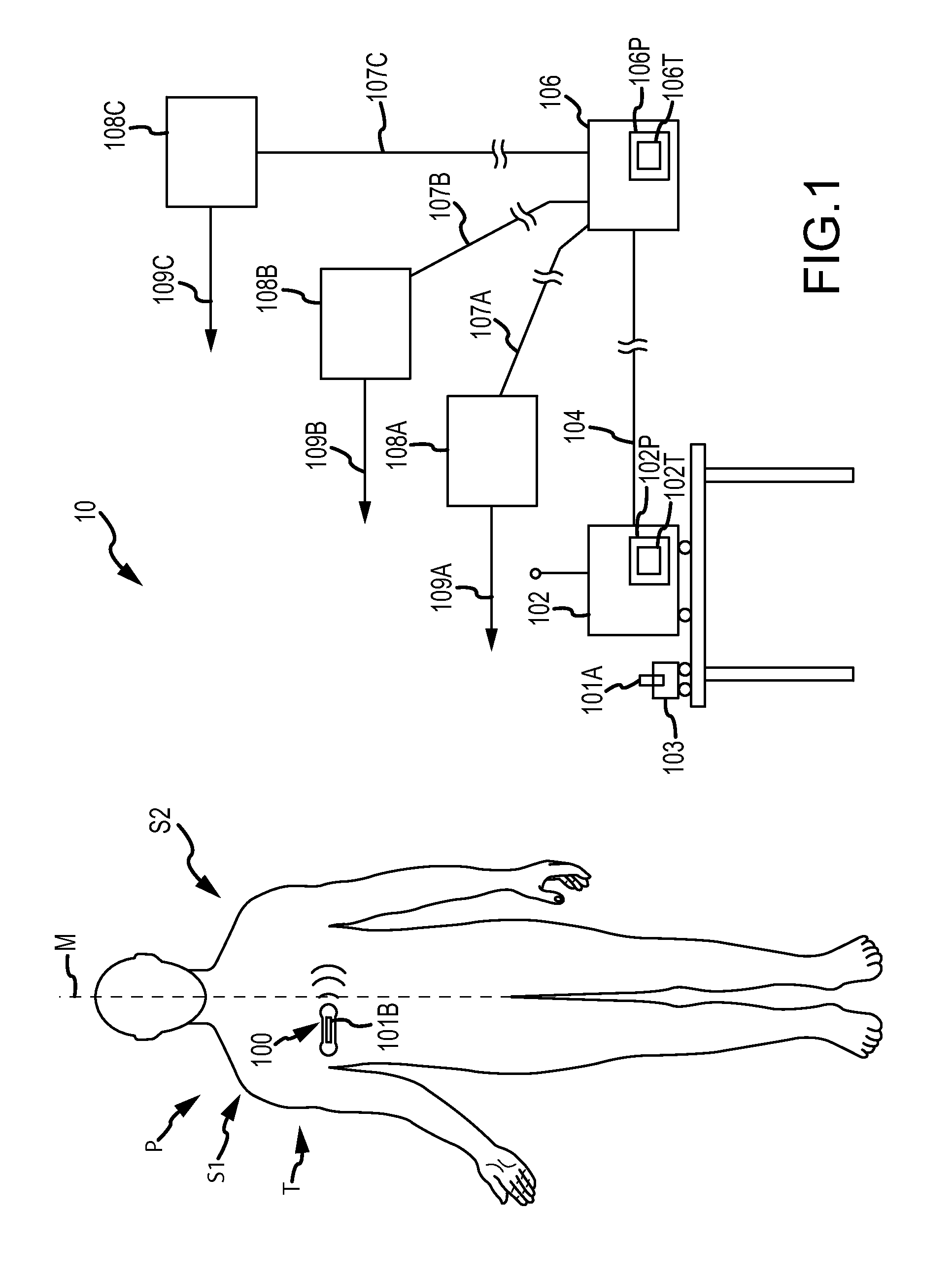
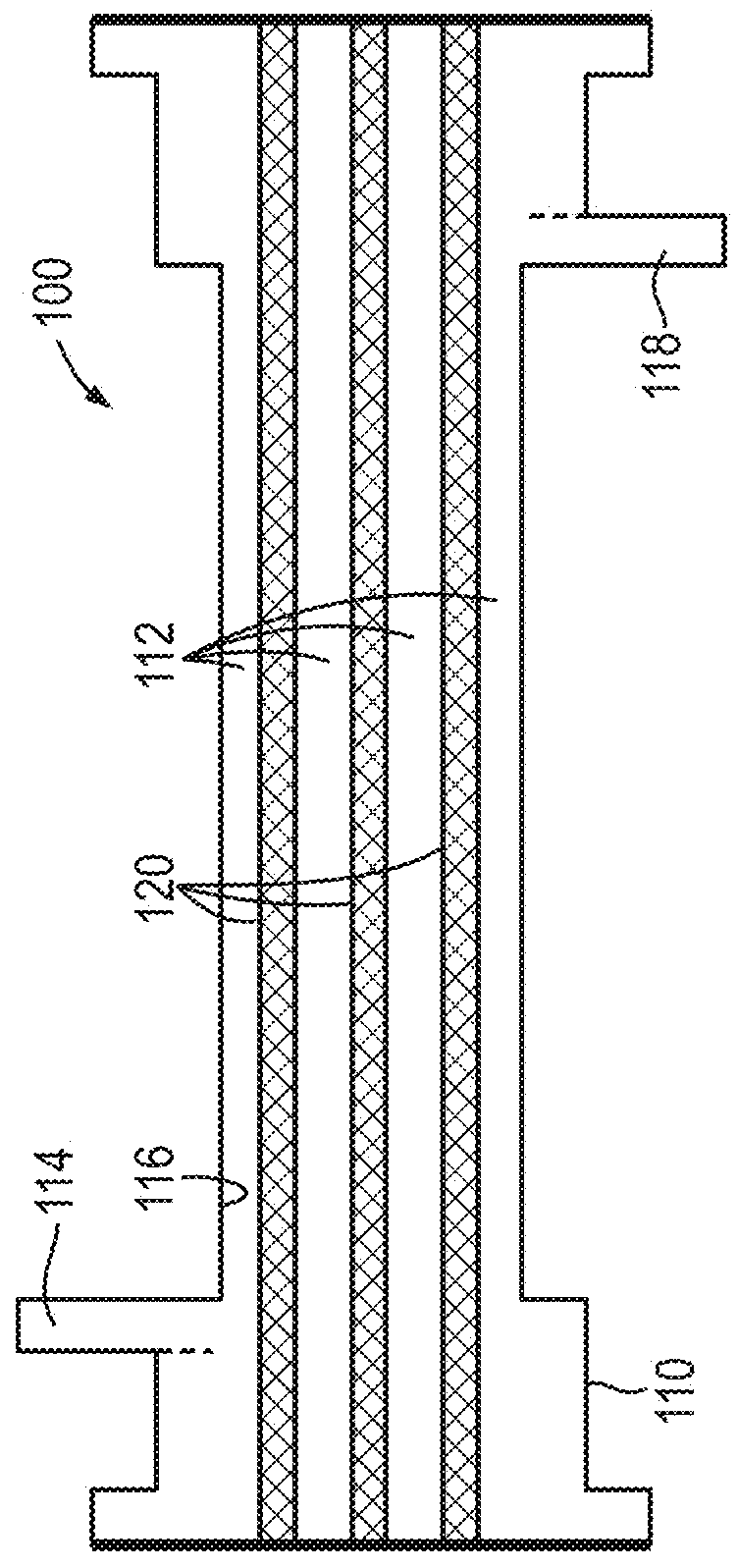
Method and apparatus for personalized physiologic parameters
InactiveUS20130096395A1ElectrotherapyDiagnostics using spectroscopyPersonalizationMultivariate prediction
Methods and apparatus combine patient measurement data with demographic or physiological data of the patient to determine an output that can be used to diagnose and treat the patient. A customized output can be determined based the demographics of the patient, physiological data of the patient, and data of a population of patients. In another aspect, patient measurement data is used to predict an impending cardiac event, such as acute decompensated heart failure. At least one personalized value is determined for the patient, and a patient event prediction output is generated based at least in part on the personalized value and the measurement data. For example, bioimpedance data may be used to establish a baseline impedance specific to the patient, and the patient event prediction output generated based in part on the relationship of ongoing impedance measurements to the baseline impedance. Multivariate prediction models may enhance prediction accuracy.
Owner:MEDTRONIC MONITORING
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS8673848B2Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
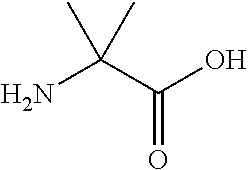
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS20130196899A1Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Synthetic linear apelin mimetics for the treatment of heart failure
