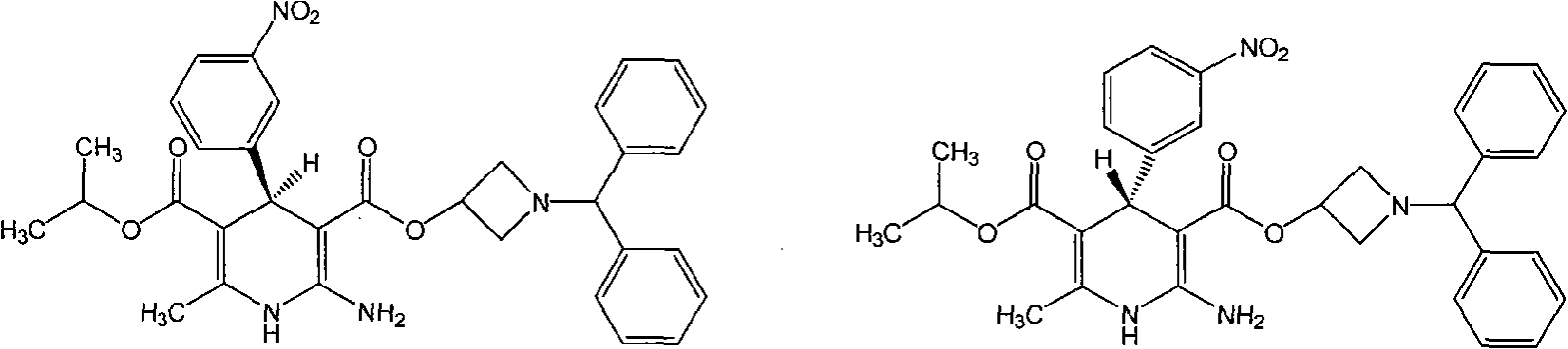
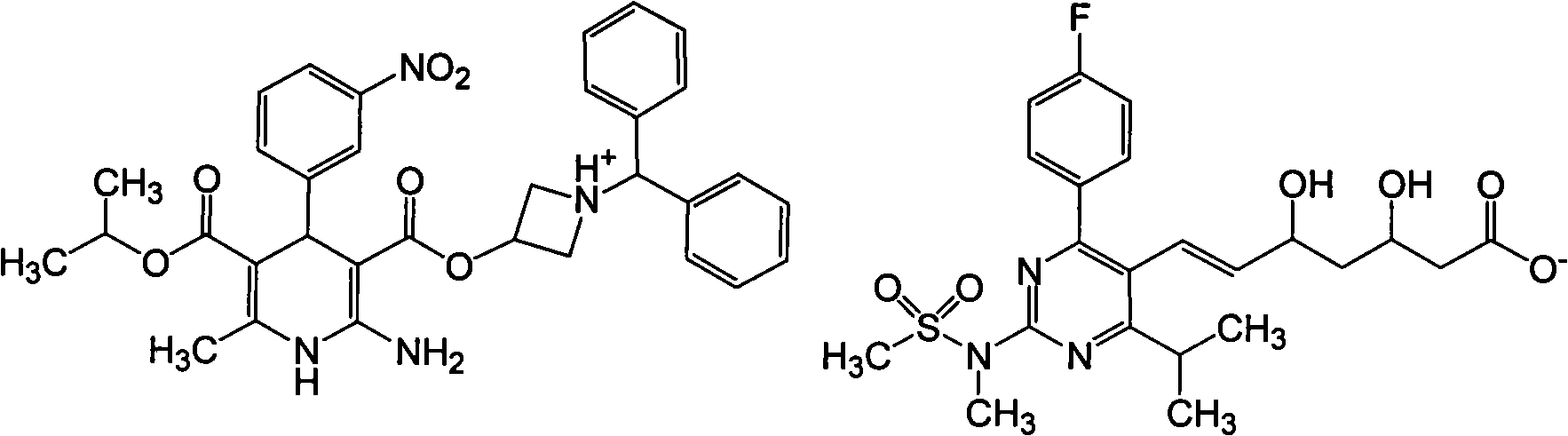
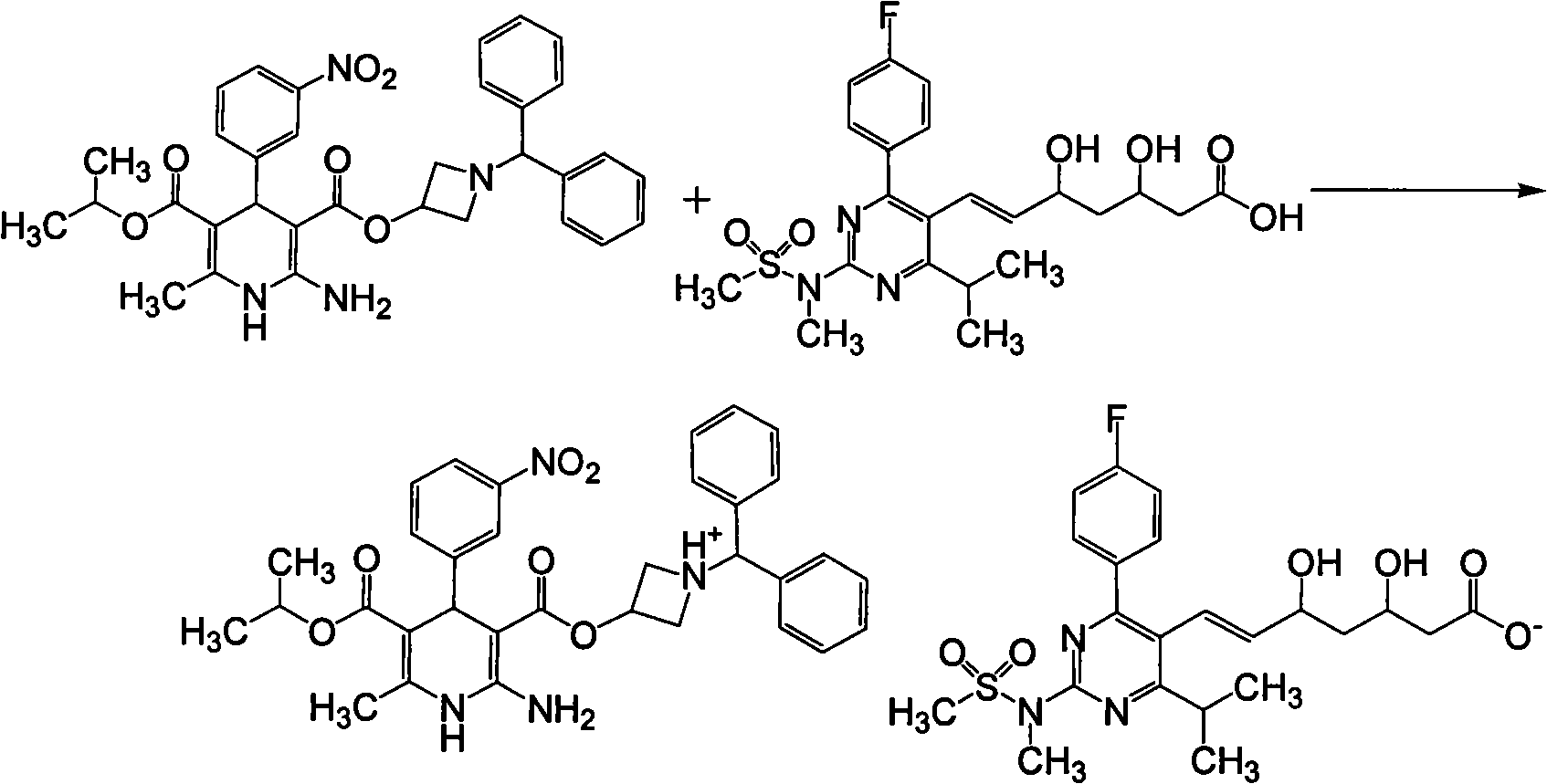
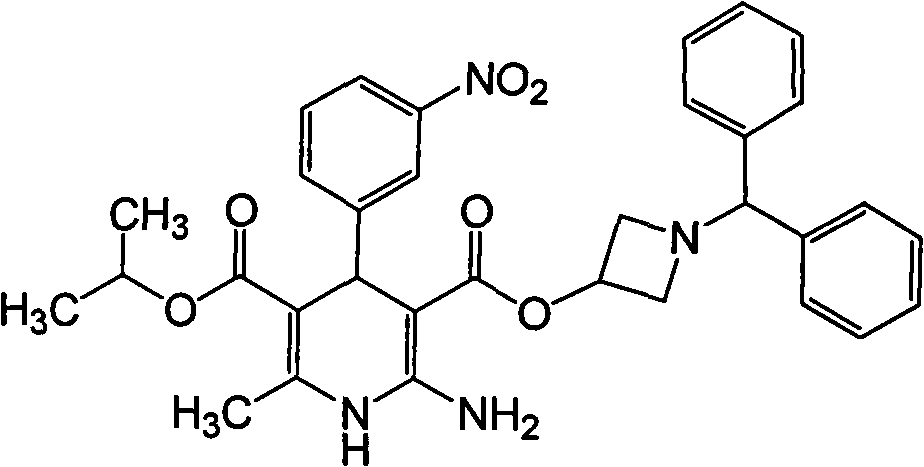
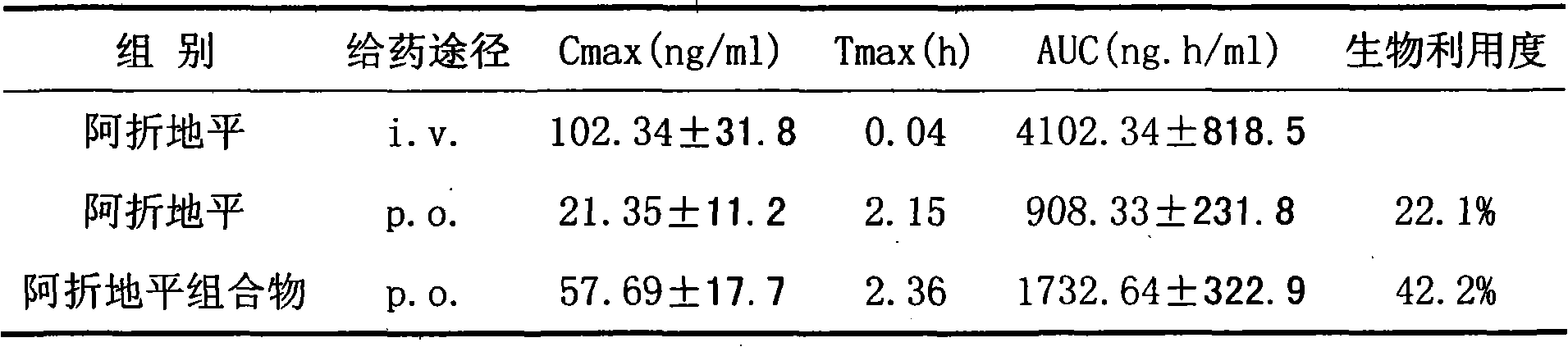Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Azelnidipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
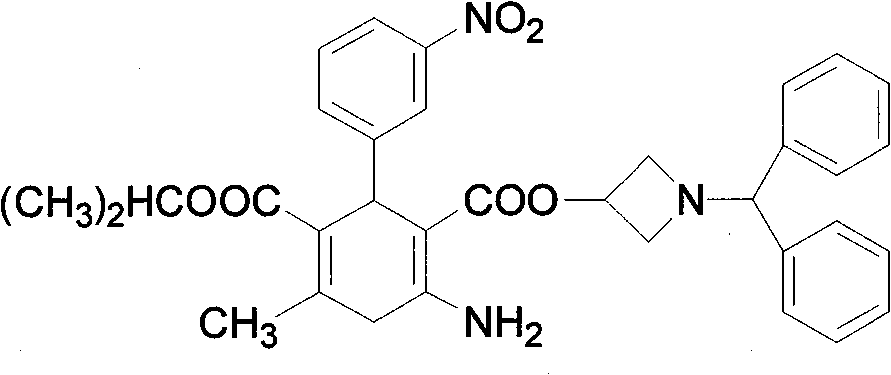
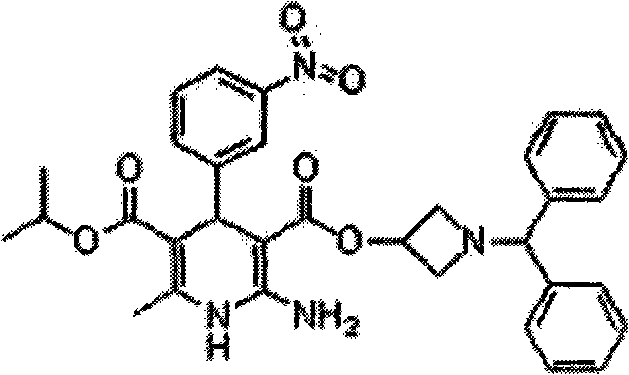
