Synthesis method for antihypertensive agent isradipine and preparation of isradipine
An antihypertensive and drug technology, applied in the field of drug isradipine preparation, can solve the problems of complicated operation, low yield, expensive solvent and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
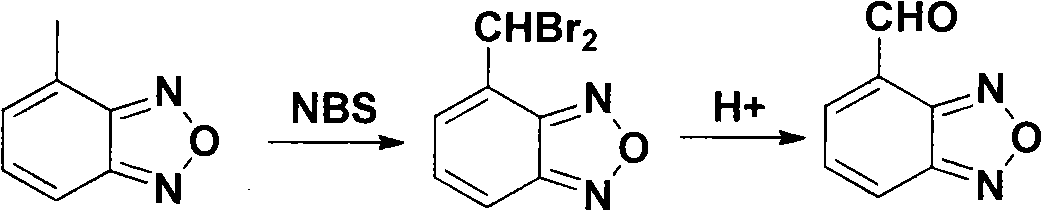
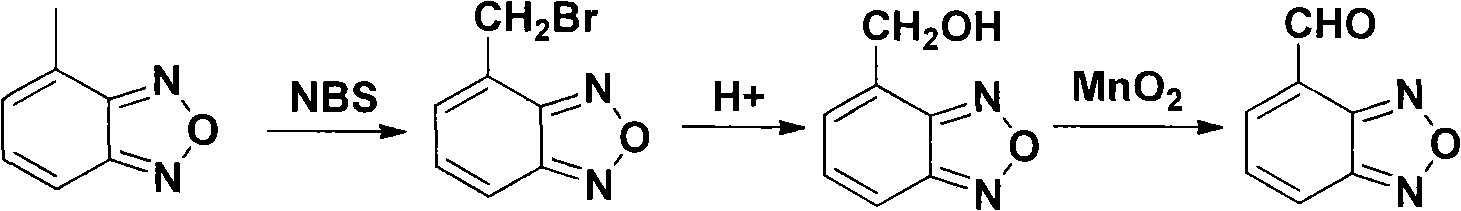
[0023] Synthesis of 4-bromomethylbenzofurazan
[0024] 4-Methylbenzofurazan (13.4g, 100mmol) was dissolved in carbon tetrachloride (150ml), then NBS (23.5g, 132mmol) and Bz202 (0.29mol) were added, and the mixture was heated to 80-85°C for reaction, After the reaction was completed, the system was lowered to 40°C, filtered, filtered and washed with chloroform, and the solvent was recovered under reduced pressure to obtain a crude product (25.6 g), which was recrystallized from petroleum ether-ethyl acetate to obtain light yellow needle crystals, 4-bromomethyl Benzofurazan (16.4 g). Yield 77%, mp 94-95°C.
Embodiment 2
[0026] Synthesis of 4-Formylbenzofurazan
[0027] 4-Bromomethylbenzofurazan (21.1 g, 0.1 mol), urotropine (21.0 g, 0.15 mol), acetic acid (80 ml) and water (80 ml) were added to the reaction flask. Heated to reflux under nitrogen protection and stirred for 1 h. Concentrated hydrochloric acid (80ml) was added and stirring was continued for 30min under reflux. After the reaction was completed, it was cooled to room temperature and extracted with ethyl acetate. The organic layers were combined and washed successively with 10% aqueous sodium carbonate solution and saturated brine. After drying with anhydrous sodium sulfate and filtering, the filtrate was evaporated to remove the solvent under reduced pressure to obtain 11.2 g of a light yellow solid, yield 75%, mp 108-109°C.
Embodiment 3
[0029] Synthesis of Methyl 2-(4-Benzofurazamethylene)-3-oxobutanoate
[0030] 4-Formylbenzofurazan (14.8g, 0.1mol) and methyl acetoacetate (11.6g, 0.1mol), stirred at room temperature until the solid was completely dissolved. Cool in an ice bath, stir and add concentrated sulfuric acid (1ml) dropwise below 10°C, continue stirring for 1h after dropping, add water (100ml), stir for 10min, filter, and wash the filter cake with 10% sodium bicarbonate solution until it becomes weakly alkaline, After washing with water to neutral and absolute ethanol, 17.5 g of solid was obtained, with a yield of 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com