InactiveUS20140155315A1Extended half-lifeIncrease constraintsNervous disorderPeptide/protein ingredientsCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′ (SEQ ID NO: 1):X1-X2-X3-R—X5-X6-X7-X8-X9-X10-X11-X12-X13 Ior an amide, an ester or a salt thereof, wherein X1, X2, X3, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
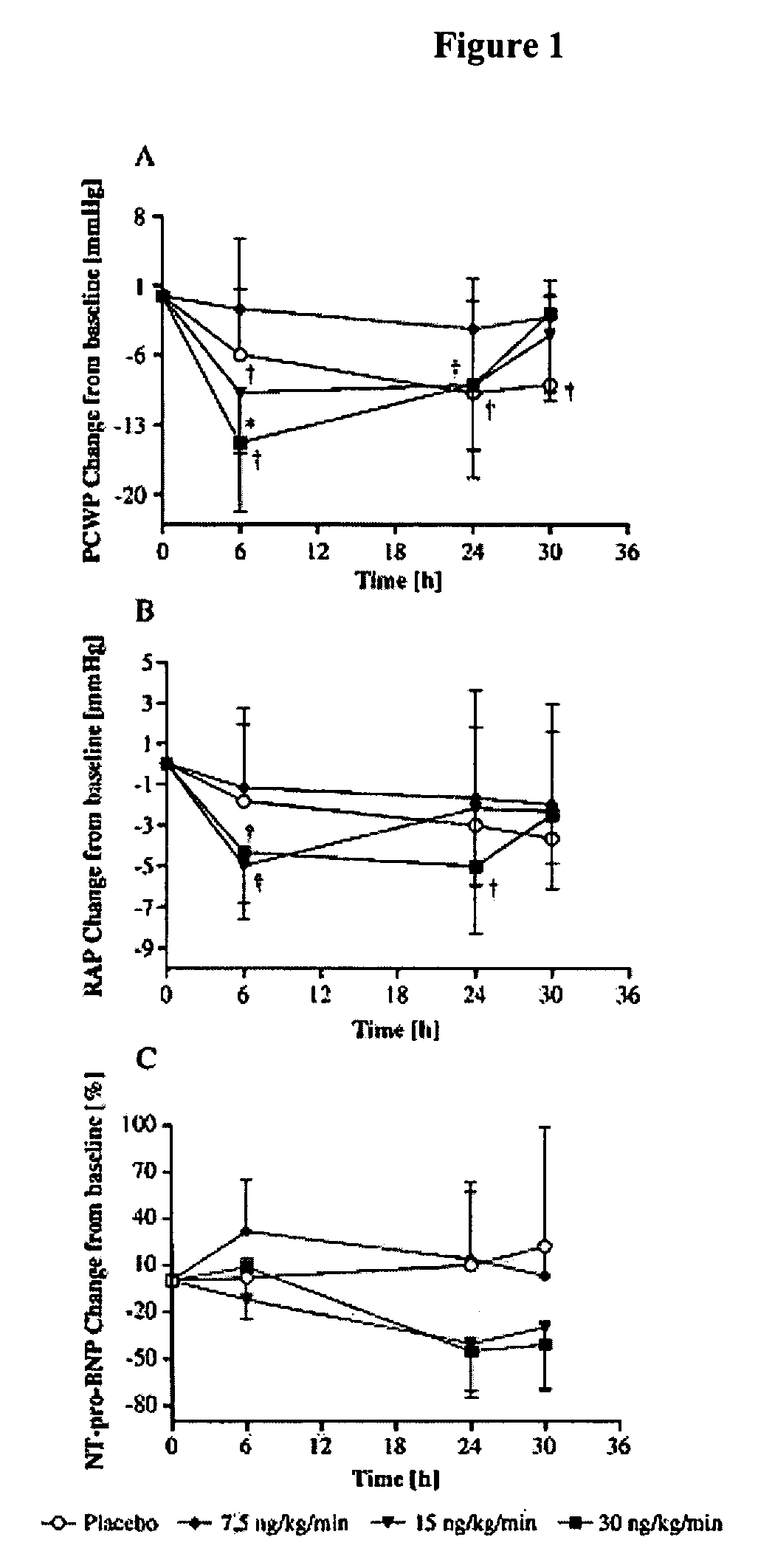
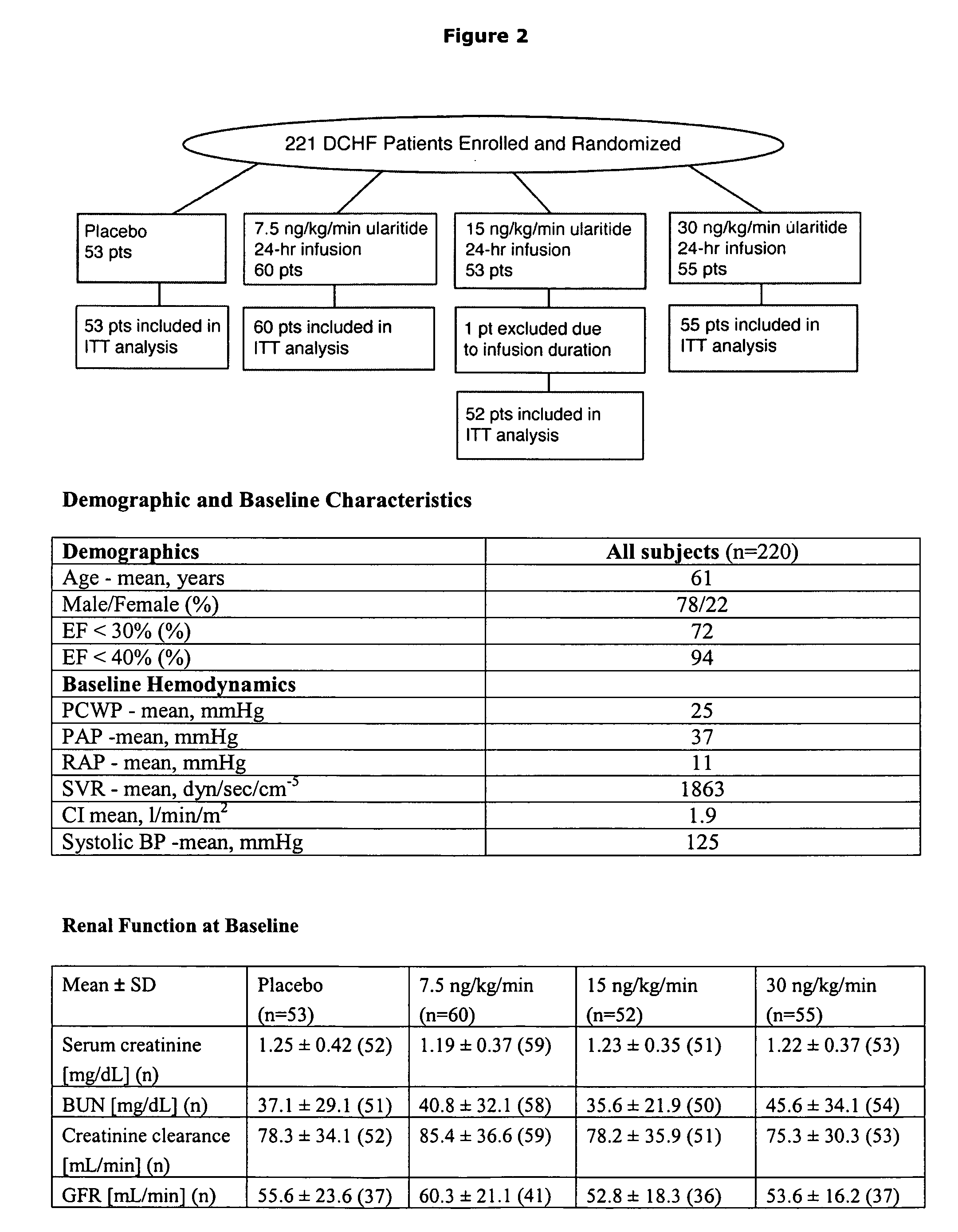
Use of natriuretic peptide for treating heart failure
Owner:CARDIORENTIS
Bioconjugates of synthetic apelin polypeptides
InactiveUS20150030594A1Extended half-lifeIncrease constraintsNervous disorderPeptide/protein ingredientsCardiac fibrosisVentricular tachycardia
The invention provides a bioconjugates comprising a synthetic polypeptide of Formula I′ (SEQ ID NO: 1):or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein and a half-life extending moiety wherein the peptide and the half-life extending moiety are covalently linked or fuse, optionally via a linker. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the bioconjugates of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Use of natriuretic peptide for treating heart failure
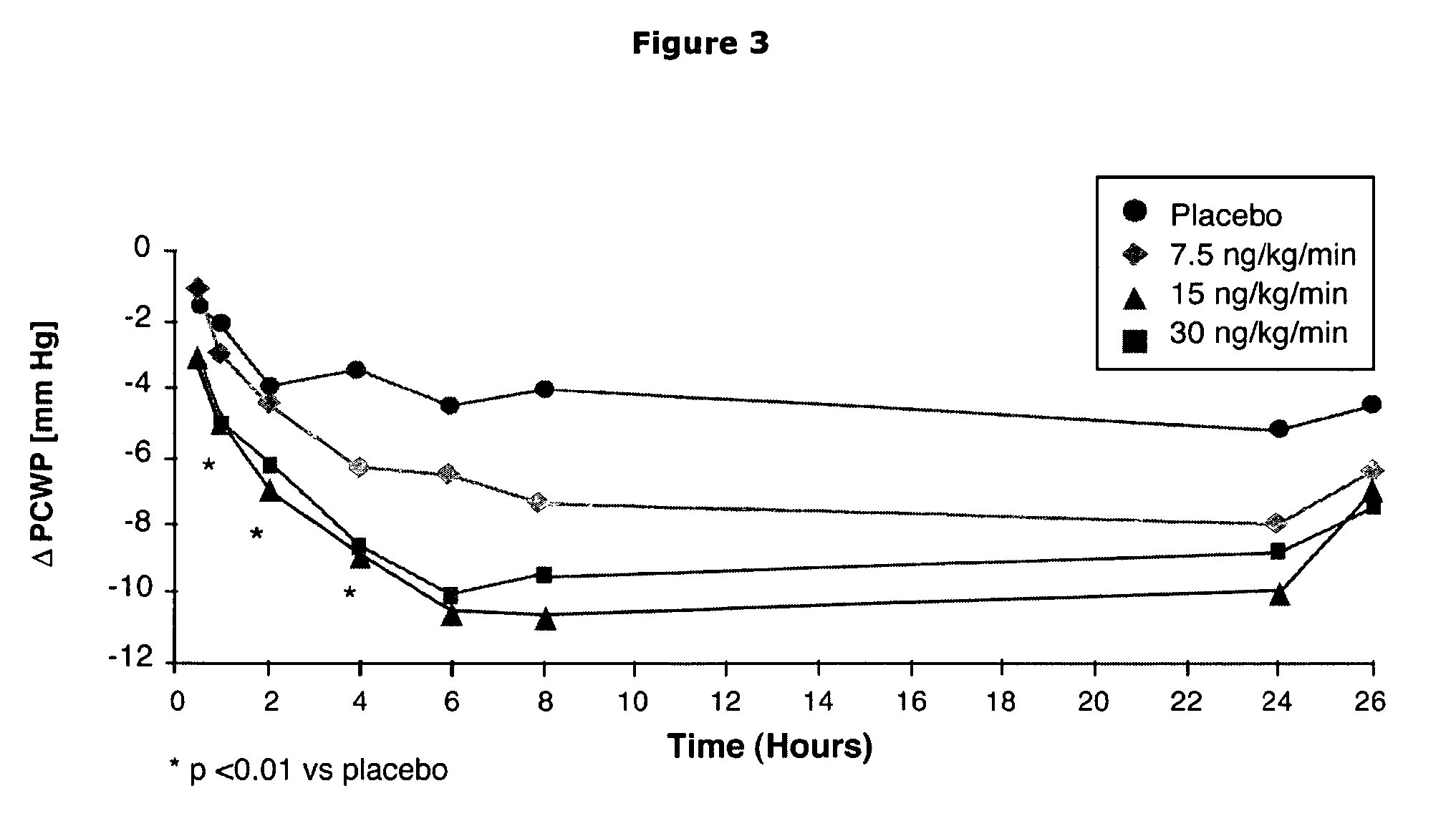
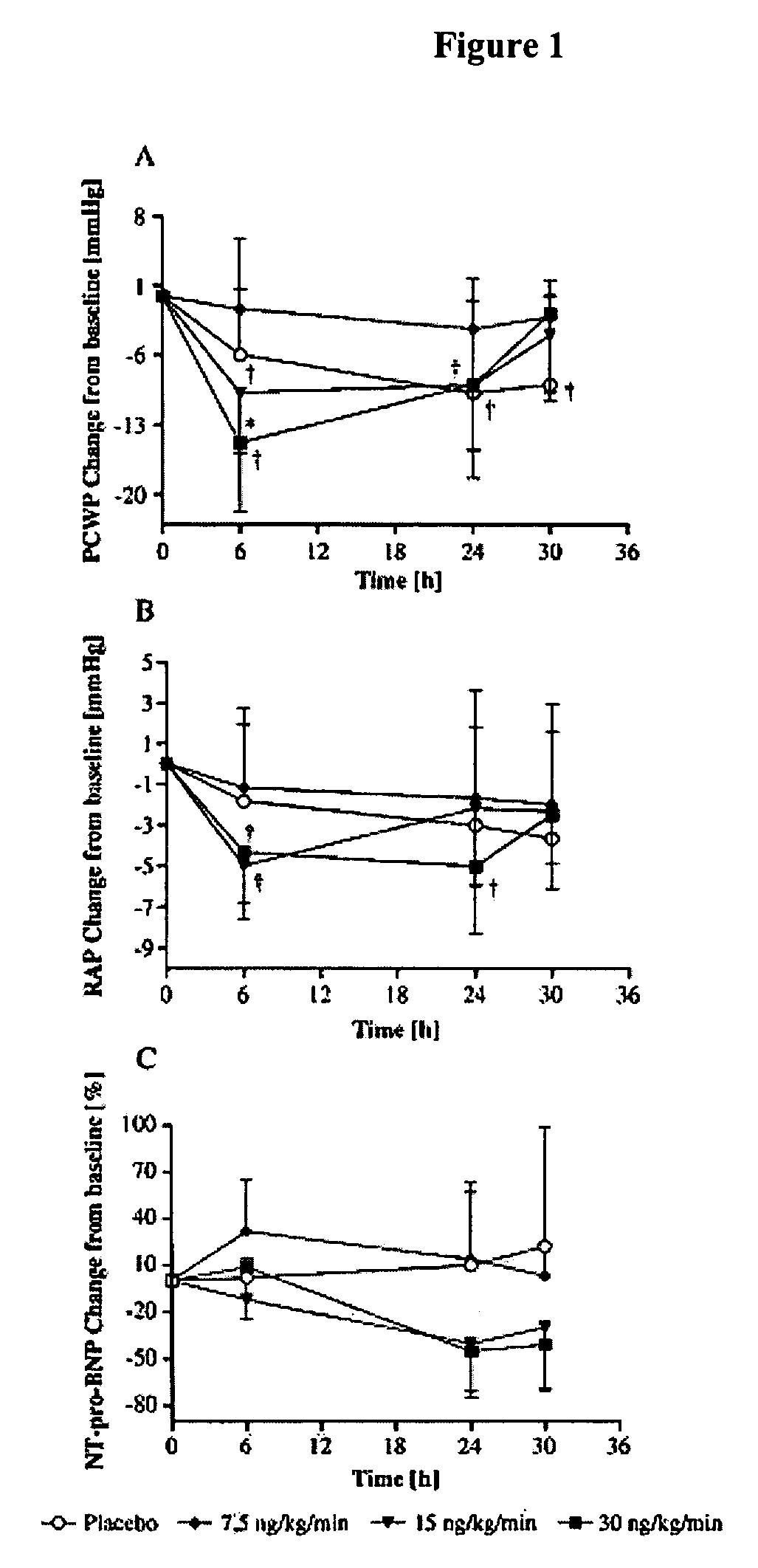
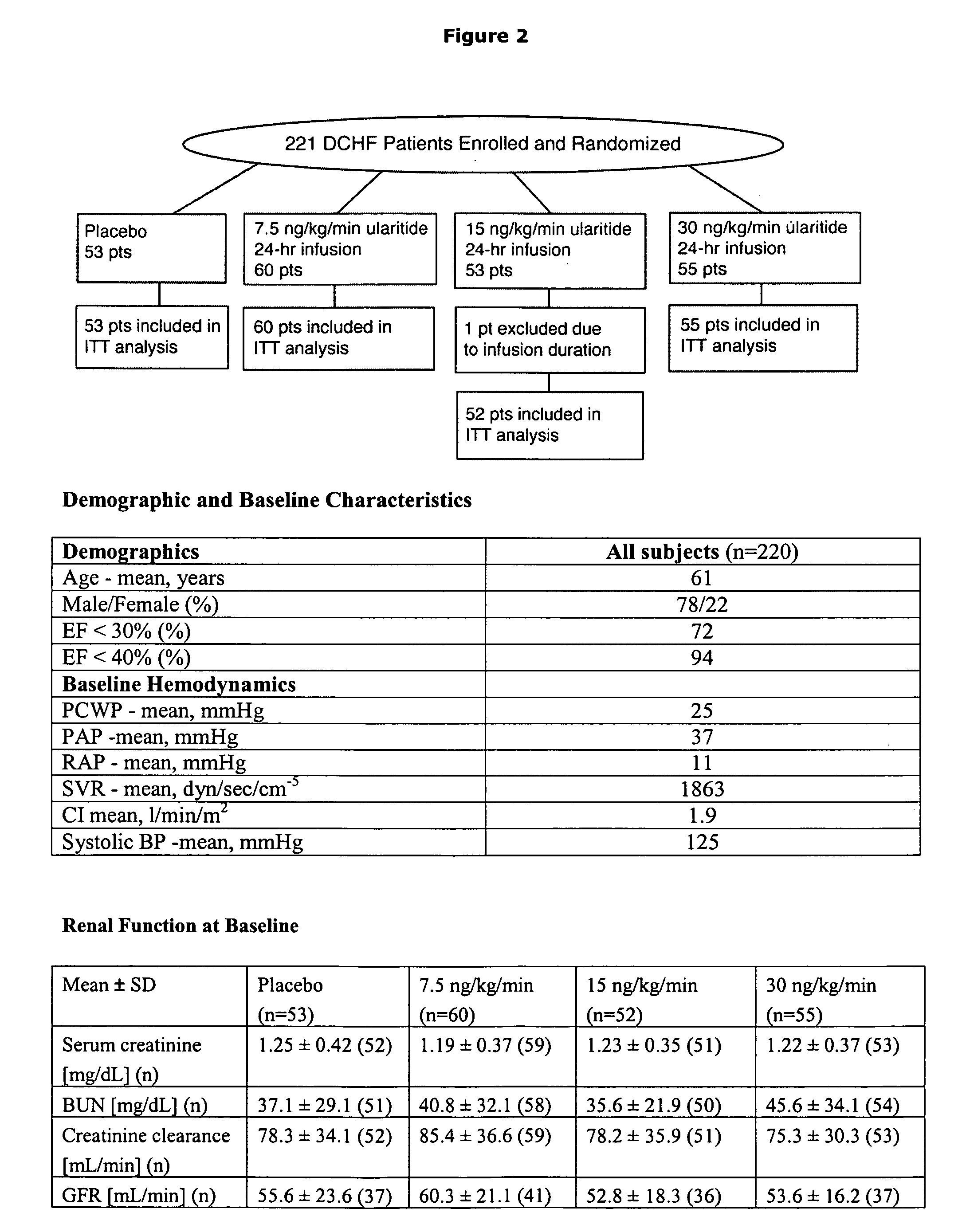
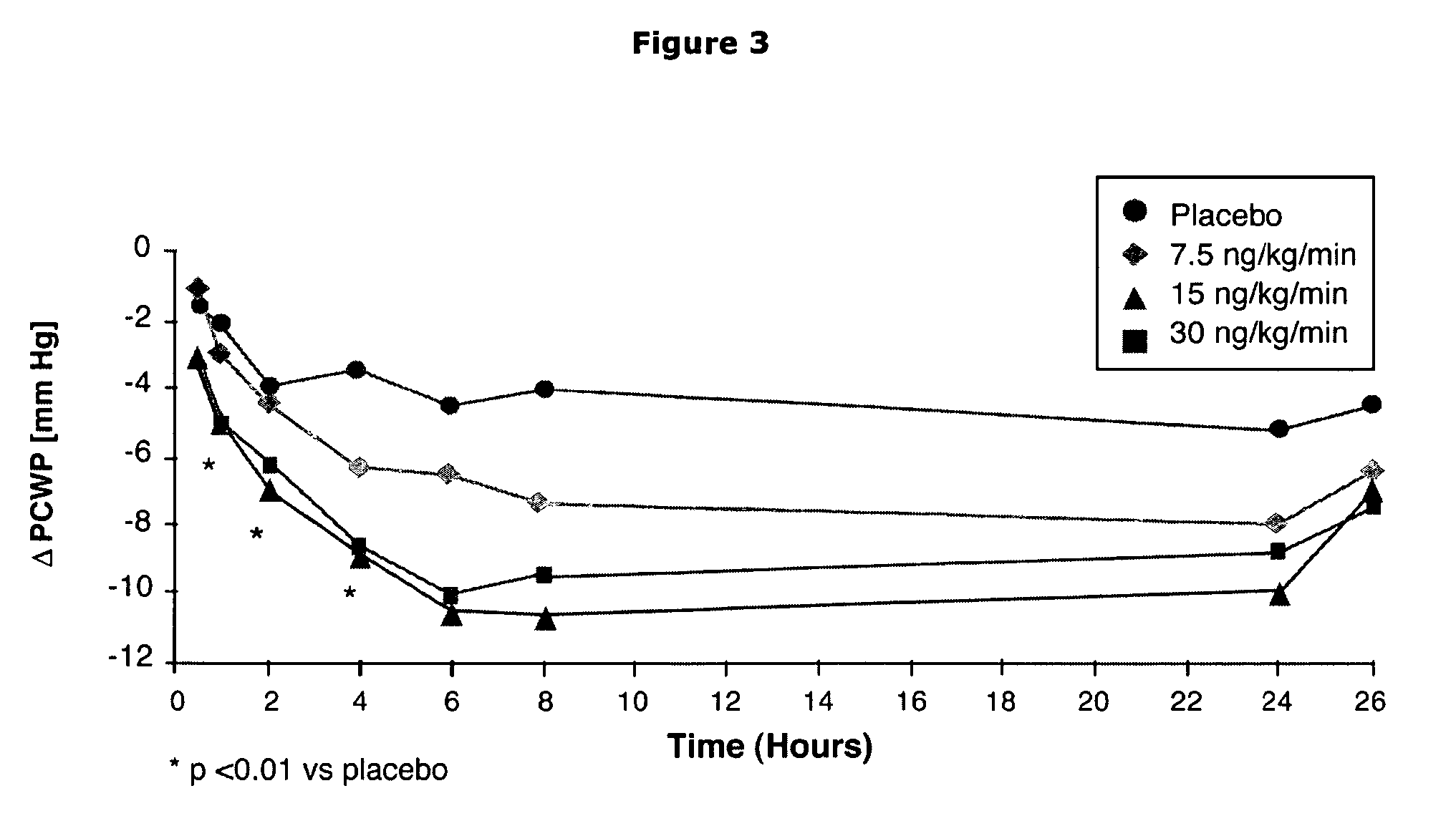
The present invention relates to the use of a natriurectic peptide, such as urodilatin, for treating a patient suffering from heart failure, such as acute decompensated heart failure. Preferably, a composition comprising an effective amount of urodilatin is intravenously administered to the patient continuously through a time period of at least 24 hours and up to 120 hours, preferably at least 48 hours.