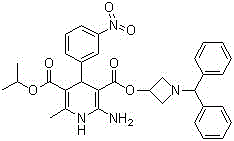
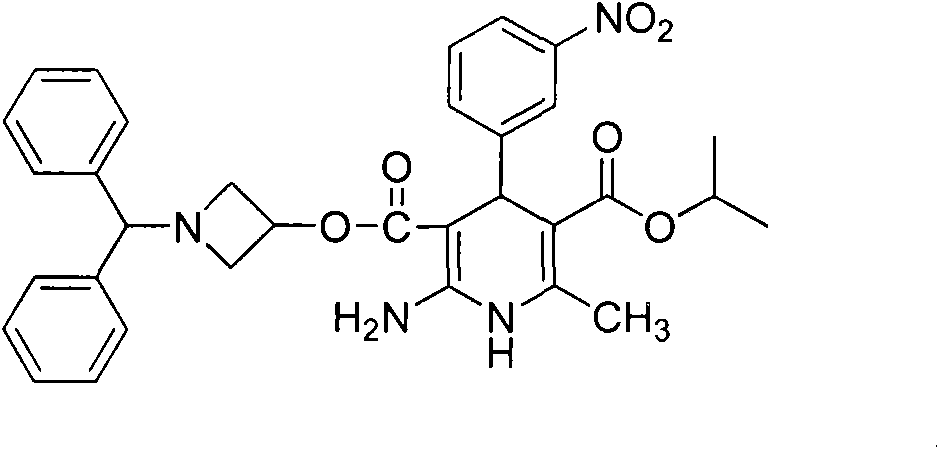
Azelnidipine (INN; marketed under the brand name CalBlock — カルブロック) is a dihydropyridine calcium channel blocker. Azelnidipine is L and T calcium channel blocker. It is sold in Japan by Daiichi-Sankyo pharmaceuticals, Inc. Unlike nicardipine, it has a gradual onset and has a long-lasting hypotensive effect, with little increase in heart rate.
