Markers for the prognosis and risk assessment of pregnancy-induced hypertension and preeclampsia
A technology for preeclampsia and risk assessment, applied in the field of clinical diagnostics, can solve problems that have not explored the prognosis of ET-1 or the application of risk assessment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
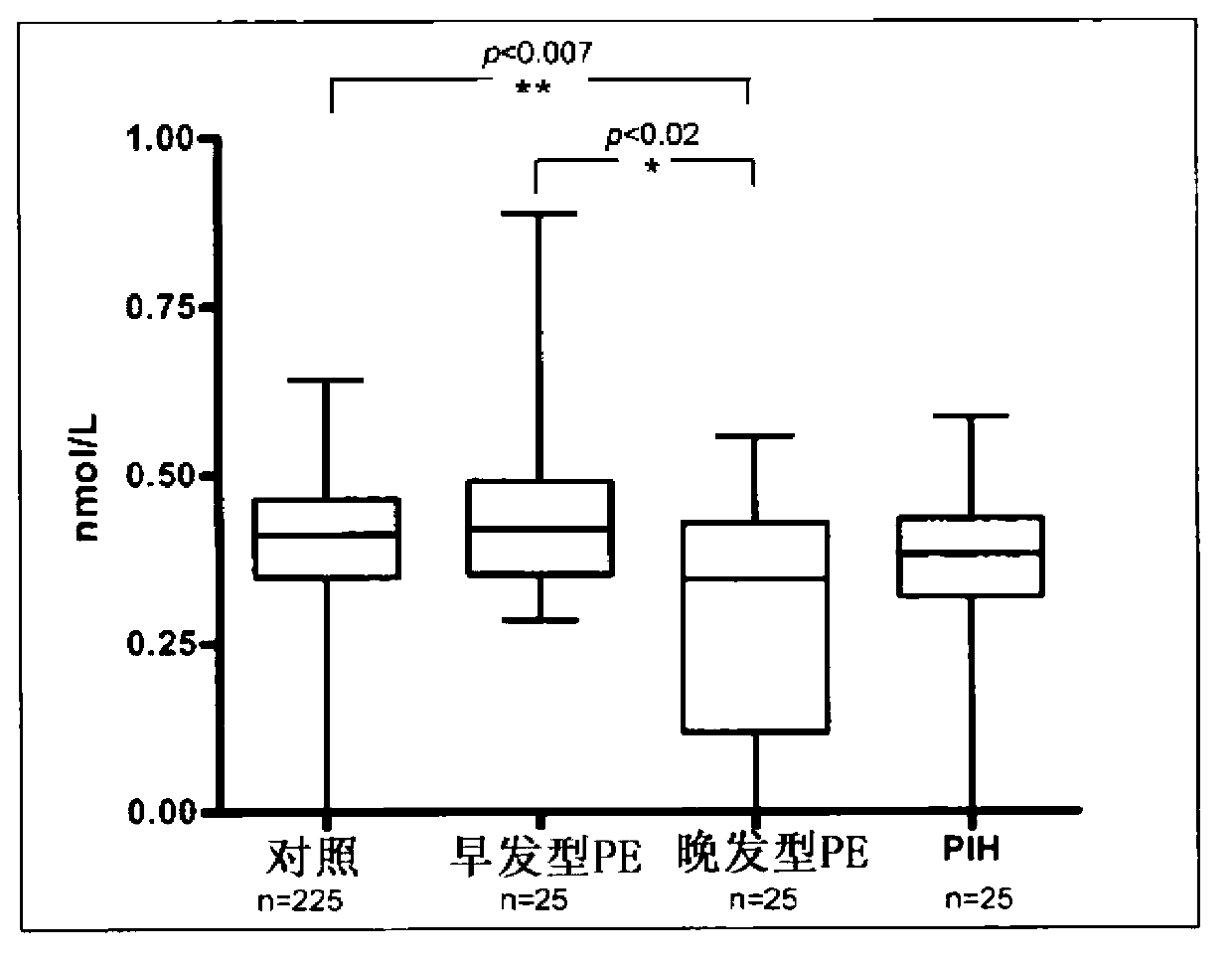
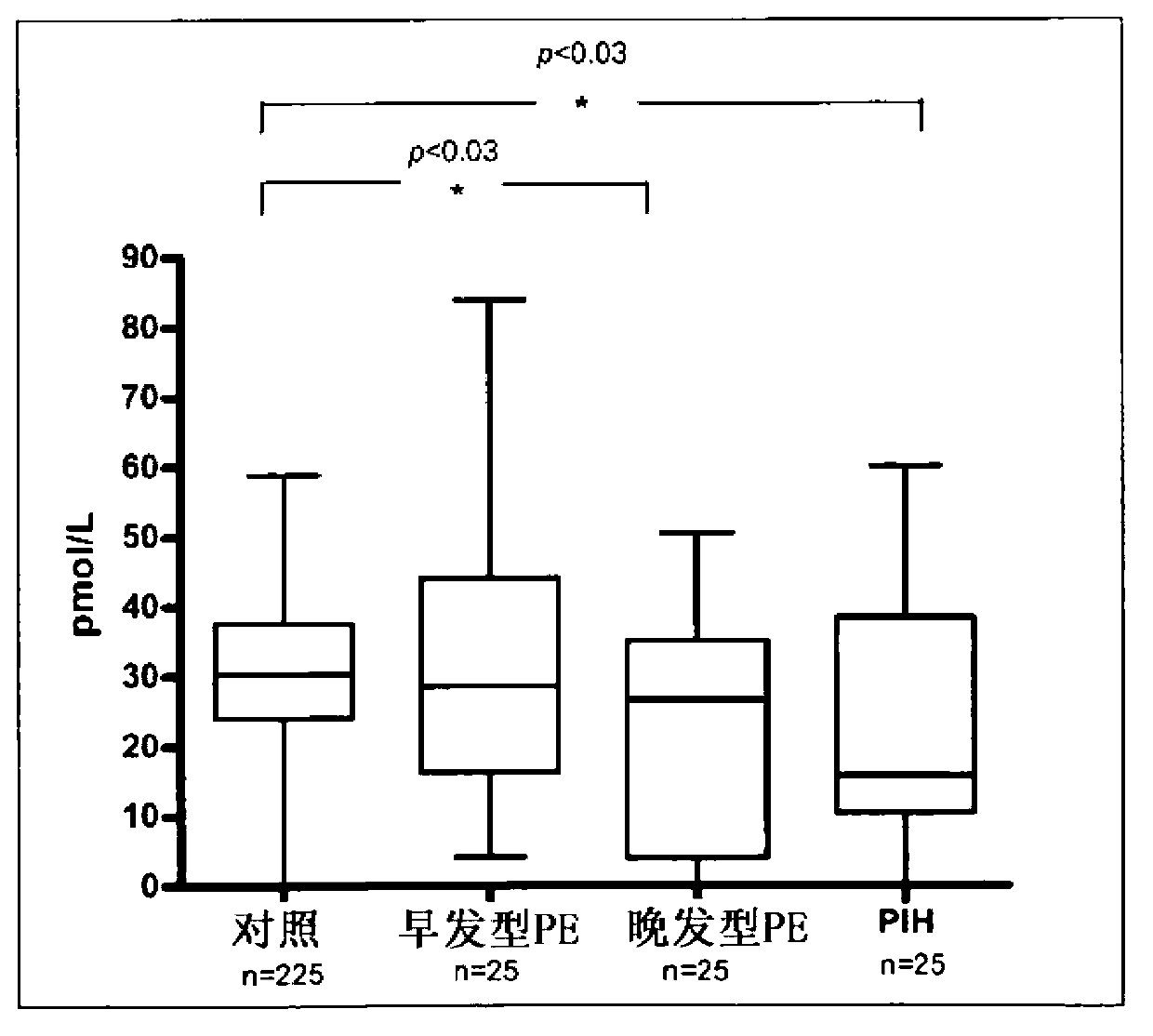
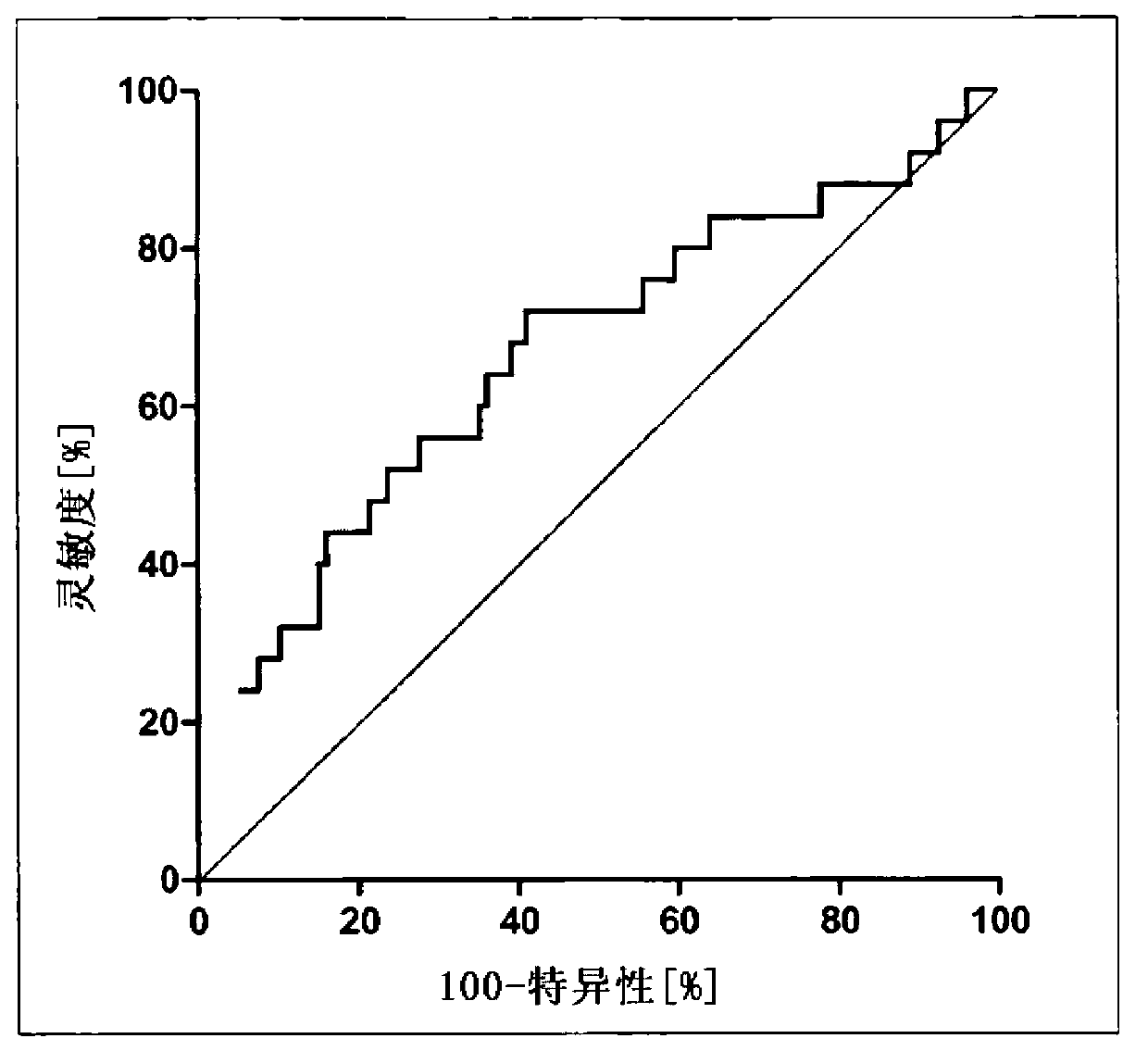
[0067] research population
[0068] A total of 323 patients were included in the retrospective study. These patients were diagnosed with early-onset preeclampsia (n=25), late-onset preeclampsia (n=25), and pregnancy-induced hypertension (PIH) (n=25). 225 pregnant women without these diseases served as controls.
[0069] EDTA-samples were collected at each prenatal visit, which was performed between 11 and 14 weeks of gestation. At that time, all patients included in the study were asymptomatic and did not show any signs or symptoms of preeclampsia or PIH. All pregnant women gave informed consent approved by the King's College Hospital Ethics Committee.
[0070] If hypertension (systolic or diastolic blood pressure ≥140 and 90 mmHg, respectively) and proteinuria (protein excretion ≥300 mg in a 24-hour urine collection, or dipstick ≥2+) are detected after 20 weeks of gestation, the patient Have been diagnosed with preeclampsia. Based on the time of onset of preeclampsia, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com