Owner:CARDIORENTIS
Methods of treating diastolic dysfunction and related conditions
InactiveUS20120214818A1Organic active ingredientsCardiovascular disorderHeart failure with preserved ejection fractionAcute decompensated heart failure
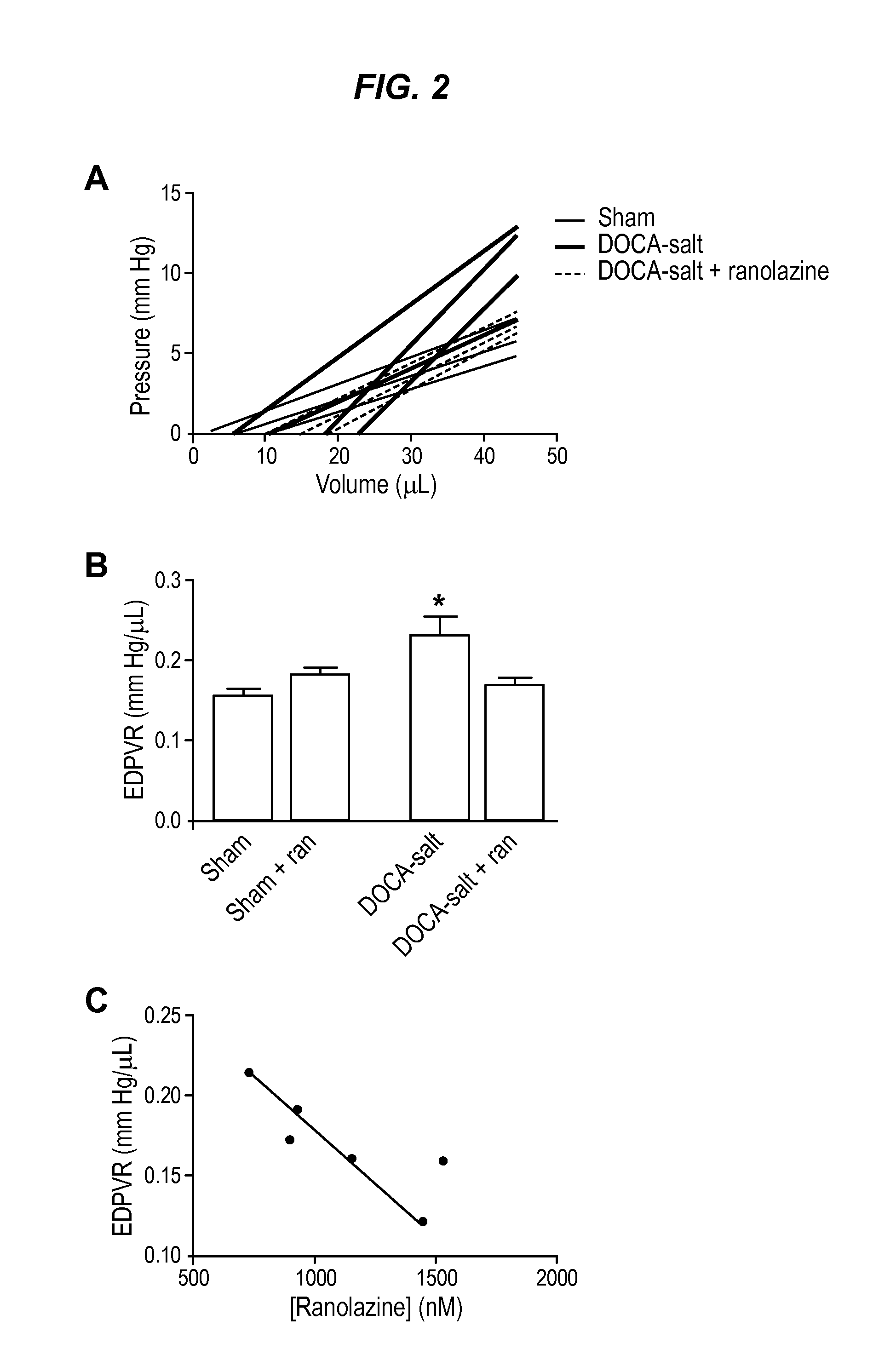
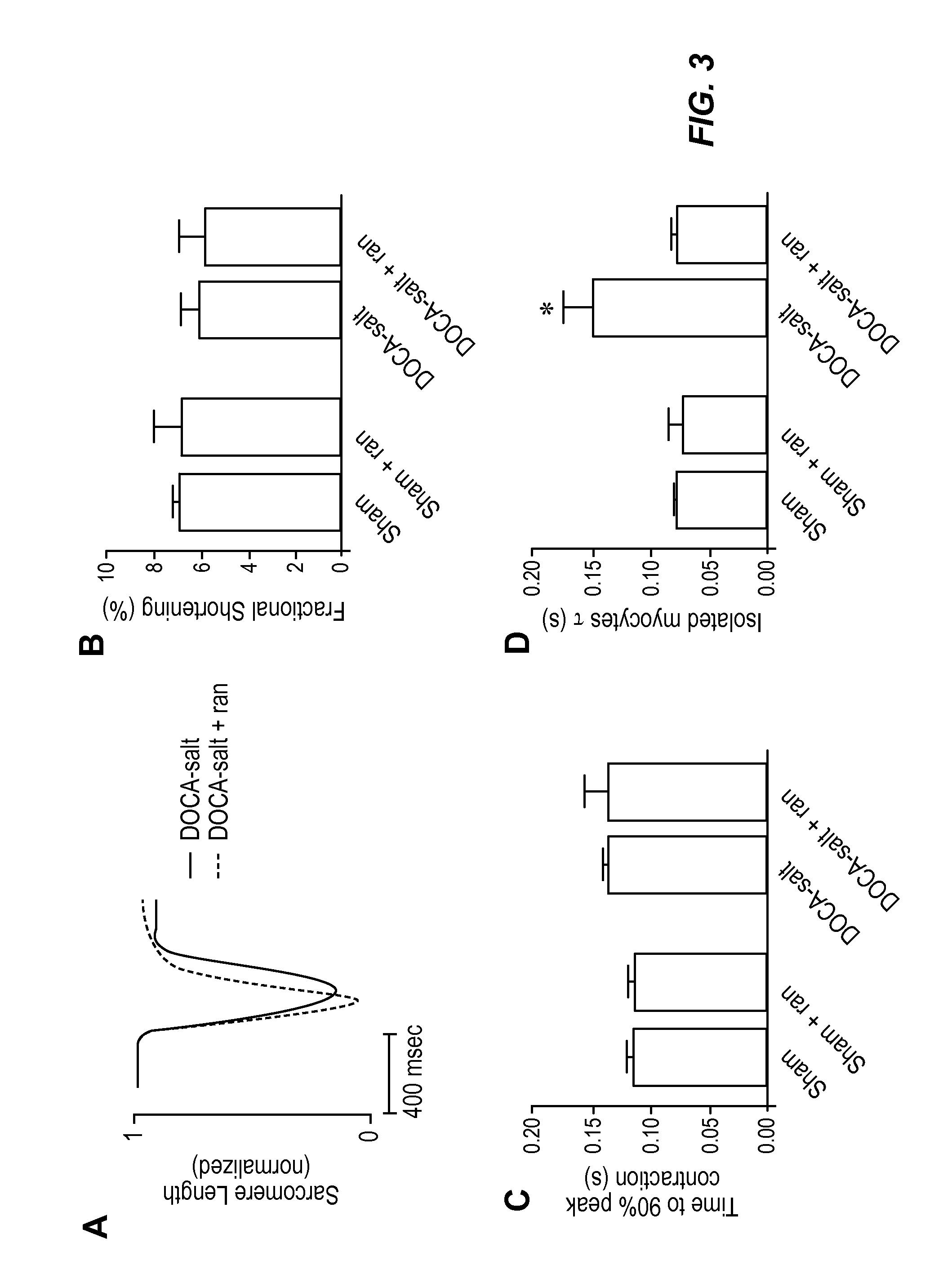
The invention provides a method of treating diastolic dysfunction, e.g., diastolic dysfunction with preserved ejection fraction, in a subject. The method comprises administering to the subject in an amount effective to treat the diastolic dysfunction a cardiac metabolic modifier, as described herein. In some embodiments, the diastolic dysfunction is characterized by (i) a lack of increased late INa in cardiomyocytes, (ii) an increase in myofilament calcium sensitivity, or (iii) a combination thereof. In some embodiments, the subject does not suffer from a cardiac injury or a structural heart disease, as described herein. Further provided are a method of treating heart failure with preserved ejection fraction in a subject, a method of treating acute decompensated heart failure, a method of modulating myofilament calcium sensitivity in a subject, and a method of treating a condition associated with or caused by increased myofilament calcium sensitivity.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS20140142022A1Extended half-lifeIncrease constraintsNervous disorderPeptide/protein ingredientsCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
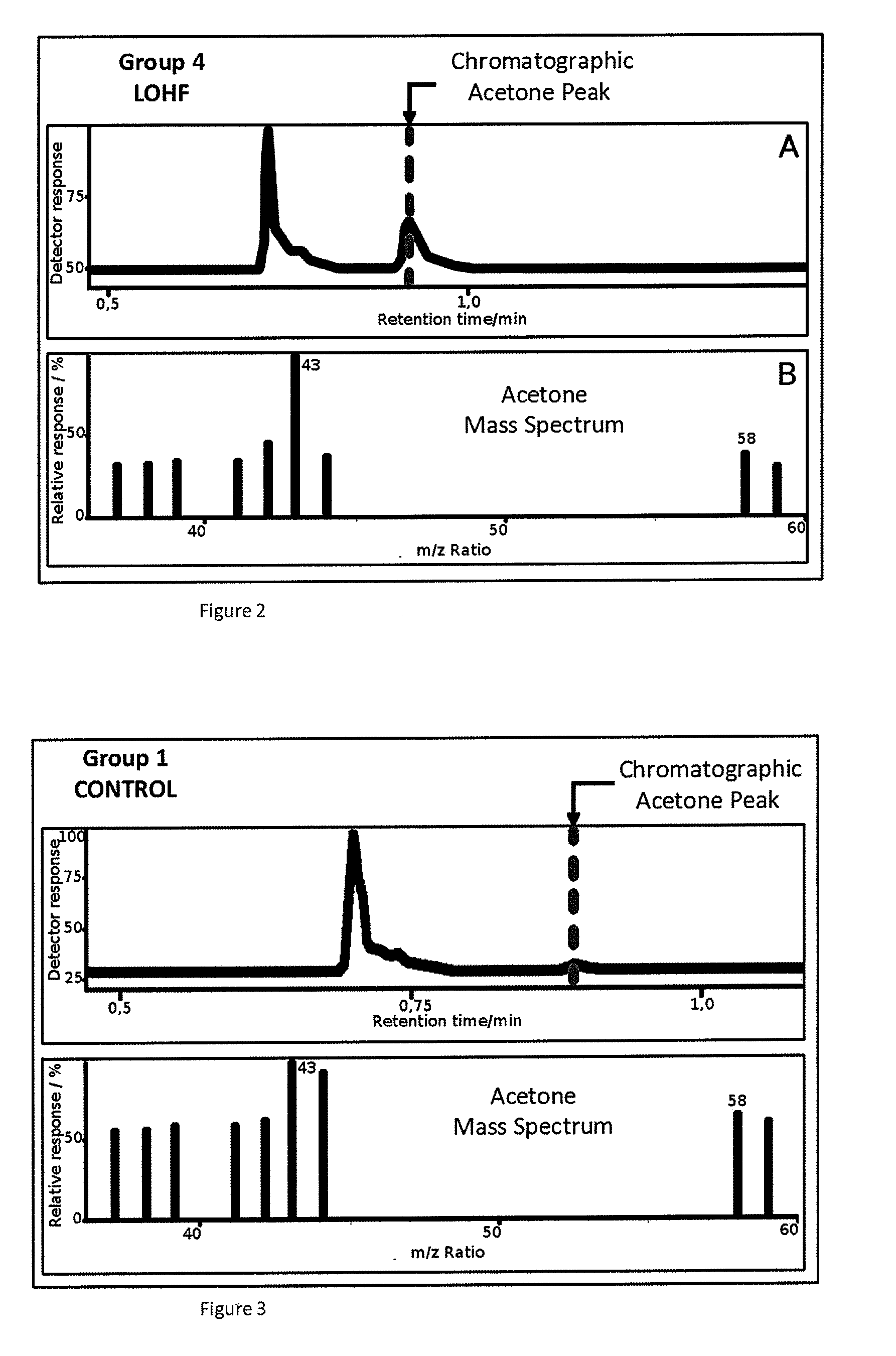
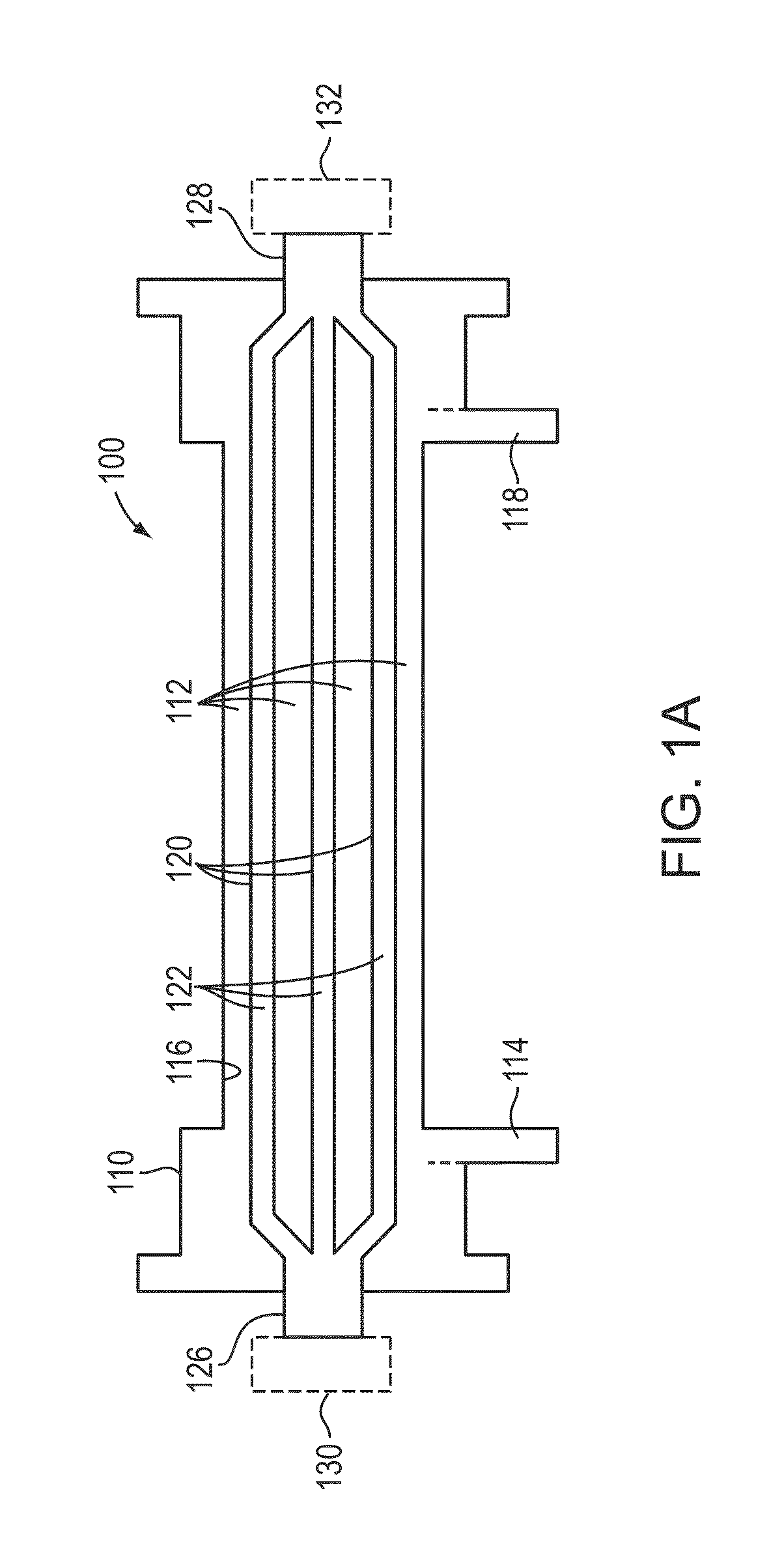
Non-invasive method for diagnosing the severity of heart failure, use of a biomarker for diagnosing decompensated heart failure, collector device for the heart failure biomarker from exhaled breath and a diagnosis kit
ActiveUS20120011918A1Improve retention efficiencyHigh affinityRadiation pyrometryOrganic chemistryDiseaseCell heart failure
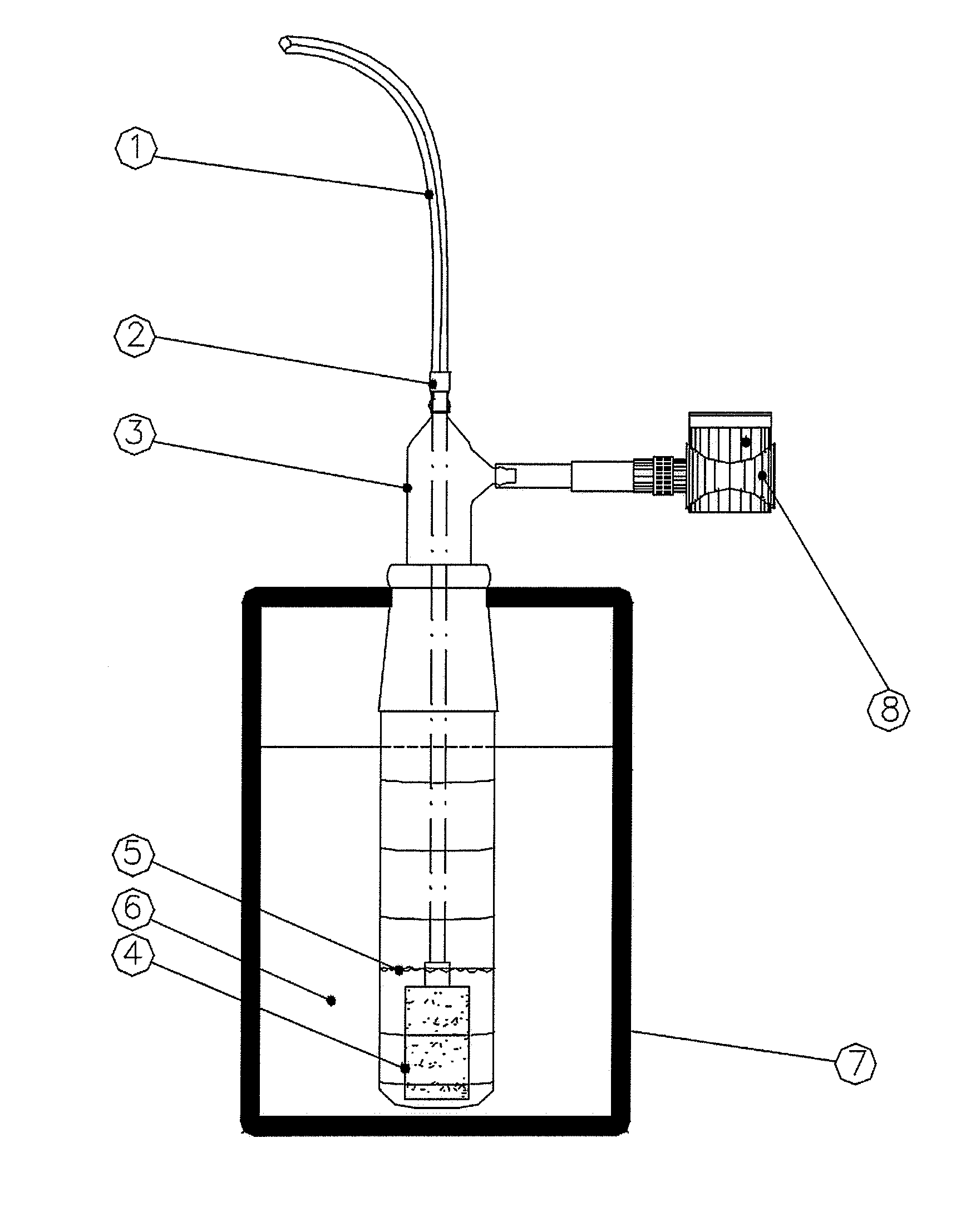
The invention application presents a fast, efficient, reproductive alternative of a non-invasive method for diagnosing the severity of heart failure based on a specific biomarker. An additional object of the present invention is a collector device for the biomarker from exhaled breath that is portable, simple, low cost and does not need to run on electric power. This invention advantageously permits the replacement of invasive diagnosis methods, favoring the patient's comfort in addition to the agility and speed of medical attention at hospitals, and may become a standard method for all suspected cases of circulatory disease and heart failure and, more specifically, decompensated heart failure.
Owner:BRAGA FABIANA GOULART MARCONDES +3
Cartridge and method for increasing myocardial function
InactiveUS20150246169A1Reduce riskReduce inflammationOrganic active ingredientsHaemofiltrationCardiac muscleAcute decompensated heart failure
The present invention relates to a cytopheretic cartridge for use in treating and / or preventing inflammatory conditions that affect myocardial function and to related methods. The cartridge can be used in treating a subject with myocardial dysfunction, such as a subject with chronic heart failure and / or acute decompensated heart failure.
Owner:SEASTAR MEDICAL INC
Cyclic polypeptides for the treatment of heart failure