Azelnidipine medicinal composition and its preparing method
InactiveCN101103979AOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionNon ionic
The invention relates to a compound for treating hypertensive, particularly relating to a calcium ion antagonist drug complex with full dissolution, good stability and high bioavailability. The invention is characterized in that the complex contains (A) calcium channel antagonist, (B) alkaline excipient with effective dose and (C) non-ionic surfactant; wherein (A):(B):(C) is ranged from 0.1:1:1 to 10:1:1.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Preparation method of alpha crystalline azelnidipine
The invention relates to a preparation method of alpha crystalline azelnidipine, which comprises the following steps that: converting an azelnidipine crude product into azelnidipine methylate; then uniformly dispersing the azelnidipine methylate in normal heptane solvent; heating, decompressing, distilling and removing the solvent; and obtaining the medicinal alpha crystalline azelnidipine by cooling, filtering, drying and the like through common methods. The method for preparing the medicinal alpha crystalline azelnidipine has the advantages of simple operation, high yield, high purity and low cost, and the yield of the alpha crystalline azelnidipine prepared by the method is about 95 percent (by the azelnidipine methylate) and the purity HPLC content thereof is more than 99.5 percent.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Preparation of Azelnidipine alpha crystal form
ActiveCN101486705AEasy to recycleReduce wasteOrganic chemistryCardiovascular disorderAlkaneWater baths
The invention discloses a preparation method of Azelnidipine of alpha crystal form, wherein, Azelnidipine of non-alpha crystal forms is completely dissolved in a two-phase organic solvent. Water-bath is heated to certain temperature and alkane and seed crystal are added into the solution under a stirring condition. A crystal is separated out by cooling and a separated crystal product is collected, namely the Azelnidipine of alpha crystal form. The two-phase organic solvent comprises two components: low boiling point esters and benzene or derivatives of benzene, with a volume ratio of 1 to 3:15. The preparation method has one-time yield up to 85 percent to 90 percent, purity of more than 99.5 percent as well as good appearance and quality. The preparation method has easily recycled organic solvent, is beneficial to environmental protection, reduces loss in Azelnidipine and / or waste in the organic solvent, greatly reduces the production costs of products and has prospect of large-scale industrialized production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
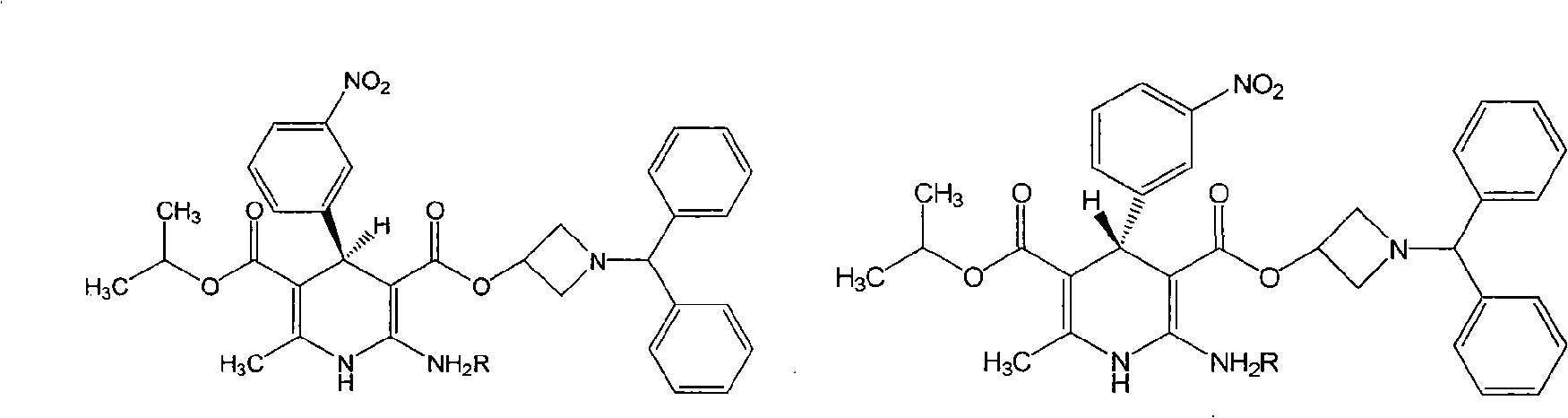
Method for preparing chiral azelnidipine and acceptable salt thereof
The invention relates to a method for preparing (S)-(+)-azelnidipine and (R)-(-)-azelnidipine as well as benzene sulfonic acid, paratoluenesulfonic acid, D-(+)-camphorsulfonic acid, L-(-)-camphorsulfonic acid, taurine and high taurine salt thereof by economically and effectively resoluting racemic azelnidipine. Compared with the prior method for preparing optically active azelnidipine and salt thereof by a high performance liquid chromatography which needs expensive instruments and equipment and has less treatment amount, the method adopts a recrystallization resolution method of the prior chiral resolution reagent and a cheap and easily-obtained solvent, has large sample treatment amount and simple operation, does not need special instruments, and is suitable for industrial production.
Owner:北京华禧联合科技发展有限公司
Rosuvastatin azelnidipine composition
InactiveCN101336921ALower blood pressureLower blood lipid levelsOrganic active ingredientsMetabolism disorderOrganic acidOrganic base
A pharmaceutical composition is an organic salt formed by an organic acid rosuvastatin and an organic base azelnidipine with a molar ratio of 1:1. The pharmaceutical composition can be made into any dosage forms together with a pharmaceutically-acceptable carrier for reducing blood pressure and blood lipid, reducing myocardial infarction and treating cerebral apoplexy. The inventive pharmaceutical composition has the advantages of stable physiochemical indexes, controllable product quality, and convenient administration.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Antihypertensive medicinal composition
InactiveCN102397278AImprove protectionReduce incidenceOrganic active ingredientsCardiovascular disorderSide effectAzelnidipine
The invention which discloses an antihypertensive medicinal composition and concretely relates to a medicinal composition containing azelnidipine or a pharmaceutically acceptable salt thereof and fimasartan belongs to the medicinal field. In the antihypertensive medicinal composition, the weight ratio of azelnidipine or the pharmaceutically acceptable salt thereof to fimasartan is 1:0.1-500, and preferably 1:1-50. The azelnidipine fimasartan medicinal composition of the invention, which has the advantages of obvious synergism, low treatment cost, small side effect and realization of stationary blood pressure reduction when the composition is used to treat hypertension, has a good clinical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Azelnidipine tablets and preparation method thereof
ActiveCN103239418AReduce typesHigh dissolution rateOrganic active ingredientsPill deliverySolvent evaporationAcrylic resin
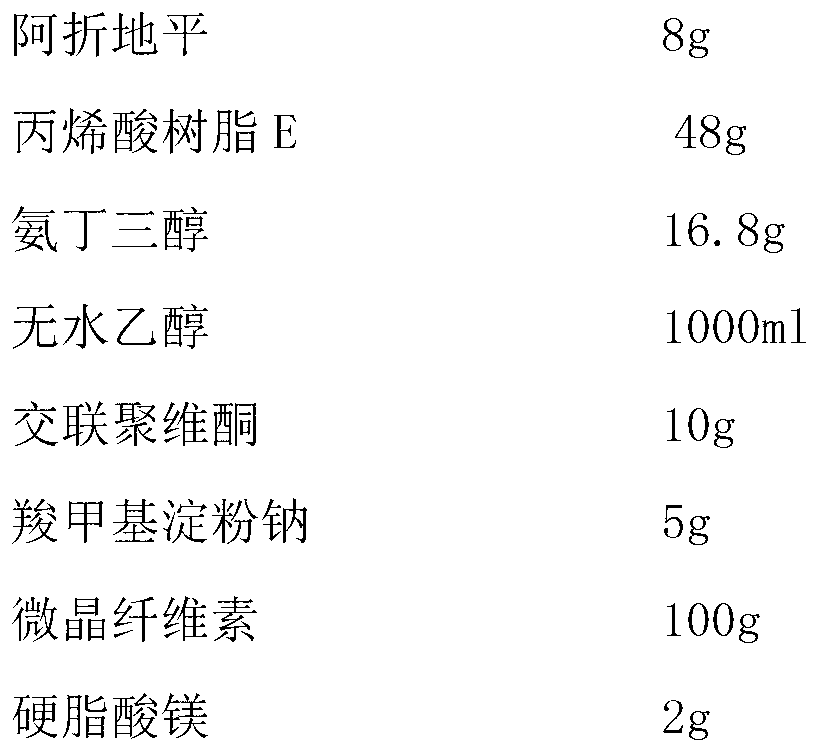
The invention discloses azelnidipine tablets and a preparation method thereof; and the azelnidipine tablets are prepared by evenly mixing a solid dispersion and pharmaceutically acceptable auxiliary materials, and then tabletting the mixture. The solid dispersion is prepared from azelnidipine, acrylic resin E and tromethamine by using a solvent evaporation method. The preparation method does not need extra alkaline matters, thus reducing the types of the auxiliary materials; the tablet dissolution rate is obviously improved; and the azelnidipine tablets are good in stability, simple in technology and easy in industrial mass production.
Owner:NANJING CHIA TAI TIANQING PHARMA
Pharmaceutical composition comprising telmisartan salt and calcium ion antagonist
InactiveCN102266559AImprove solubilityHigh dissolution rateOrganic active ingredientsSenses disorderMagnesium saltDiltiazem
The present invention relates to a kind of pharmaceutical composition, it comprises telmisartan salt and calcium ion antagonist or its pharmaceutically acceptable salt and pharmaceutically acceptable carrier; Described telmisartan salt is selected from telmisartan Sodium salt, potassium salt, calcium salt, magnesium salt or amine salt of sartan, described calcium ion antagonist is selected from amlodipine, lacidipine, cilnidipine, lercanidipine, nisoldipine, nica Dipine, azedipine, barnidipine, manidipine, benidipine, verapamil, diltiazem, or a pharmaceutically acceptable salt thereof. The composition is used for preventing, delaying progress or treating patients with hypertension, angina pectoris, atherosclerosis, stroke, cardiac insufficiency, dyslipidemia, diabetes, renal function damage or hypertension accompanied by Alzheimer's disease, reducing Reduce the morbidity and / or mortality of cardiovascular and cerebrovascular diseases, reduce adverse drug reactions, and improve patients' compliance with medication.
Owner:王丽燕
Medicine for prevention of and treatment for arteriosclerosis and hypertension
InactiveUS20060252806A1Avoid remodelingInhibit progressBiocideNervous disorderOlmesartanHeart disease
A pharmaceutical composition comprising the following active ingredients: (A) an angiotensin II receptor antagonist selected from the group consisting of a compound having a formula (I), a pharmacologically acceptable ester thereof and a pharmacologically acceptable salt thereof (for example, olmesartan medoxomil); and (B) a calcium channel blocker selected from the group consisting of a 1,4-dihydropyridine compound and a pharmacologically acceptable salt thereof (for example, azelnidipine), wherein the composition does not include the combination of olmesartan medoxomil and amlodipine. The composition is useful for prophylaxis and / or treatment of arteriosclerosis, hypertension, heart diseases, renal diseases and cerebrovascular diseases.