ActiveUS20150031604A1Extended half-lifeIncrease constraintsNervous disorderAntibody mimetics/scaffoldsCardiac fibrosisVentricular tachycardia
The invention provides a cyclic polypeptide of Formula I (SEQ ID NO: 1):X1-R-X3-X4-L-S-X7-X8-X9-X10-X11-X12-X13 Ior an amide, an ester or a salt thereof, or a bioconjugate thereof, wherein X1, X3, X4, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention or bioconjugates thereof, and their therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Cyclic polypeptides for the treatment of heart failure
ActiveUS9266925B2Extended half-lifeIncrease constraintsAntibody mimetics/scaffoldsSerum albuminCardiac fibrosisVentricular tachycardia
The invention provides a cyclic polypeptide of Formula I (SEQ ID NO: 1):X1-R-X3-X4-L-S-X7-X8-X9-X10-X11-X12-X13 Ior an amide, an ester or a salt thereof, or a bioconjugate thereof, wherein X1, X3, X4, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention or bioconjugates thereof, and their therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Cartridge and method for increasing myocardial function
InactiveUS20150129497A1Reduce riskReduce inflammationOrganic active ingredientsOther blood circulation devicesCardiac muscleAcute decompensated heart failure
The present invention relates to a cytopheretic cartridge for use in treating and / or preventing inflammatory conditions that affect myocardial function and to related methods. The cartridge can be used in treating a subject with myocardial dysfunction, such as a subject with chronic heart failure and / or acute decompensated heart failure.
Owner:SEASTAR MEDICAL INC
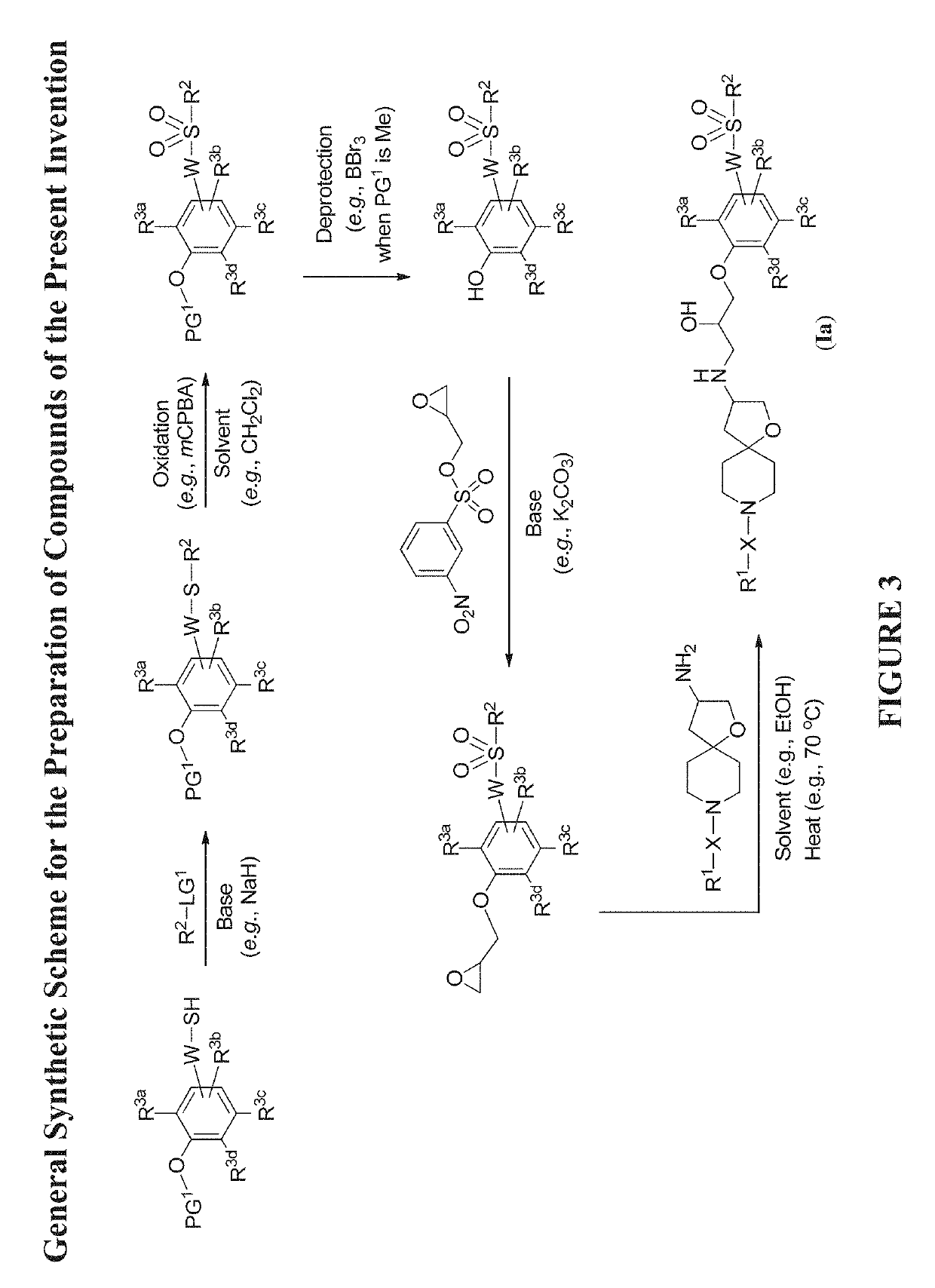
Modulators of the beta-3 adrenergic receptor useful for the treatment or prevention of disorders related thereto
InactiveUS20190284200A1Inhibition of contractilityImproves contractile functionOrganic active ingredientsOrganic chemistryLeft ventricular sizeKidney
The present invention relates to compounds of Formula (Ia) and pharmaceutical compositions thereof that modulate the activity of the beta-3 adrenergic receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of a beta-3 adrenergic receptor-mediated disorder, such as, heart failure; cardiac performance in heart failure; mortality, reinfarction, and / or hospitalization in connection with heart failure; acute heart failure; acute decompensated heart failure; congestive heart failure; severe congestive heart failure; organ damage associated with heart failure (e.g., kidney damage or failure, heart valve problems, heart rhythm problems, and / or liver damage); heart failure due to left ventricular dysfunction; heart failure with normal ejection fraction; cardiovascular mortality following myocardial infarction; cardiovascular mortality in patients with left ventricular failure or left ventricular dysfunction; left ventricular failure; left ventricular dysfunction; class II heart failure using the New York Heart Association (NYHA) classification system; class III heart failure using the New York Heart Association (NYHA) classification system; class IV heart failure using the New York Heart Association (NYHA) classification system; LVEF<40% by radionuclide ventriculography; LVEF≤35% by echocardiography or ventricular contrast angiography; and conditions related thereto.