Owner:DAIICHI SANKYO CO LTD
Azelnidipine composition
ActiveCN101401942AImprove bioavailabilityImproved and enhanced bioavailabilityOrganic active ingredientsMacromolecular non-active ingredientsBioavailabilityAzelnidipine
The invention discloses an Azelnidipine composition, which consist of Azelnidipine and beta-cyclodextrin, wherein the dosage by weight of the beta-cyclodextrin is 3 to 15 times of that of the Azelnidipine, and the Azelnidipine is enveloped in a hydrophobic cavity of the cyclodextrin. The bioavailability of the Azelnidipine composition is obviously improved compared with a common preparation of the Azelnidipine, which lays a foundation for further development of a new oral preparation of the Azelnidipine.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Azelnidipine quick-releasing drug preparation and preparation method
ActiveCN102512391AReduce drying and other processesAvoid destructionOrganic active ingredientsPill deliveryDrugs preparationsDissolution
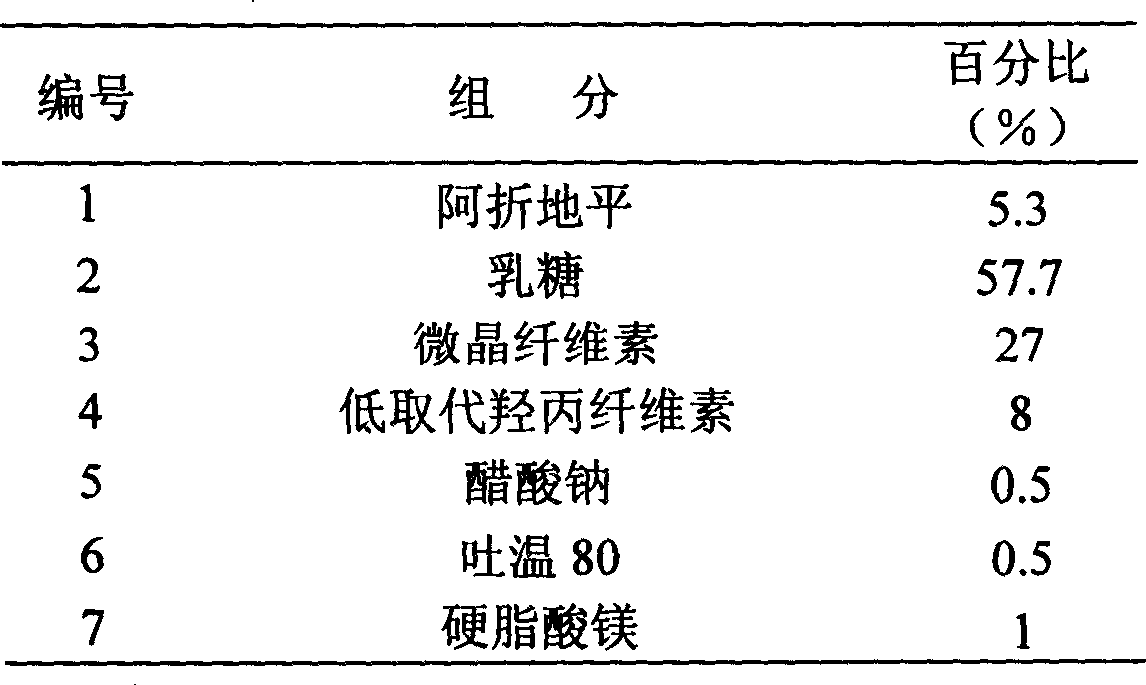
The invention discloses an azelnidipine quick-releasing drug preparation, which comprises the following components by mass percent: 2-12 percent of azelnidipine, 25-45 percent of anhydrous lactose, 40-60 percent of microcrystalline cellulose, 2-10 percent of disintegrating agent and 1-3 percent of lubricating agent. The invention also discloses a preparation method of azelnidipine quick-releasing tablets. The azelnidipine quick-releasing drug preparation prepared by the invention has high dissolution, and the dissolution can be more than 10 percent higher than that of a sample prepared by the common wet and dry granulation. The azelnidipine quick-releasing drug preparation can achieve an effect of quick releasing and has better application prospect in the clinical.
Owner:CHENGDU HENGRUI PHARMA
Composition for treating cardiovascular diseases
InactiveCN101884634AEasy to carryEasy to takeMetabolism disorderCardiovascular disorderVascular diseaseBlood pressure
The invention relates to a medicinal composition containing azelnidipine and atorvastatin calcium, which is used for reducing blood pressure and blood fat and reducing risks of myocardial infarction and cerebral apoplexy.
Owner:北京华禧联合科技发展有限公司
Medicine for prevention of and treatment for arteriosclerosis and hypertension
A pharmaceutical composition comprising the following active ingredients: (A) an angiotensin II receptor antagonist selected from the group of a compound having a formula (I), a pharmacologically acceptable ester thereof and a pharmacologically acceptable salt thereof (for example, olmesartan medoxomil); and (B) a calcium channel blocker selected from the group consisting of a 1,4-dihydropyridine compound and a pharmacologically acceptable salt thereof (for example, azelnidipine), for the prophylaxis and / or treatment of arteriosclerosis, hypertension, heart diseases, renal diseases or cerebrovascular diseases.
Owner:DAIICHI SANKYO CO LTD
Granule for treating hypertension
InactiveCN102580090AProtectiveImprove bioavailabilityOrganic active ingredientsGranular deliveryMedicinal herbsSide effect