Owner:ARENA PHARMA
Synthetic linear apelin mimetics for the treatment of heart failure
InactiveUS8921307B2Extended half-lifeIncrease constraintsPeptide/protein ingredientsMetabolism disorderCardiac fibrosisVentricular tachycardia
Owner:NOVARTIS AG
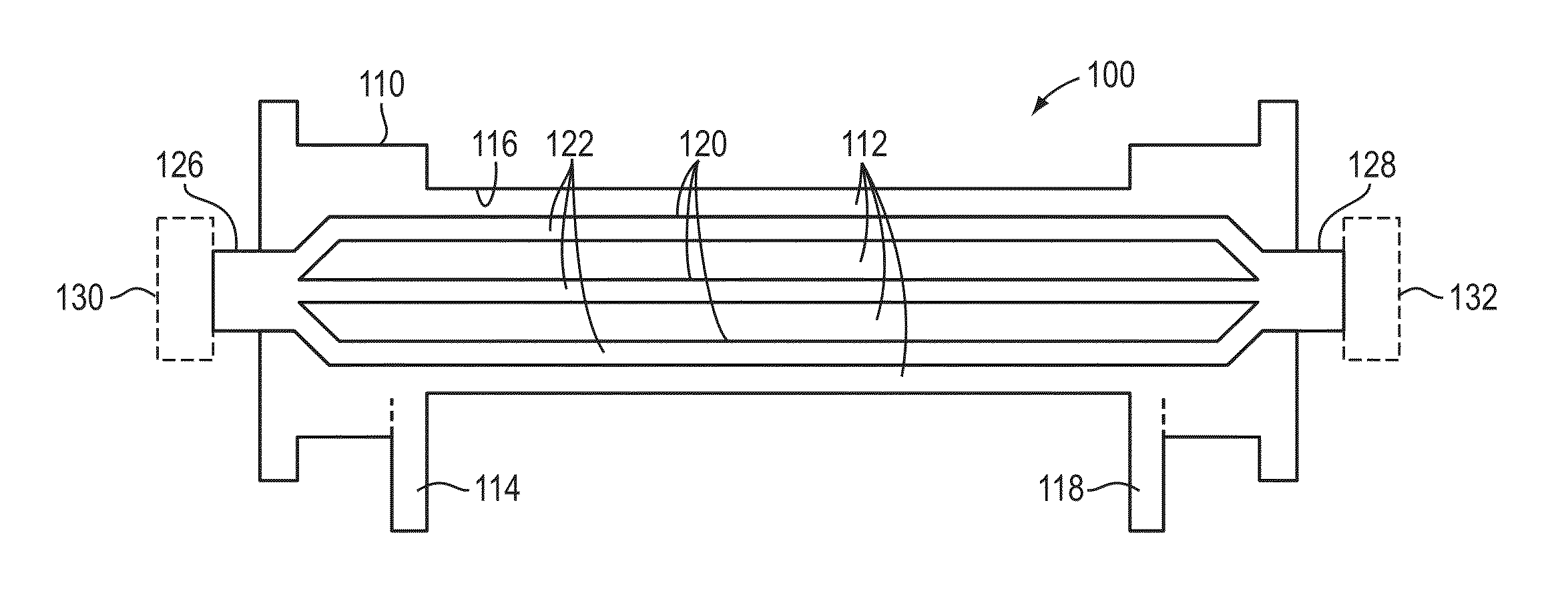
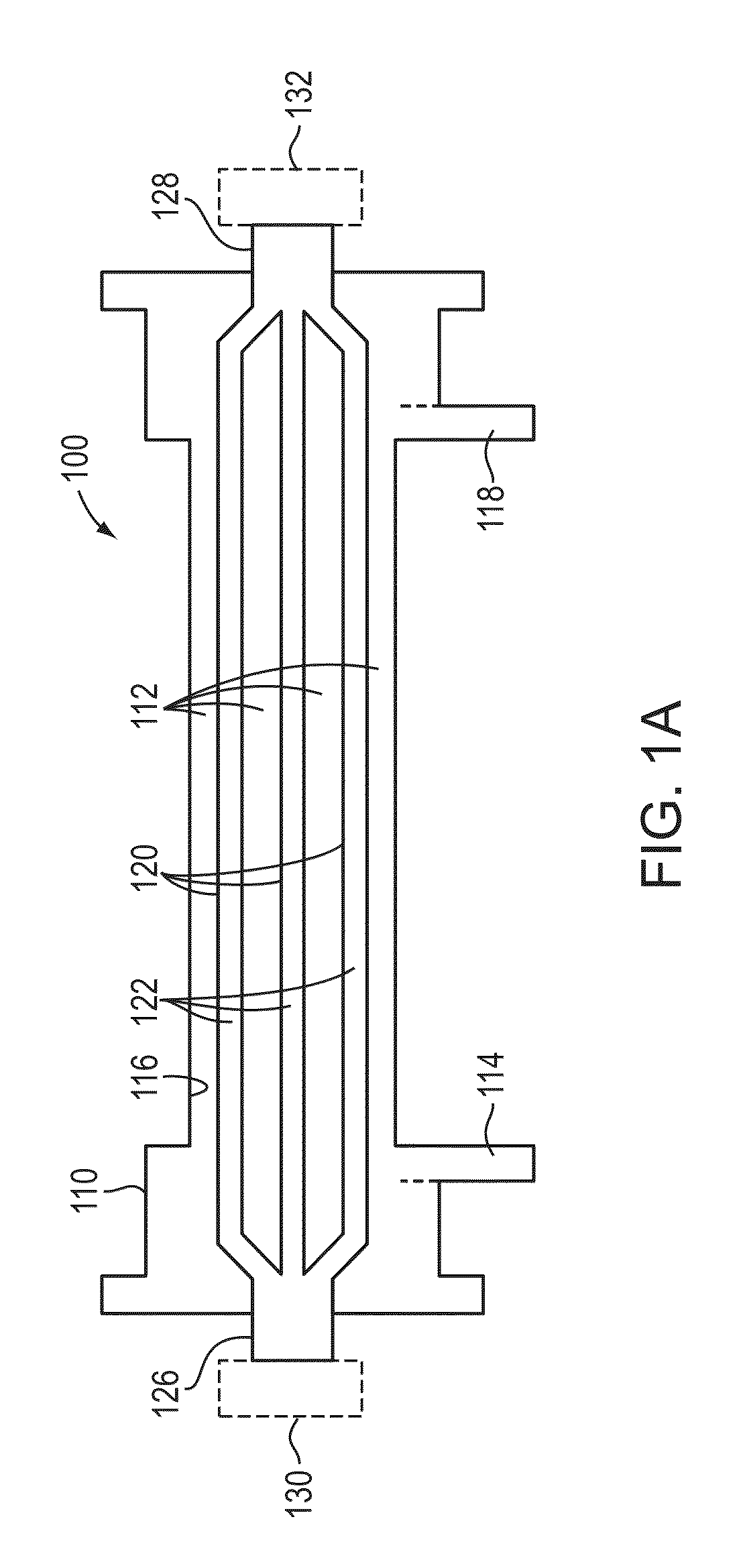
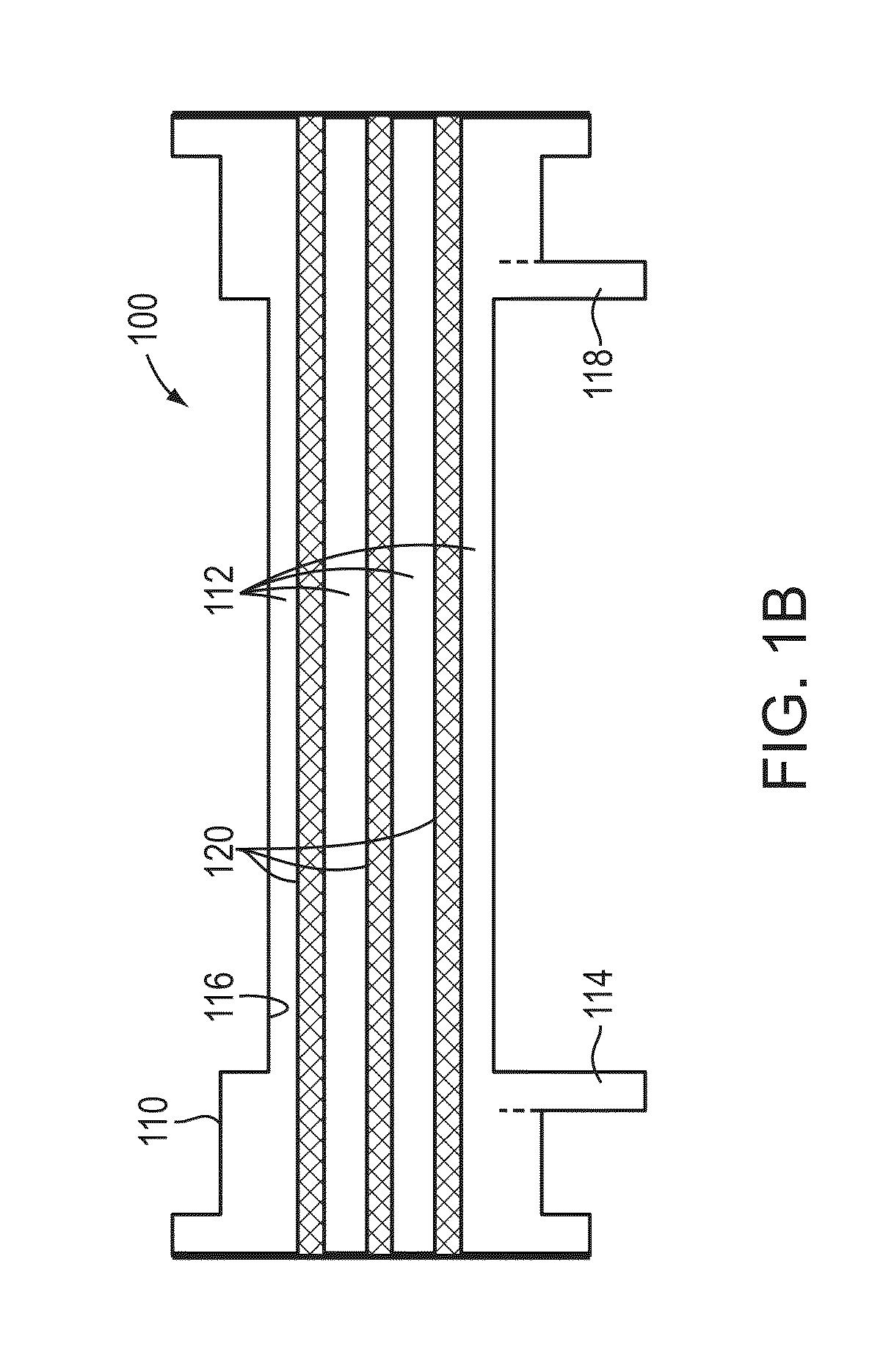
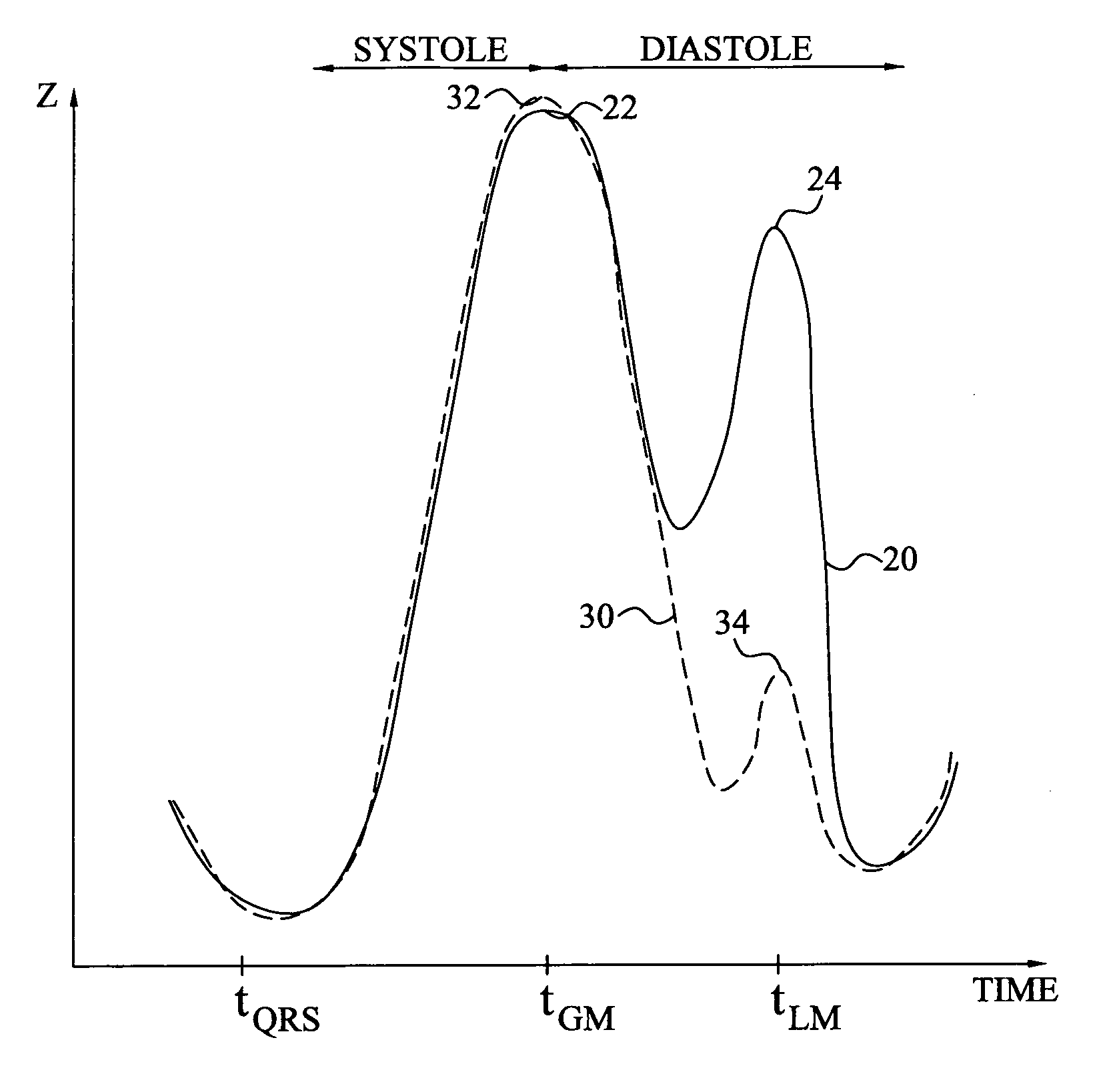
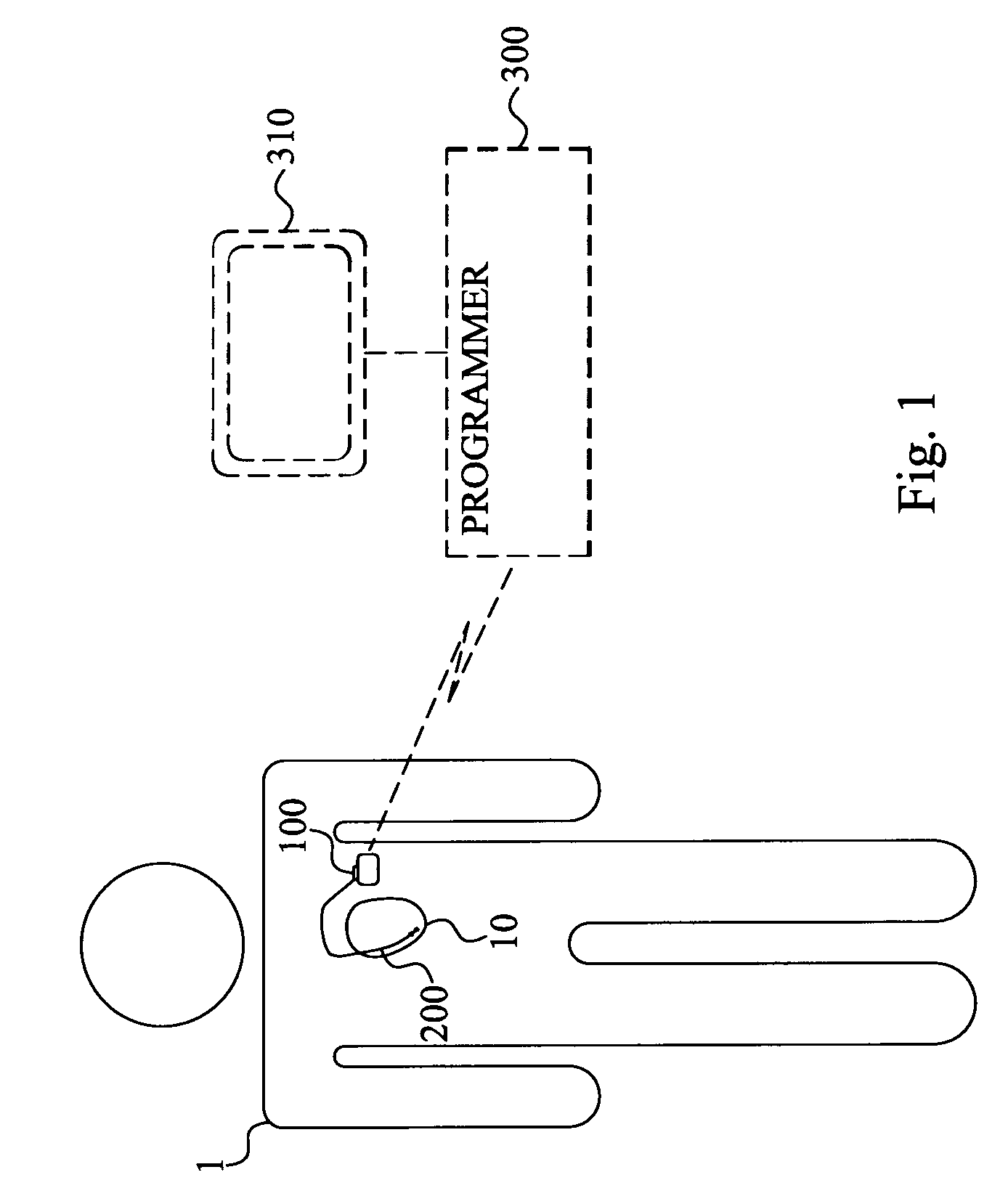
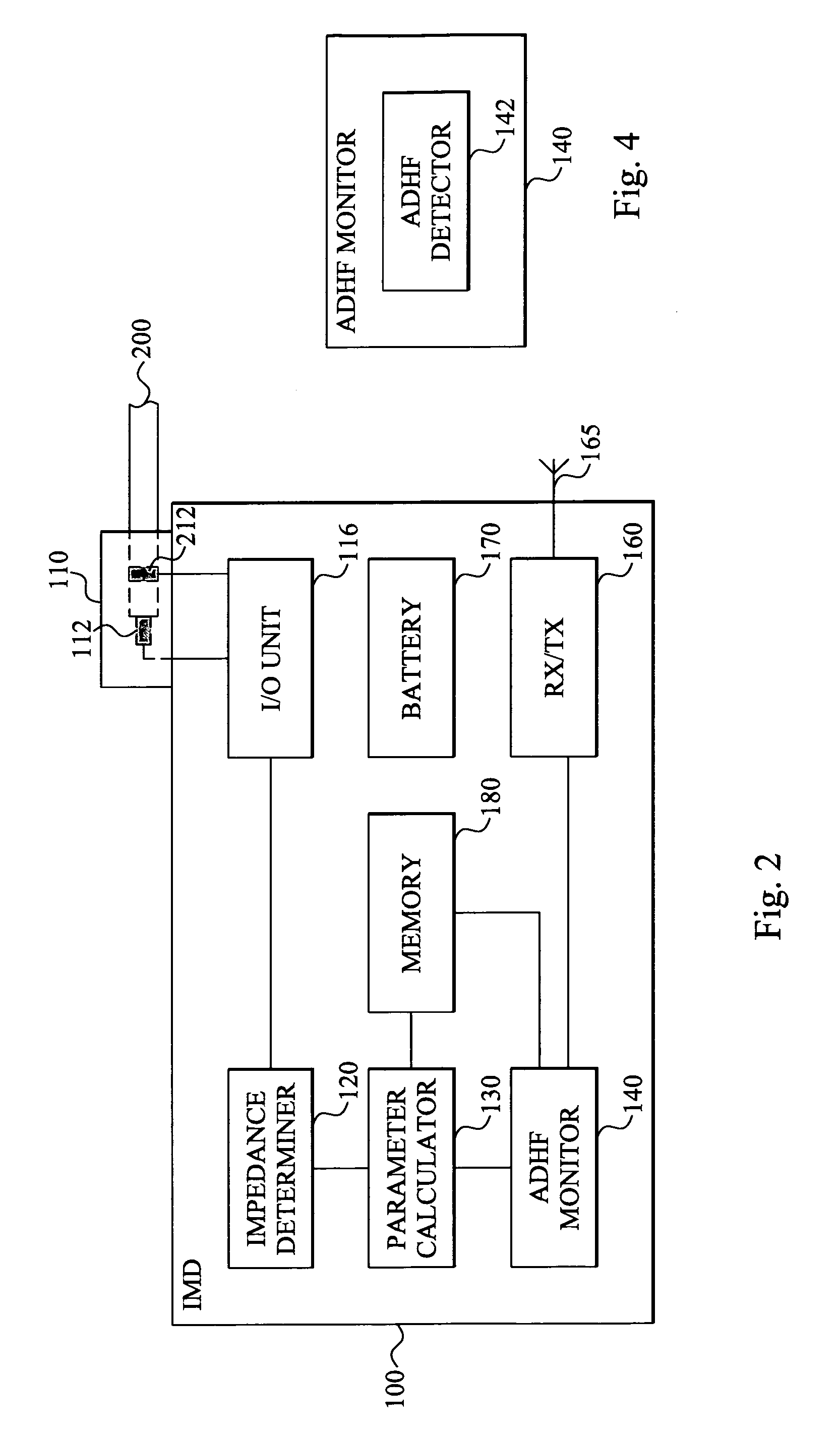
Method and device for monitoring acute decompensated heart failure
InactiveUS8565866B2Detection is simple and fastElectrocardiographyCatheterCardiac cycleAcute decompensated heart failure
An implantable medical device has an impedance determiner for determining a cardiogenic impedance signal based on electric signals sensed by connected electrodes. A parameter calculator processes the impedance signal to calculate an impedance parameter representative of the cardiogenic impedance in connection with the diastolic phase of a heart cycle. This parameter is then employed by the device for monitoring acute decompensated heart failure status of a subject.
Owner:ST JUDE MEDICAL
Cartridge and method for increasing myocardial function
ActiveUS20180093028A1Improve myocardial functionInhibition releaseOrganic active ingredientsOther blood circulation devicesCardiac muscleAcute decompensated heart failure
The present invention relates to a cytopheretic cartridge for use in treating and / or preventing inflammatory conditions that affect myocardial function and to related methods. The cartridge can be used in treating a subject with myocardial dysfunction, such as a subject with chronic heart failure and / or acute decompensated heart failure.
Owner:SEASTAR MEDICAL INC
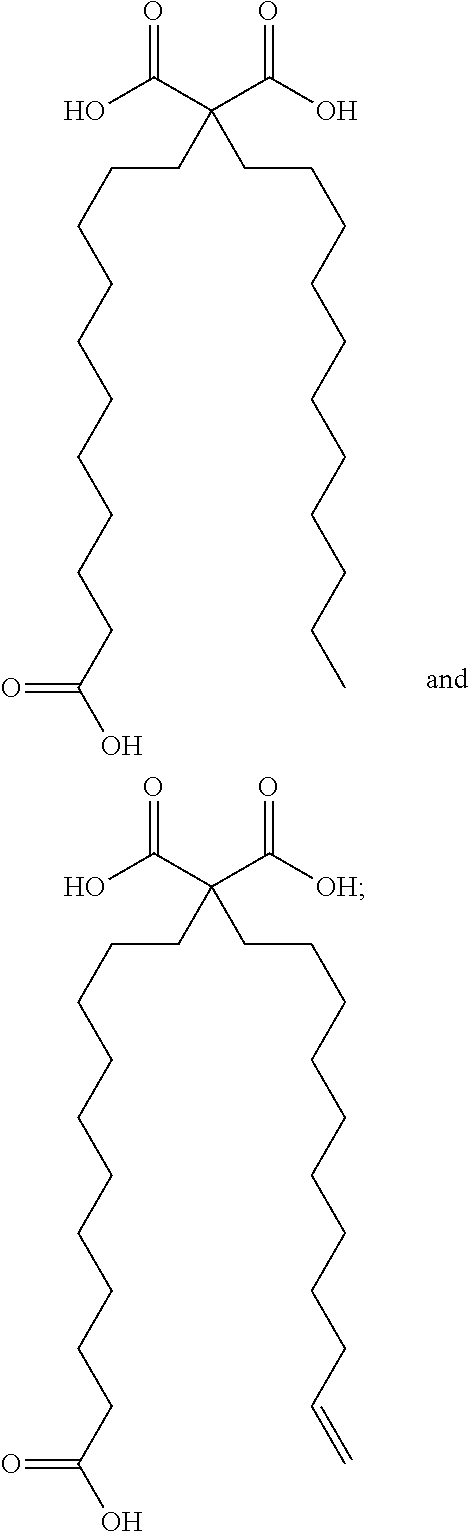
Synthetic apelin fatty acid conjugates with improved half-life
ActiveUS9931372B2Extended half-lifeExcellent agonistic activityNervous disorderMetabolism disorderCardiac fibrosisVentricular tachycardia
The invention provides a conjugate, or a pharmaceutically acceptable salt thereof, comprising a synthetic polypeptide of Formula I:Q-R-P-R-L-C*-H-K-G-P-(Nle)-C*-F (I)or a amide or ester thereof; and a fatty acid selected from:wherein said fatty acid is covalently linked to the N-terminus of the peptide via one of its carboxylic acid functionality, optionally via a polyethylene glycol linker; and wherein the two cysteine amino acids labeled with “*” form a disulfide bond between the thiol functionalities of their side chain. The conjugates are agonist of the APJ receptor. The invention also relates to a method for manufacturing the conjugates of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Cartridge and method for increasing myocardial function
ActiveUS20180126059A1Reduce riskReduce inflammationOrganic active ingredientsOther blood circulation devicesCardiac muscleAcute decompensated heart failure
The present invention relates to a cytopheretic cartridge for use in treating and / or preventing inflammatory conditions that affect myocardial function and to related methods. The cartridge can be used in treating a subject with myocardial dysfunction, such as a subject with chronic heart failure and / or acute decompensated heart failure.