The invention relates to a granule for treating hypertension. The granule is characterized by comprising a calcium channel blocker, a beta receptor inhibitor, Chinese medicinal herbs and a medicinal carrier, and is prepared from the following components in percentage by weight: 0.1-60 percent of isradipine, 3-15 percent of labetalol, 10-20 percent of yellowmouth dutchmanspipe root and 7-70 percent of the medicinal carrier. The granule has the advantages of high safety, high synergistic effect among raw material components, high bioavailability, and capability of effectively reducing the side effects of separate azelnidipine on the health of a patient.
Owner:北京水圣木科技有限责任公司
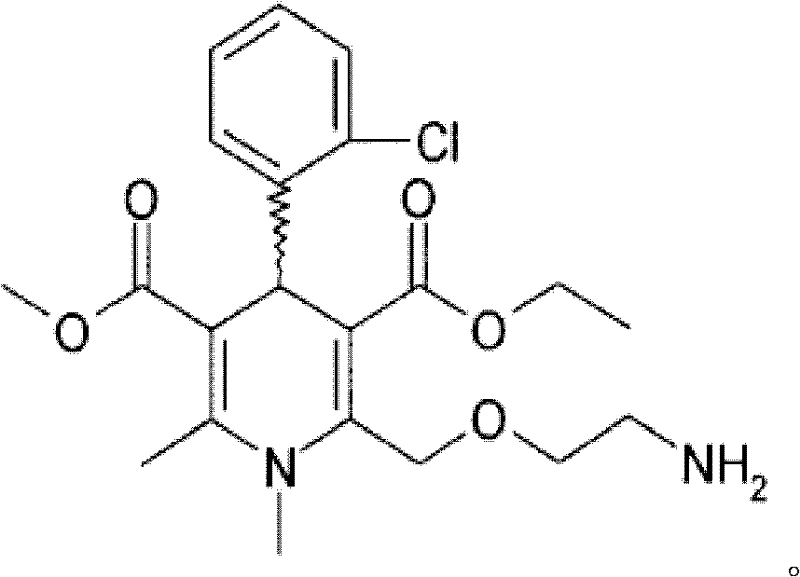
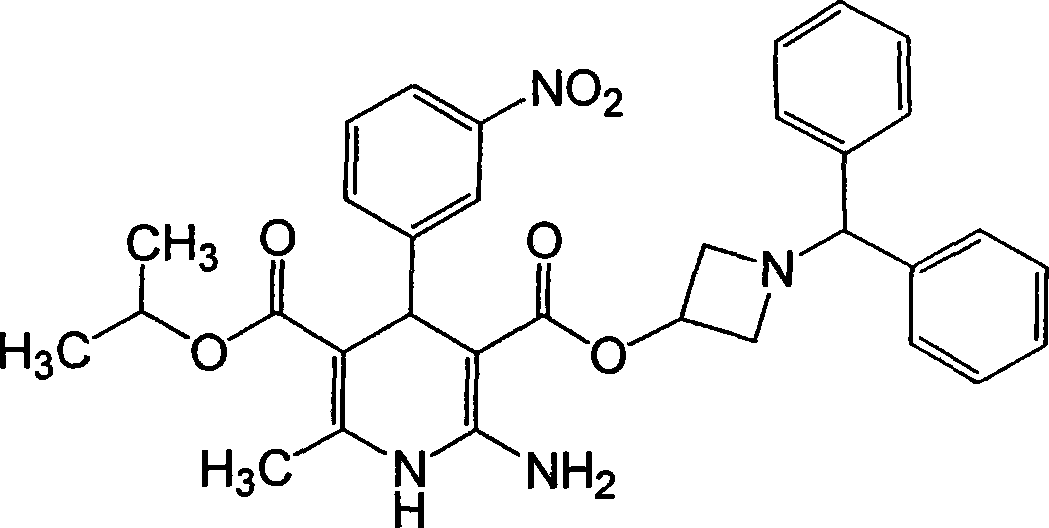
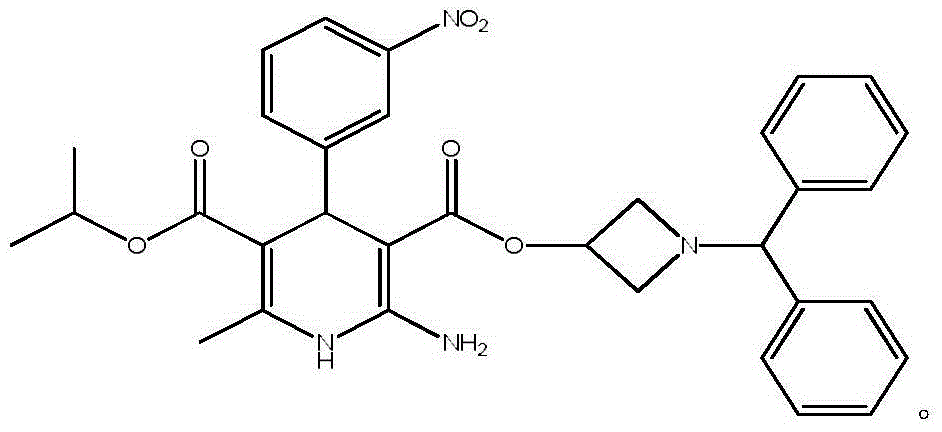
Production method of azelnidipine intermediate
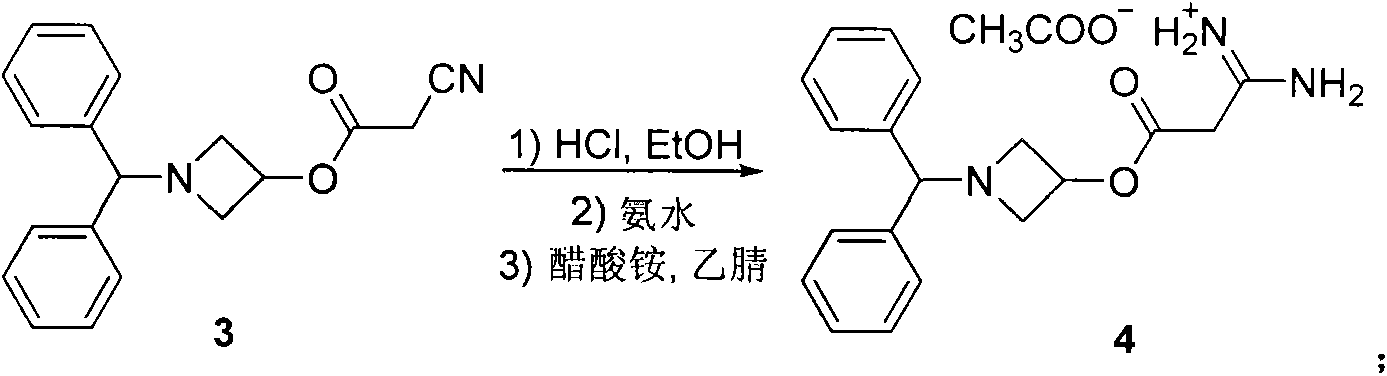
The invention discloses a production method of an azelnidipine intermediate. The production method comprises the following steps of 1 an esterification reaction; 2 a Pinner reaction, wherein a solvent A is added into a microreactor through a pump with the flow velocity of 100-200 ml / min, heating is conducted until the temperature is 220 DEG C-260 DEG C, absolute ethyl alcohol and dichloromethane are slowly and dropwise added into the microreactor, stirring is conducted for 3-5 h, liquid nitrogen is introduced, cooling is conducted until the temperature is 15 DEG C-25 DEG C, dry hydrogen chloride gas is introduced for 2-6 h, the microreactor is sealed, stirring is conducted for 20-30 h, and mixed liquid B is prepared for standby application; 3 a neutralization reaction; 4 an amidine generation reaction, wherein the azelnidipine intermediate-3-amino-3-aminoproionic acid-1-(diphenylmethyl)-3-azetidinyl ester acetate compound is obtained. According to the production method, the temperature state of a reaction system and the usage quantity of the reactants in all the steps are strictly controlled, synthesis of the intermediate is stable, the product yield is increased, the product purity is improved, side reactions are decreased, the purpose sof reducing the production cost and shortening the reaction cycle are achieved, and the production method is more suitable for industrialized large-scale production.
Owner:XUCHANG HAOFENG CHEM TECH
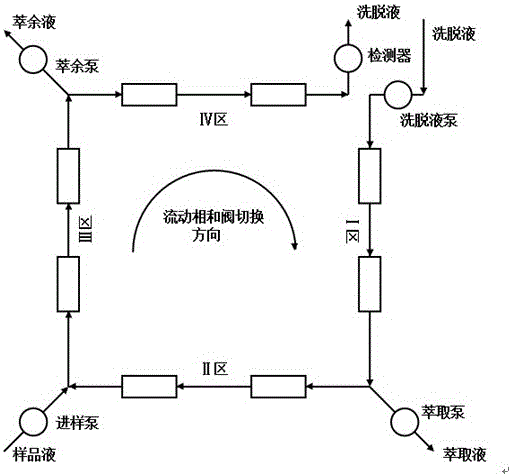
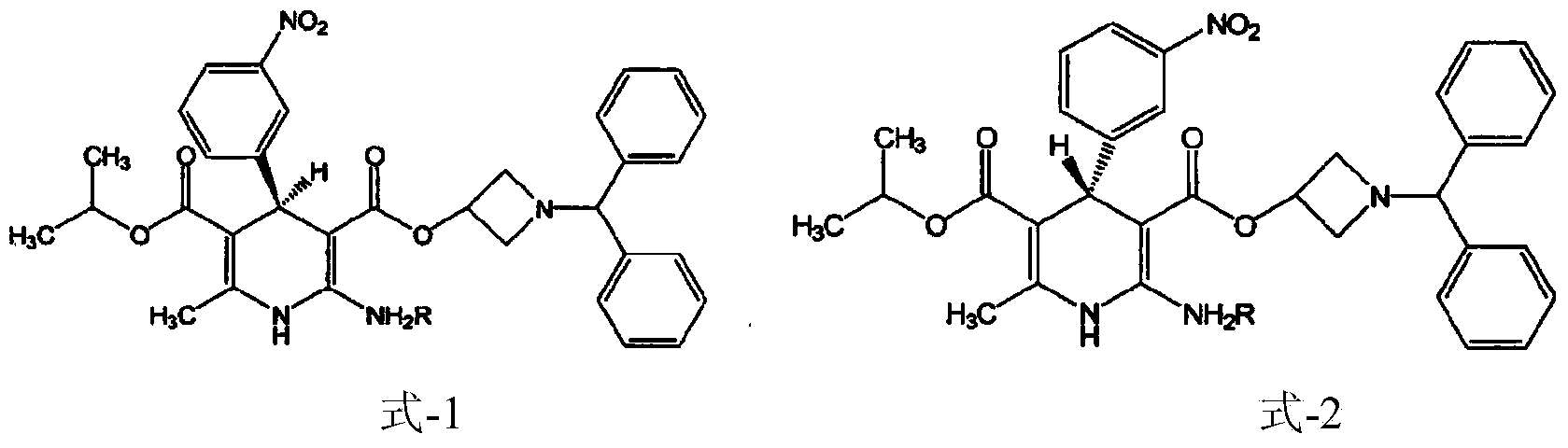
Method for separating chiral drug azelnidipine enantiomer through simulated moving bed chromatography technique
A method for separating the enantiomers of the chiral drug Azedipine by simulated moving bed chromatography, using simulated moving bed chromatography to separate, and the product with a purity of 98% R-enantiomer can be obtained at the extraction port of one of the two outlets solution, the separation process is a continuous process. After simulated moving bed chromatographic separation, under suitable operating conditions, the purity of R-enantiomer can reach more than 98%, and the yield can reach 95%. The solvent can be recovered, the cost is low, and the pollution is less. Continuous production can be realized, which is beneficial to guarantee the product stable quality.