Owner:SEASTAR MEDICAL INC
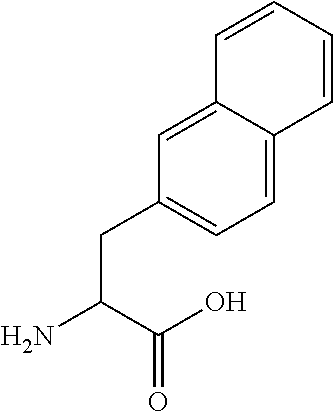
Natriuretic peptide, as well as gene and use thereof
ActiveCN103159847AHormone peptidesBacteriaAcute Anterior Wall Myocardial InfarctionCoronary arteries
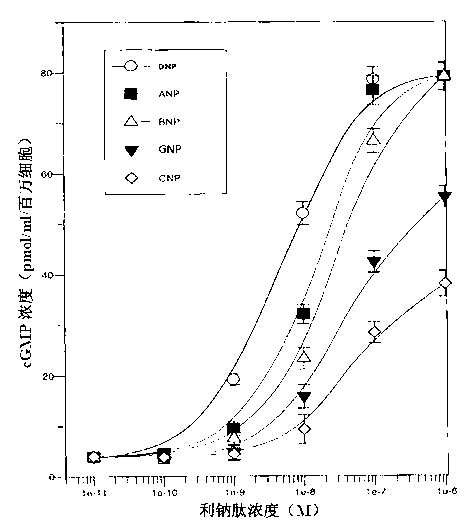
The invention relates to a natriuretic peptide from a snake and a gene encoding the polypeptide. The natriuretic peptide disclosed by the invention is the natriuretic peptide from dendroaspis angusticeps. The natriuretic peptide disclosed by the invention is the brand new natriuretic peptide and a new member in a natriuretic peptide family, and is further called as GNP, namely G type natriuretic peptide. The invention further provides a preparation method of the recombinant natriuretic peptide. The natriuretic peptide provided by the invention comprises the recombinant natriuretic peptide and an artificially synthesized natriuretic peptide, which can be applied to preparation of medicaments for treating patients with acute heart failure, acute decompensated heart failure, acute myocardial infarction after coronary intervention, chronic heart failure, and acute anterior wall myocardial infarction with systolic heart failure of old people.
Owner:叶亮 +2
Cyclic apelin derivatives for the treatment of heart failure
InactiveUS20160159871A1Extended half-lifeIncrease constraintsMuscular disorderCyclic peptide ingredientsCardiac fibrosisActive agent
The invention provides a synthetic polypeptide of Formula I′: X1-R-X3-R-L-X6-X7-K-X9-P-X11-X12-X13 or an amide, an ester, a salt thereof, or a bioconjugate thereof, wherein X1, X3, X6, X7, X9, X11, X12 and X13 are defined herein. The polypeptides and bioconjugates are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides and bioconjugates of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Modulators of the beta-3 adrenergic receptor useful for the treatment or prevention of disorders related thereto
ActiveUS20190292196A1Lower performance requirementsImproves contractile function and hemodynamic statusOrganic active ingredientsOrganic chemistryLeft ventricular sizeKidney
The present invention relates to compounds of Formula (Ia) and pharmaceutical compositions thereof that modulate the activity of the beta-3 adrenergic receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of a beta-3 adrenergic receptor-mediated disorder, such as, heart failure; cardiac performance in heart failure; mortality, reinfarction, and / or hospitalization in connection with heart failure; acute heart failure; acute decompensated heart failure; congestive heart failure; severe congestive heart failure; organ damage associated with heart failure (e.g., kidney damage or failure, heart valve problems, heart rhythm problems, and / or liver damage); heart failure due to left ventricular dysfunction; heart failure with normal ejection fraction; cardiovascular mortality following myocardial infarction; cardiovascular mortality in patients with left ventricular failure or left ventricular dysfunction; left ventricular failure; left ventricular dysfunction; class II heart failure using the New York Heart Association (NYHA) classification system; class III heart failure using the New York Heart Association (NYHA) classification system; class IV heart failure using the New York Heart Association (NYHA) classification system; LVEF<40% by radionuclide ventriculography; LVEF≤35% by echocardiography or ventricular contrast angiography; and conditions related thereto.
Owner:ARENA PHARMA
Cyclic apelin derivatives for the treatment of heart failure
InactiveUS9908919B2Extended half-lifeIncrease constraintsPeptide/protein ingredientsMetabolism disorderCardiac fibrosisActive agent
The invention provides a synthetic polypeptide of Formula I (SEQ ID NO: 1):X1-R-X3-R-L-X6-X7-K-X9-P-X11-X12-X13 Ior an amide, an ester, a salt thereof, or a bioconjugate thereof, wherein X1, X3, X6, X7, X9, X11, X12 and X13 are defined herein. The polypeptides and bioconjugates are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides and bioconjugates of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Disulfide cyclic polypeptides for the treatment of heart failure
InactiveUS9683018B2Extended half-lifeIncrease constraintsNervous disorderMetabolism disorderCardiac fibrosisVentricular tachycardia
Owner:NOVARTIS AG
Disulfide cyclic polypeptides for the treatment of heart failure
InactiveUS20160145309A1Extended half-lifeIncrease constraintsNervous disorderPeptide/protein ingredientsCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′: X1-R-P-R-X5-X6-X7-K-X9-P-X11-X12-X13 or an amide, an ester, a salt or a bioconjugate thereof, wherein X1, X5, X6, X7, X9 and X11 to X13 are defined herein. The polypeptides and bioconjugates are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides or bioconjugates of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
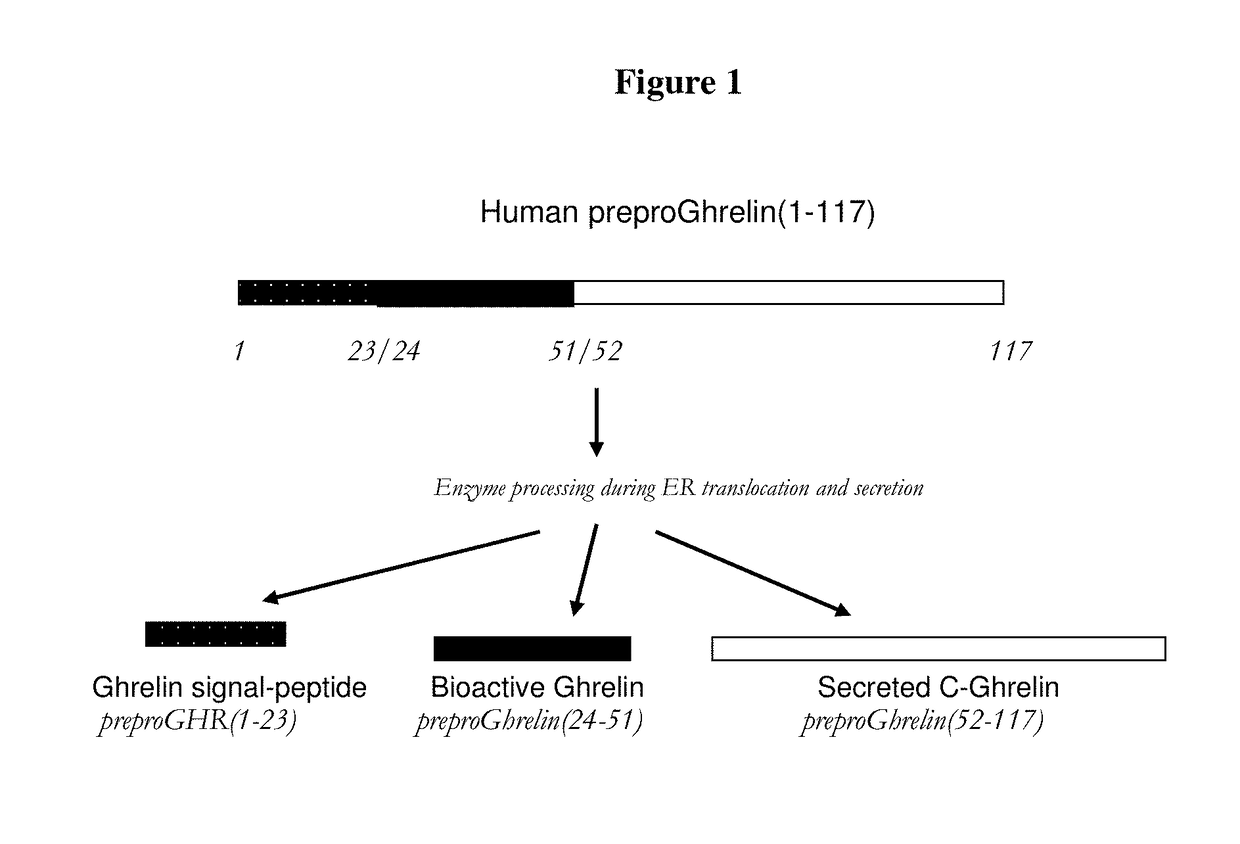
Biomarkers for pneumonia and acute decompensated heart failure
Ghrelin signal peptide fragment assays and kits useful in the diagnosis, prognosis, risk stratification, assessing, staging, monitoring, categorizing and determination of further diagnoses and treatment regimens in subjects with various disorders, diseases and conditions including, pneumonia, heart failure, or pneumonia and heart failure or suspected pneumonia, heart failure, or pneumonia and heart failure, and methods for monitoring treatment.
Owner:UPSTREAM MEDICAL TECH LTD
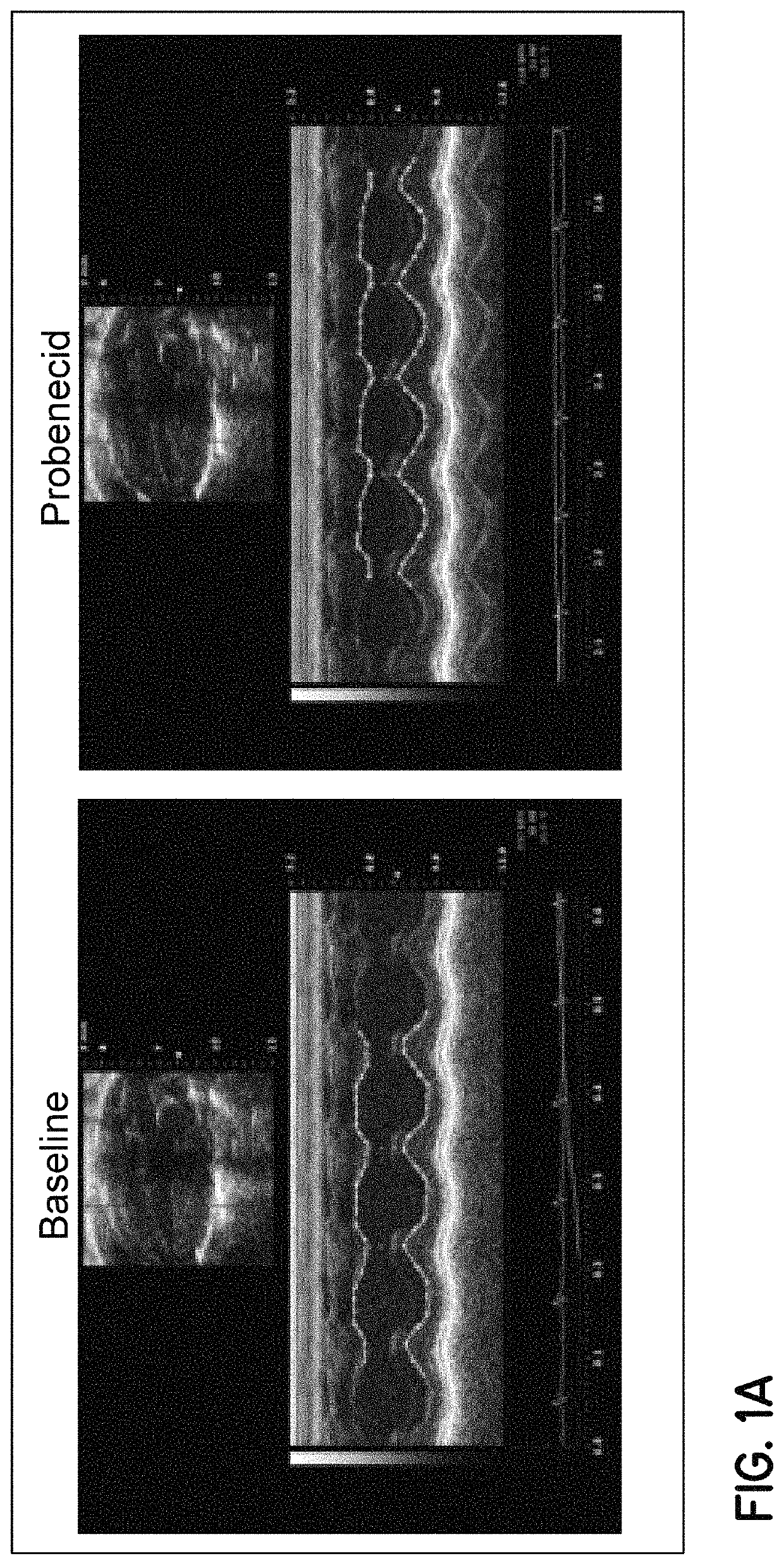
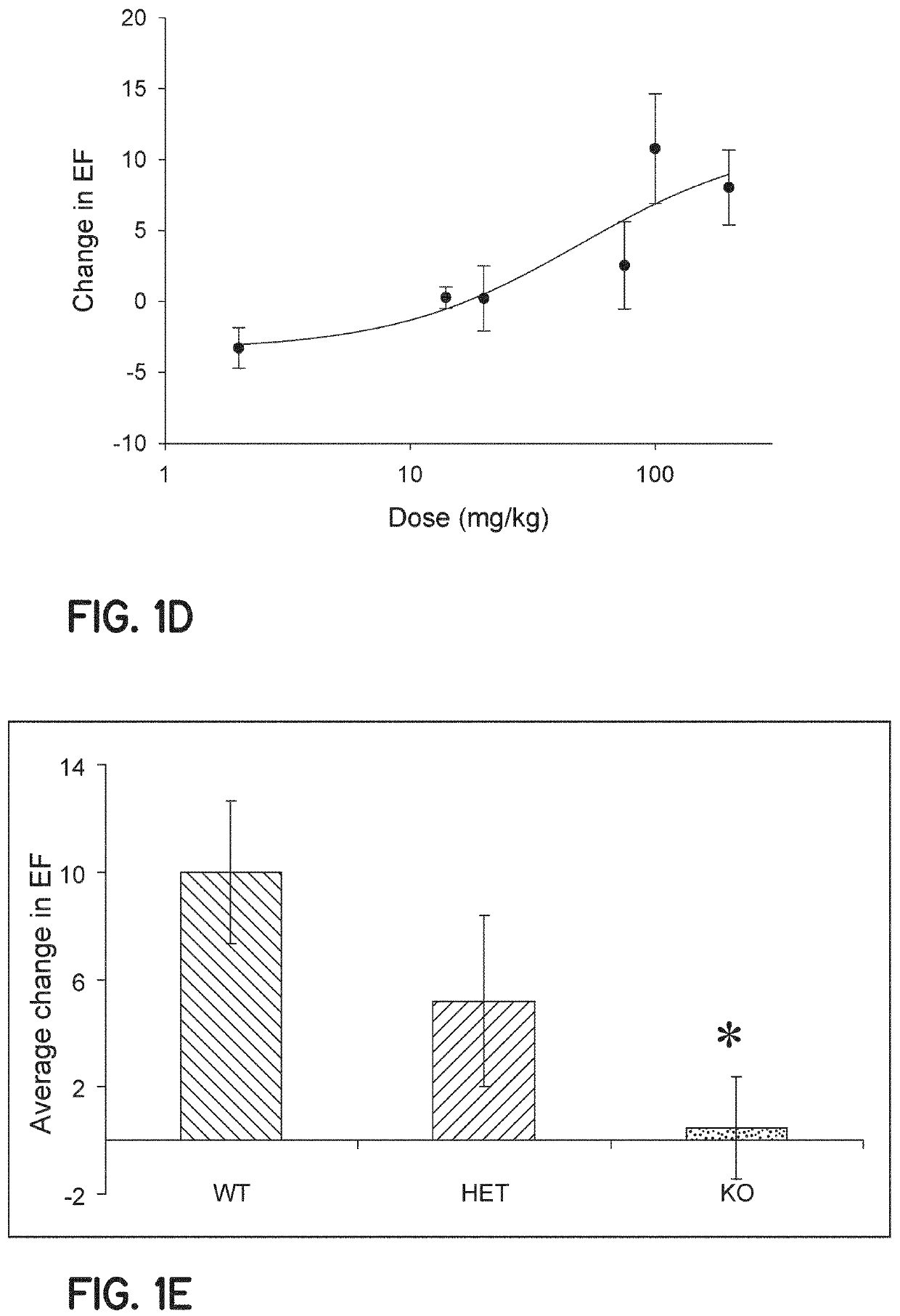
Method of treating acute decompensated heart failure with probenecid
ActiveUS10806711B2Organic active ingredientsPeptide/protein ingredientsCardiac dysfunctionAcute decompensated heart failure
Described are inventions directed to methods of treating a cardiac dysfunction in a subject that includes administering an amount of probenecid effective to treat a symptom of cardiac dysfunction. The probenecid may be administered in at least one of an injection, orally, or transdermally. The amount of probenecid is sufficient to result in an improved performance on a standardized 6 minute walk test, an improved New York Heart Association (NYHA) classification, a lower diuretic dose requirement, a lower serum BNP levels, a normalization of serum sodium concentrations, and combinations thereof. In an embodiment, probenecid is administered over a period of about 8 hours to about 24 hours. Probenecid may be used for short term treatments, i.e., less than a week, or it may be administered in a long term manner, i.e., over a period of weeks, months, or even years.