Owner:WENZHOU UNIVERSITY
Valsartan/azelnidipine oral preparation and preparation method thereof
InactiveCN105213388AGood curative effectMedication conveniencePill deliveryCapsule deliveryValsartanAngiotensin receptor
The invention discloses a valsartan / azelnidipine oral preparation and a preparation method thereof. The oral preparation is characterized by comprising a main medicine and auxiliary materials, wherein the main medicine comprises valsartan and azelnidipine; the auxiliary materials include a filling agent, a disintegrating agent, an adhesive and a lubricating agent and glidant. The oral preparation is prepared through the processing steps of raw material selection, preparation, mixing, soft material preparation, granulation, drying, granule straightening, total mixing, finished product preparation, and the like. The valsartan / azelnidipine oral preparation composition for treating hypertension has the beneficial effects that valsartan is an orally effective angiotensin II(AT1) receptor antagonist with specificity; azelnidipine belongs to a dihydropyridine calcium channel antagonist; the product is a compound preparation of two kinds of anti-hypertension branded medicines, is suitable for the patients whose blood pressures can not be controlled by using a single medicine, has good curative effects, is convenient to use and has the effect of greatly improving the medicine use compliance of the patients.
Owner:HARBIN SHENGJI PHARMA
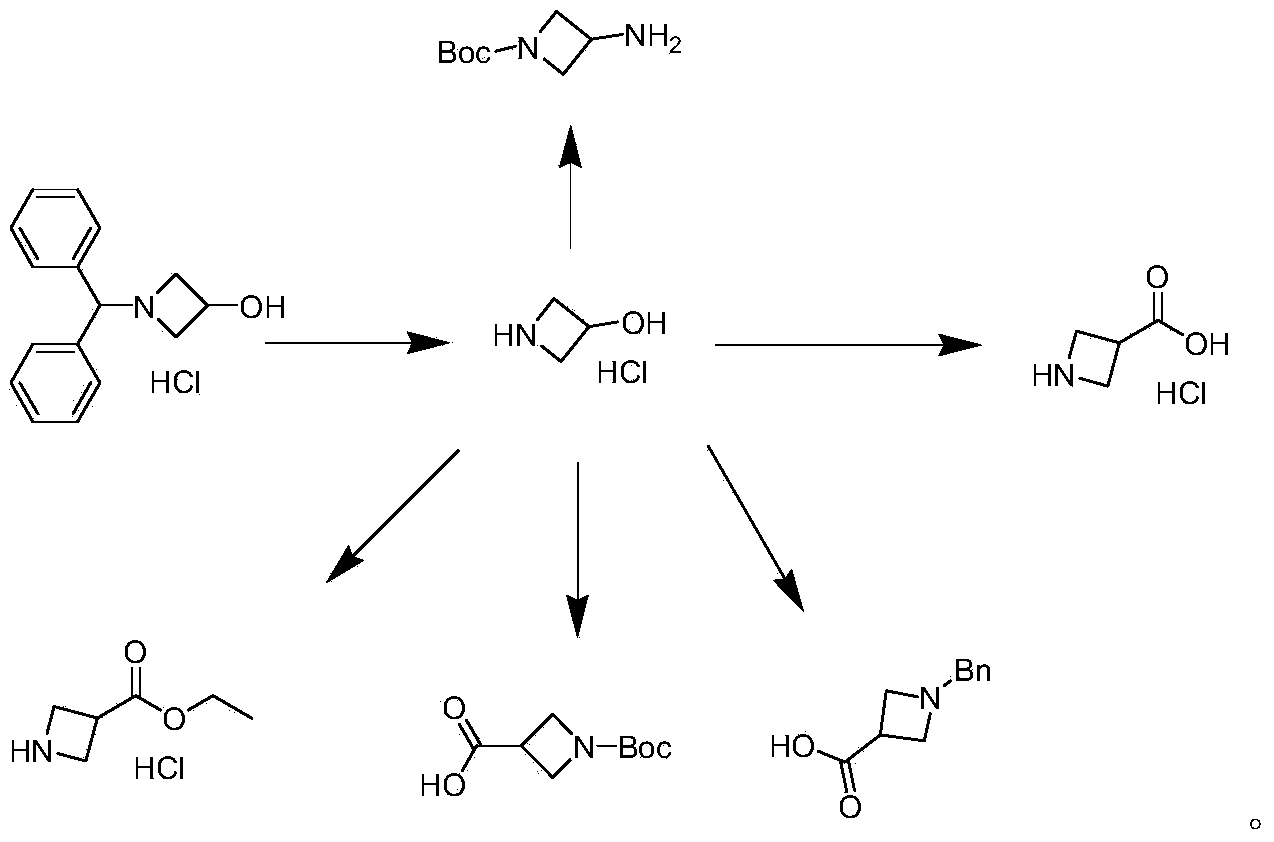
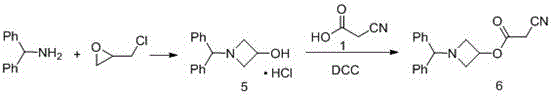
Preparation method of 1-benzhydryl-3-hydroxylazetidine hydrochloride
InactiveCN104356040AIncrease profitNo magnification effectOrganic chemistryMicroreactorOrganic solvent
The invention provides a preparation method of an azelnidipine intermediate, namely 1-benzhydryl-3-hydroxylazetidine hydrochloride. The preparation method comprises the following steps: 1) preparation of a reaction solution I: mixing dimethylaniline and epoxy chloropropane in proportion and adding an organic solvent to prepare the reaction solution I; 2) preparation of a reaction solution II: letting the reaction solution I react at 0-60 DEG C to prepare the reaction solution II; and 3) preparation of 1-benzhydryl-3-hydroxylazetidine hydrochloride: adding the reaction solution II into a microreactor by the use of a pump with flwo velocity of 1-200ml / min, heating to 60-250 DEG C at pressure of 0-2 MPa, completely reacting, and carrying out separation purification to obtain white crystals, namely 1-benzhydryl-3-hydroxylazetidine hydrochloride.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Azelnidipine composition
ActiveCN101401942BImproved and enhanced bioavailabilityReduce difficultyOrganic active ingredientsMacromolecular non-active ingredientsBioavailabilityAzelnidipine
The invention discloses an Azelnidipine composition, which consist of Azelnidipine and beta-cyclodextrin, wherein the dosage by weight of the beta-cyclodextrin is 3 to 15 times of that of the Azelnidipine, and the Azelnidipine is enveloped in a hydrophobic cavity of the cyclodextrin. The bioavailability of the Azelnidipine composition is obviously improved compared with a common preparation of the Azelnidipine, which lays a foundation for further development of a new oral preparation of the Azelnidipine.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Pharmaceutical composition of azelnidipine
ActiveCN104473888ALong validity periodRelaxed storage conditionsOrganic active ingredientsPill deliveryAzelnidipinePolymer chemistry
The invention relates to a pharmaceutical composition of azelnidipine and particularly relates to a composition of jointly applying three alkaline auxiliary materials. The medicine has good storage stability and solves the problem of poor stability of azelnidipine and short storage time.
Owner:NANJING CHIA TAI TIANQING PHARMA
Method for preparing chiral azelnidipine and acceptable salt thereof
The invention relates to a method for preparing (S)-(+)-azelnidipine and (R)-(-)-azelnidipine as well as benzene sulfonic acid, paratoluenesulfonic acid, D-(+)-camphorsulfonic acid, L-(-)-camphorsulfonic acid, taurine and high taurine salt thereof by economically and effectively resoluting racemic azelnidipine. Compared with the prior method for preparing optically active azelnidipine and salt thereof by a high performance liquid chromatography which needs expensive instruments and equipment and has less treatment amount, the method adopts a recrystallization resolution method of the prior chiral resolution reagent and a cheap and easily-obtained solvent, has large sample treatment amount and simple operation, does not need special instruments, and is suitable for industrial production.
Owner:北京华禧联合科技发展有限公司
Methods for prevention and treatment of arteriosclerosis, hypertension and restenosis
InactiveUS20080176909A1Avoid remodelingInhibit progressBiocideNervous disorderOlmesartanPharmacometrics
Methods for the prophylaxis and / or treatment of arteriosclerosis, hypertension, restenosis, heart diseases, renal diseases and cerebrovascular diseases by administering a pharmaceutical composition comprising the following active ingredients: (A) an angiotensin II receptor antagonist selected from the group consisting of a compound having a formula (I), a pharmacologically acceptable ester thereof and a pharmacologically acceptable salt thereof (for example, olmesartan medoxomil), the compound having the following formula:and (B) a calcium channel blocker selected from the group consisting of a 1,4-dihydropyridine compound and a pharmacologically acceptable salt thereof (for example, azelnidipine), wherein the composition does not include the combination of olmesartan medoxomil and amlodipine or amlodipine besylate.
Owner:DAIICHI SANKYO CO LTD
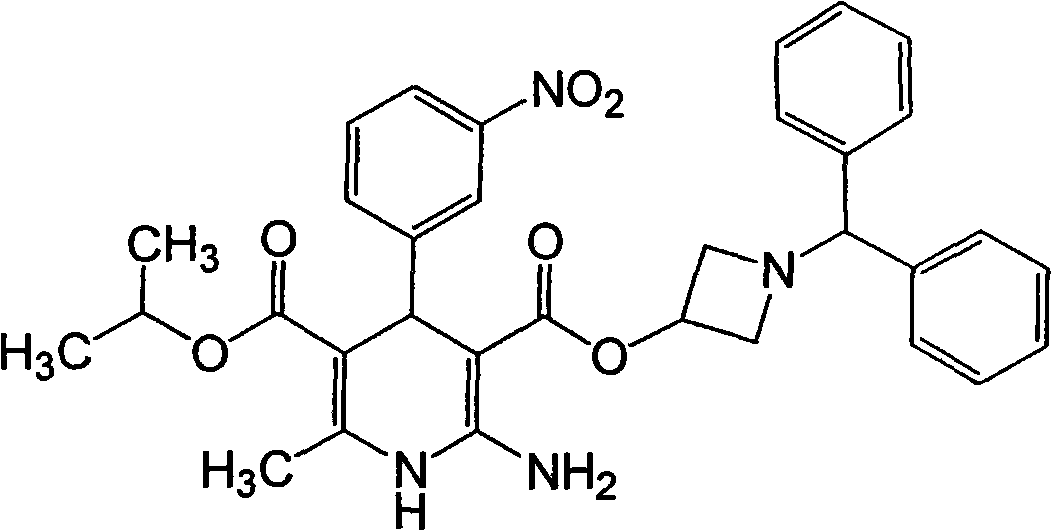
Preparation method for azelnidipine
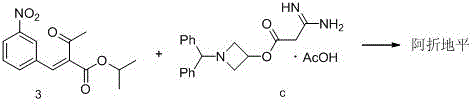
The invention relates to a preparation method for an antihypertensive drug, azelnidipine, which belongs to the field of medicine. The synthesis condition of amidine 4 in traditional synthetic route is improved by the method; and the original anhydrous reaction condition is changed into the reaction in water, so that the operation is convenient; and the requirement for the anhydrous degree of the reaction solvent is reduced. Therefore, the preparation method is very suitable for industrial production.
Owner:迪嘉药业集团股份有限公司
Preparation method of azelnidipine
InactiveCN106279109AGreat purification difficultyLow yieldOrganic chemistryAcetic acidDihydropyridine
The invention relates to a preparation method of azelnidipine, belongs to the field of medicines and opens up a new way for synthesizing dihydropyridine calcium antagonists. With cyanoacetic acid as a raw material, the method comprises the following sequential steps: synthesizing isopropyl 2-amino-3-carboxyl-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-5-formate through a Pinner reaction and a Hantzsch reaction; and performing DCC dehydration esterification with 1-diphenylmethyl-3-hydroxyl azetidine hydrochloride to obtain a crude product of azelnidipine; and performing further crystal transformation to obtain alpha-crystal type azelnidipine.
Owner:WEIHAI DISU PHARMA CO LTD +1
Azelnidipine and isosorbide dinitrate compound composition
ActiveCN102058592AIncrease the effective ratioEasy to takePill deliveryCardiovascular disorderBlood pressureAngina symptoms
The invention relates to an azelnidipine and isosorbide dinitrate compound composition, which consists of an azelnidipine common layer and an isosorbide dinitrate slow-release layer. Each unit preparation comprises 2-8mg of azelnidipine and 5-40mg of isosorbide dinitrate and each tablet is 0.1g theoretically. Azelnidipine and isosorbide dinitrate are organically combined to reduce the dosage of medical accessories to a maximum extent, which can not only relieve angina and reduce the blood pressure stably, but also improve the survival rate of the patients. The compound composition has high medical effective ratio and is convenient to take and beneficial for price reduction and industrialized production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Preparation method of azelnidipine alpha crystal form
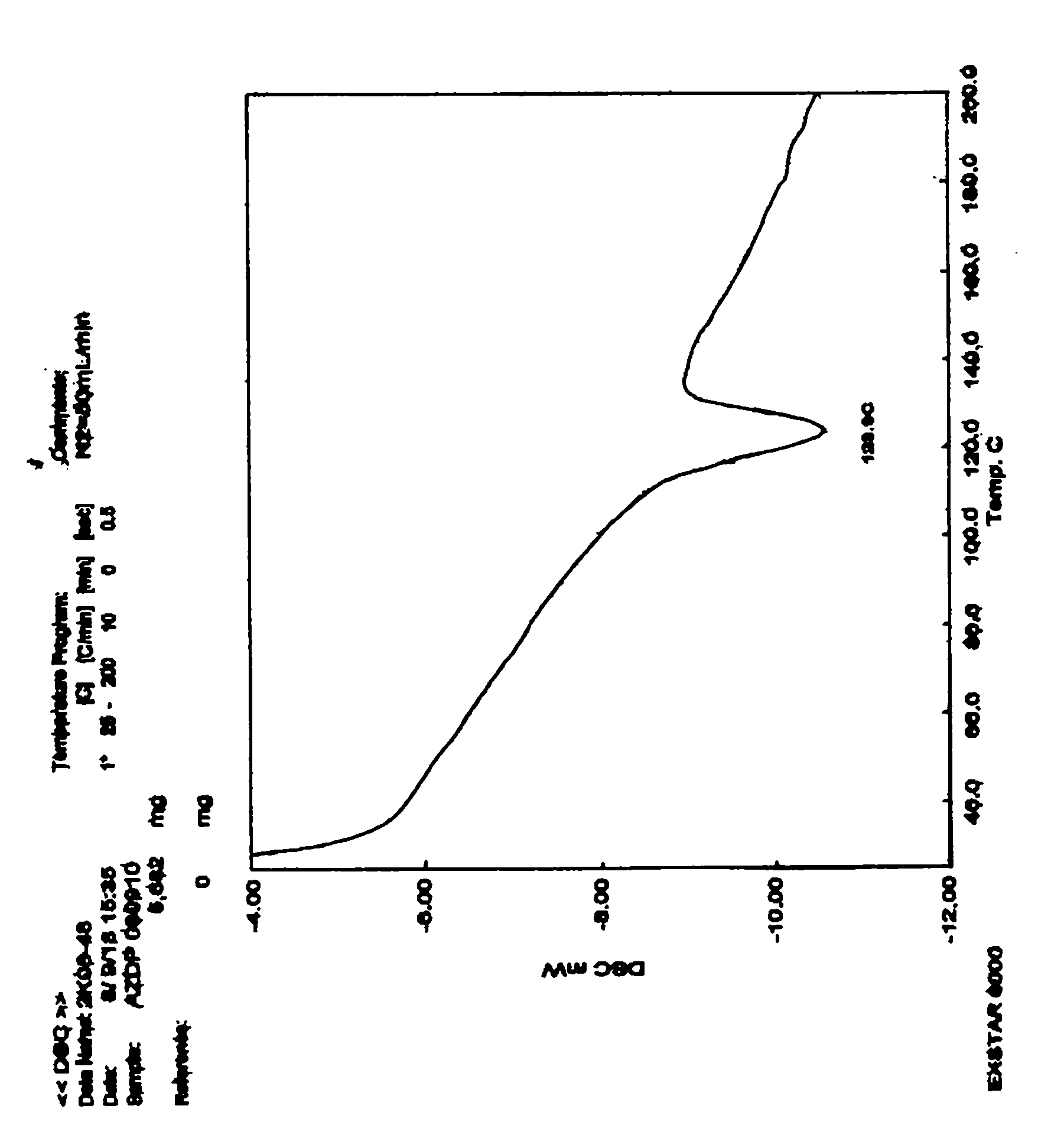
InactiveCN107188885AEasy to recycleQuality improvementOrganic chemistry methodsAzelnidipineVacuum drying
The invention relates to a preparation method of an azelnidipine alpha crystal form. According to the technical scheme, the preparation method comprises the following steps; I, adding non-alpha-crystal-form azelnidipine or crude azelnidipine of other forms into isopropanol of which the amount is not less than 5 times the mass of the non-alpha-crystal-form azelnidipine or the crude azelnidipine, heating for dissolved clarification, cooling to the temperature of 40 DEG C, observing whether crystallization occurs or not, if so, replenishing the isopropanol for dissolved clarification once again, cooling to the temperature of 40 DEG C or less for crystallization, preserving heat at the temperature of 10 DEG C below zero to 0 DEG C for 6 to 12 hours, and filtering to obtain an azelnidipine-diisopropanol compound; II, putting the azelnidipine-diisopropanol compound solid prepared by filtering in the step I into a crystal transformation kettle, adding cyclohexane in an amount which is not less than 6 times the mass of the azelnidipine-diisopropanol compound solid for crystal transformation with stirring, stirring for not less than 3 hours, finishing crystal transformation, filtering, and performing vacuum drying under a reduced pressure to obtain the azelnidipine alpha crystal form. By adopting the method, the crude azelnidipine and the non-alpha-crystal-form azelnidipine can be transformed into the azelnidipine alpha crystal form.