Owner:UNIVERSITY OF CINCINNATI
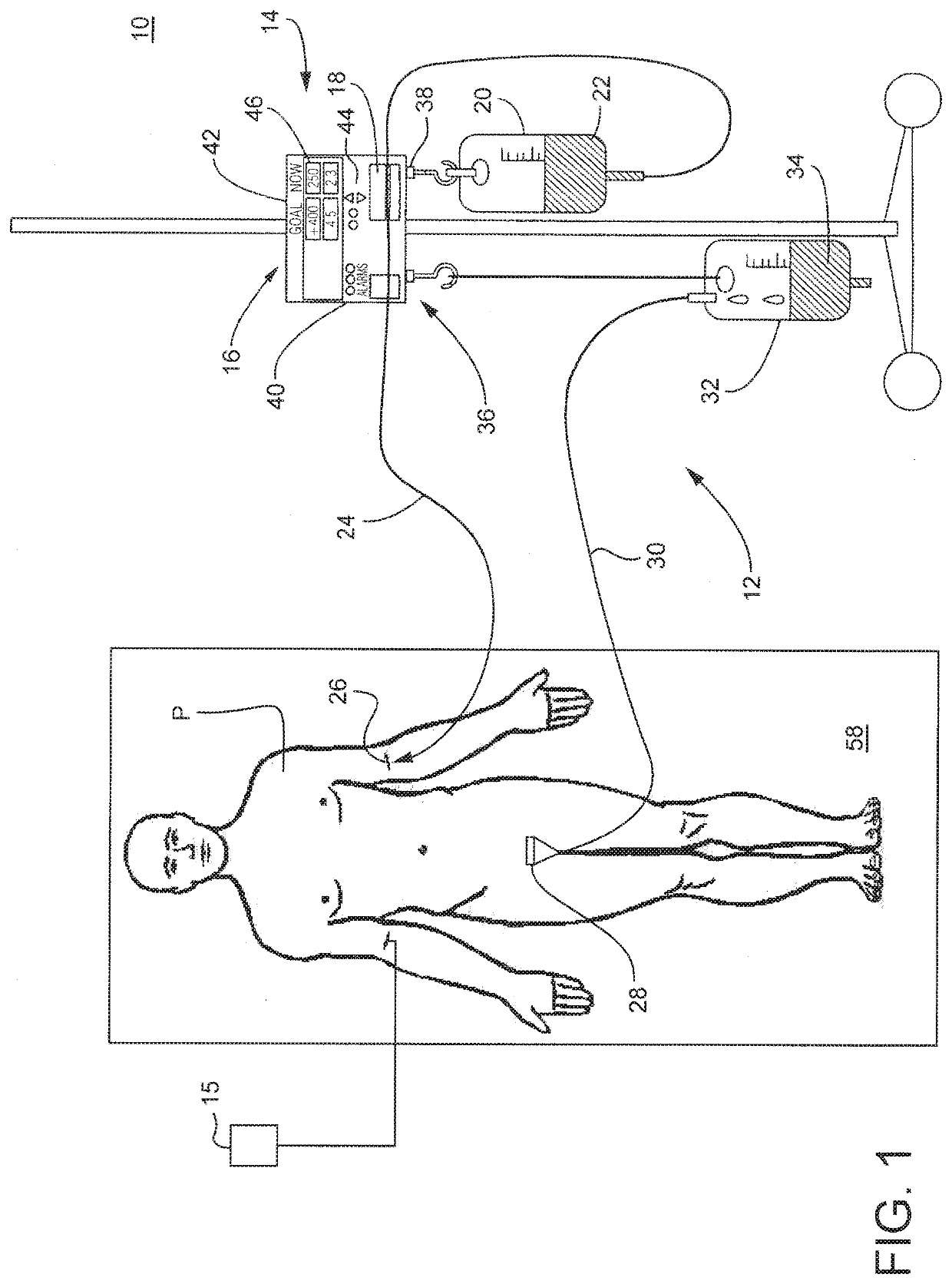
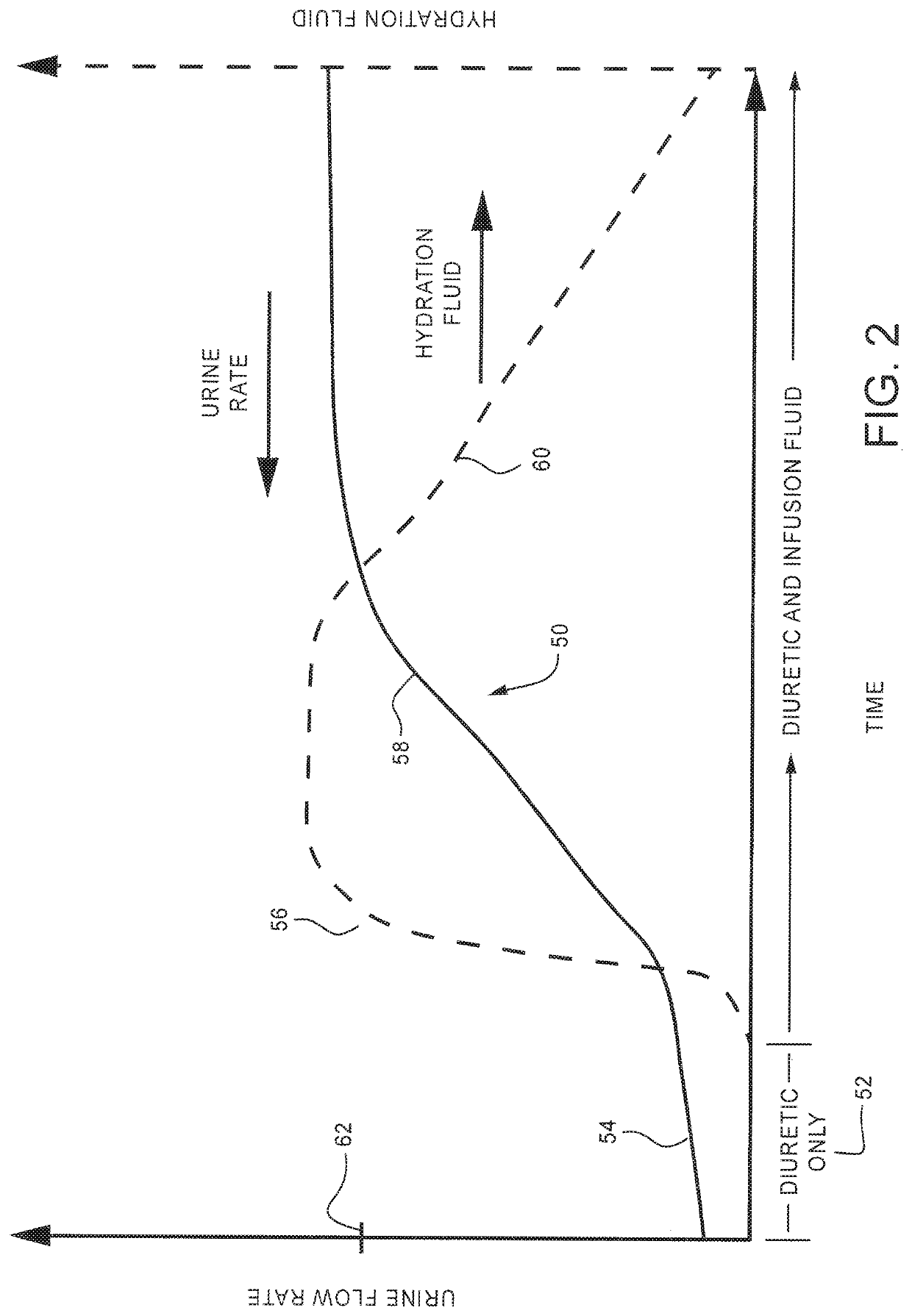
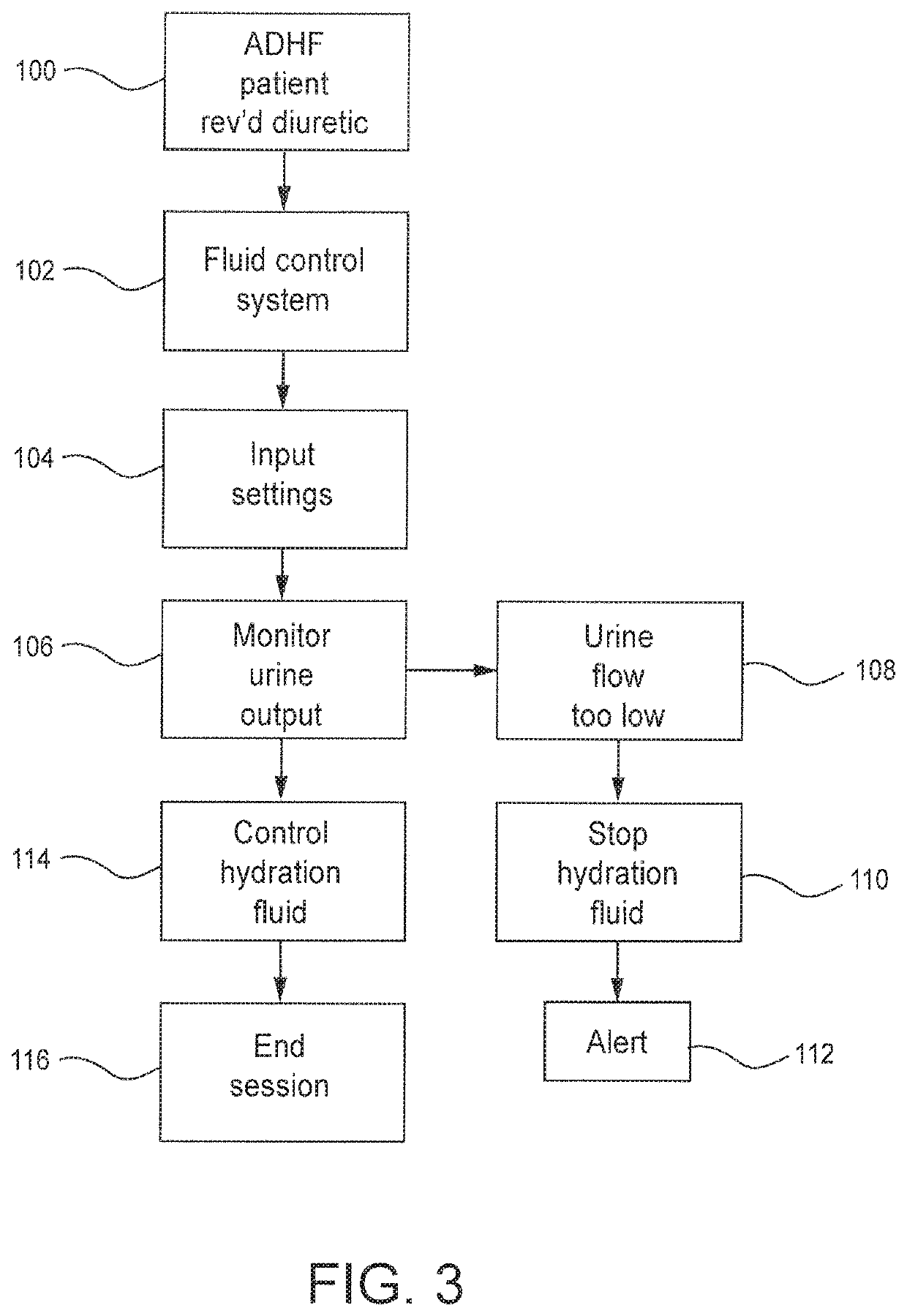
Method and system to treat acute decompensated heart failure
PendingUS20210236727A1Prevent dehydrationReduce the amount requiredDrug and medicationsMedical devicesAcute decompensated heart failureDecompensation
A apparatus and method to treat patients with acute decompensated heart failure (ADHF), heart failure or another fluid overload condition, that includes: administrating a diuretic to the patient to increase urine output of the patient; monitoring a rate or amount of urine output by the patient after administration of the diuretic; infusing a hydration liquid into the patient to induce an increase in the urine output; and adjusting the rate or amount of the hydration liquid infused into the patient to achieve a target fluid loss in the patient.
Owner:REPRIEVE CARDIOVASCULAR INC
Bioconjugates of synthetic apelin polypeptides
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com