Owner:WEIHAI DISU PHARMA CO LTD +1
A kind of preparation method of azedipine impurity b
The invention relates to a method for preparing an azelnidipine impurity B and belongs to the technical field of preparation of bulk drugs. According to the technical scheme, the method disclosed by the invention comprises the following steps: carrying out an oxidizing reaction on azelnidipine and metachloroperbenzoic acid, and generating an intermediate of formula I; performing silica gel columnchromatography on the intermediate of the formula I, and generating the impurity B under catalysis of silica. The invention provides a preparation method of the high-purity azelnidipine impurity B.
Owner:迪嘉药业集团股份有限公司
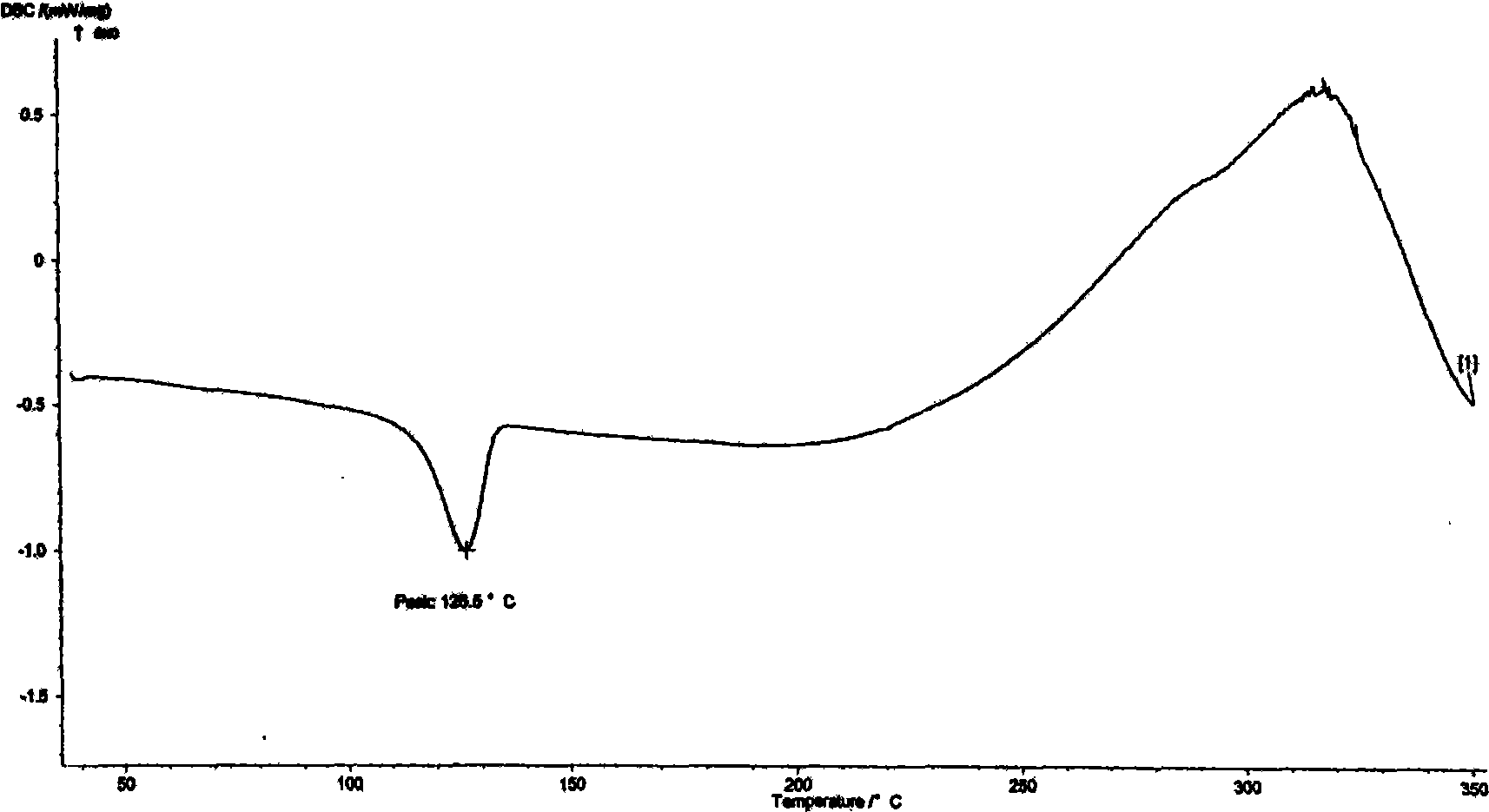
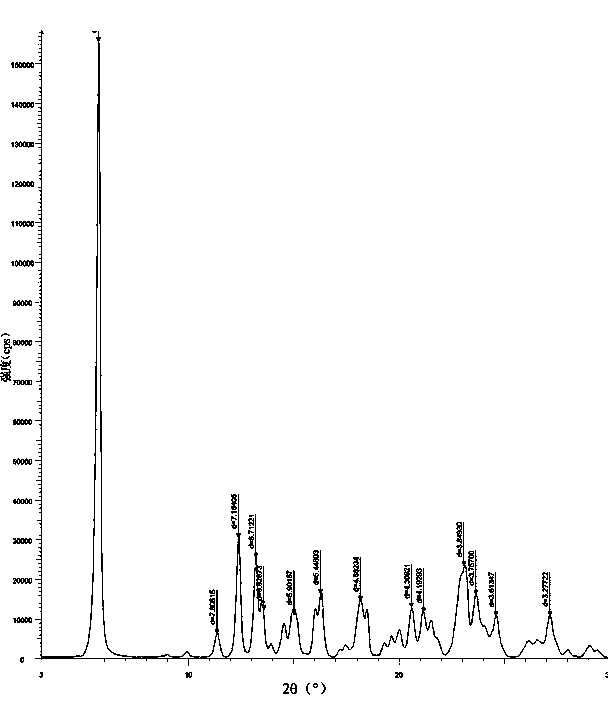
Azelnidipine crystal form, preparation method for same and officinal composition thereof
ActiveCN102503935BImprove stabilityEasy to storeOrganic active ingredientsOrganic chemistryActivated carbonFiltration
The invention provides a novel azelnidipine crystal form, a preparation method for the same and an officinal composition thereof. The X-ray powder diffraction characteristic absorption peak value 2theta of the novel azelnidipine crystal form is 5.68, 11.33, 12.34, 13.18, 13.55, 15.00, 16.26, 18.16, 20.60, 21.50, 23.09, 23.66, 24.62 and 27.19. The preparation method for the novel azelnidipine crystal form includes the steps: mixing amorphous azelnidipine with methylbenzene according to amount ratio ranging from 1:4 to 1:6 (w / v) prior to heating, adding activated carbon with stirring and filtering, adding cyclohexane into filtrate with the amount ratio of the cyclohexane to the methylbenzene ranging from 1:2 to 2:1 (v / v), and obtaining the novel azelnidipine crystal form by means of crystallization, filtration and drying. The novel azelnidipine crystal form is durable in drug properties, small in static electricity, higher in stability, high in yield and suitable for mass production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Antihypertensive medicine
InactiveCN102125553ALower blood pressureReduce first pass effectOrganic active ingredientsCardiovascular disorderSide effectPatient compliance
The invention relates to a medicine for treating hypertension, which is a compound preparation consisting of telmisartan angiotensin converting enzyme receptor inhibitor and azelnidipine calcium antagonist. When the compound preparation disclosed by the invention is used, the dose and application times of the medicine are reduced, side effects are reduced, the patient compliance is improved and the treatment effect is enhanced.
Owner:北京华禧联合科技发展有限公司
Azelnidipine medicinal composition and its preparing method
InactiveCN101103979BOrganic active ingredientsPharmaceutical non-active ingredientsActive agentPharmaceutical drug
The invention relates to a compound for treating hypertensive, particularly relating to a calcium ion antagonist drug complex with full dissolution, good stability and high bioavailability. The invention is characterized in that the complex contains (A) calcium channel antagonist, (B) alkaline excipient with effective dose and (C) non-ionic surfactant; wherein (A):(B):(C) is ranged from 0.1:1:1 to 10:1:1.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com