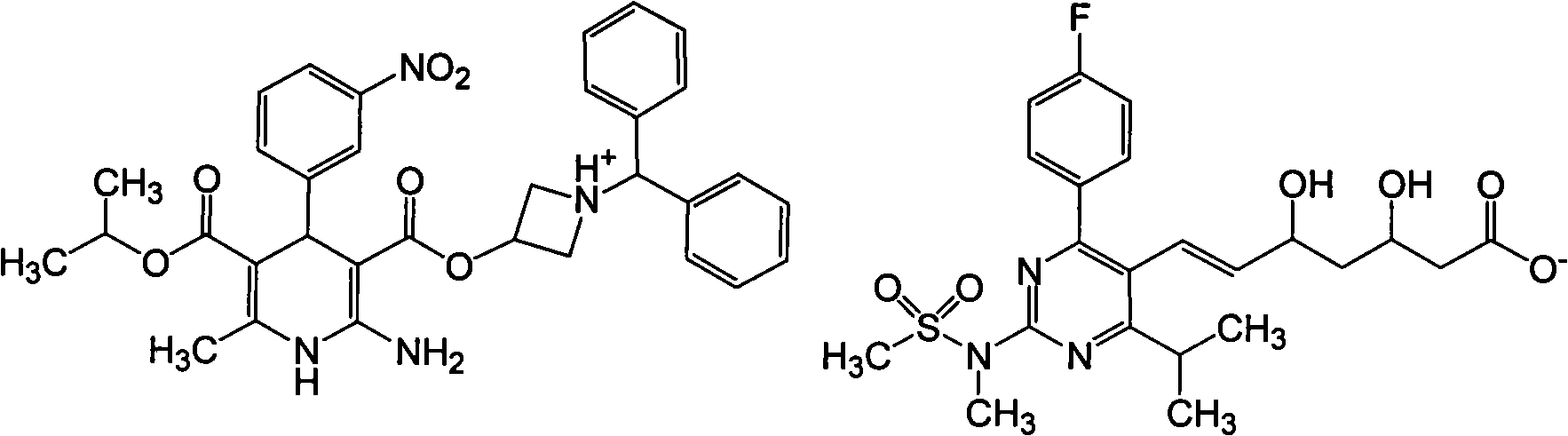
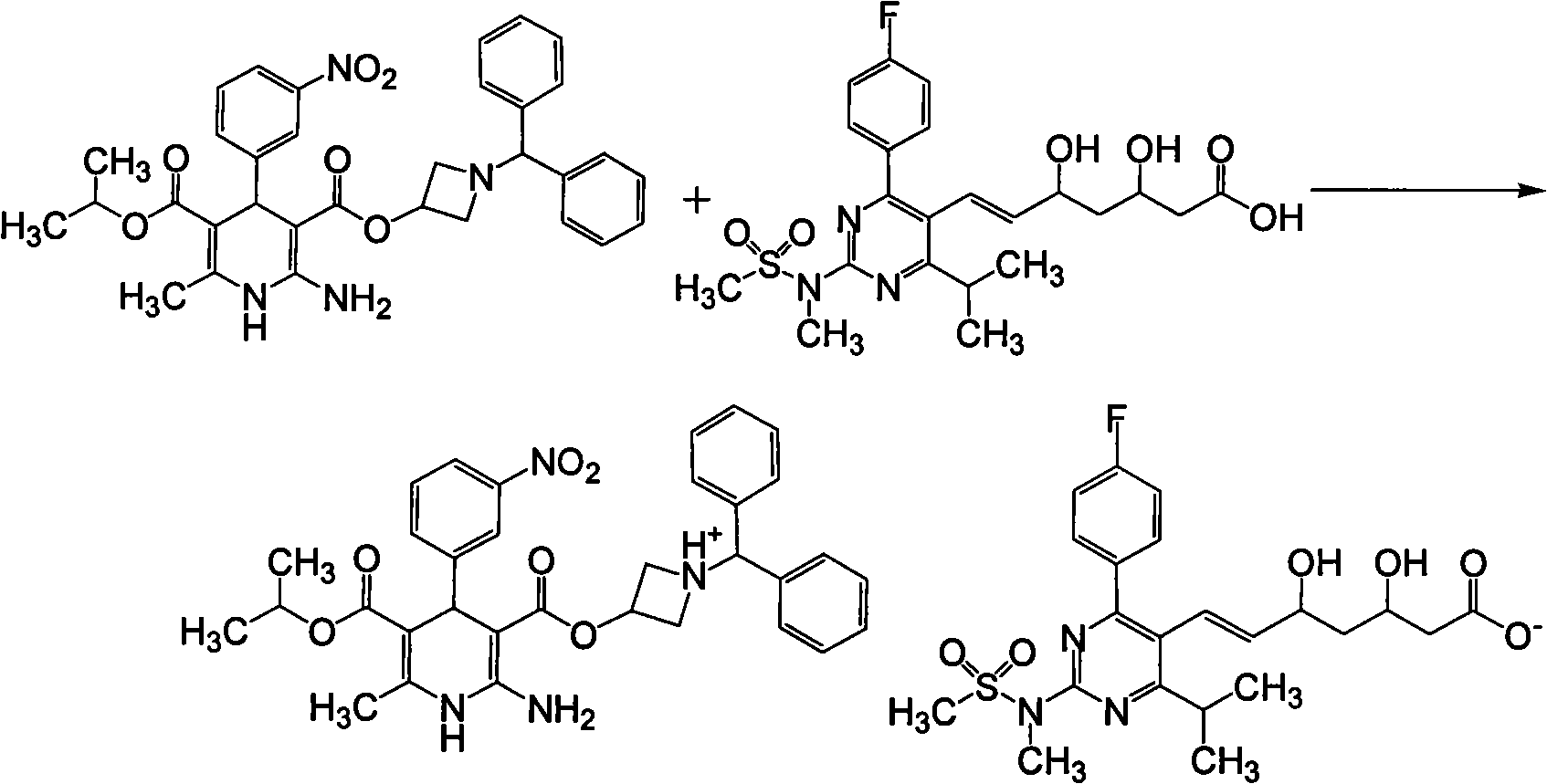
Rosuvastatin azelnidipine composition
A technology of rosuvastatin and azedipine, which is applied in the direction of drug combination, metabolic disease, cardiovascular system disease, etc., can solve the problems of difficult control of drug effect and inconvenient taking, and achieve convenient taking, stable physical and chemical indicators, and reduced The effect of myocardial infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
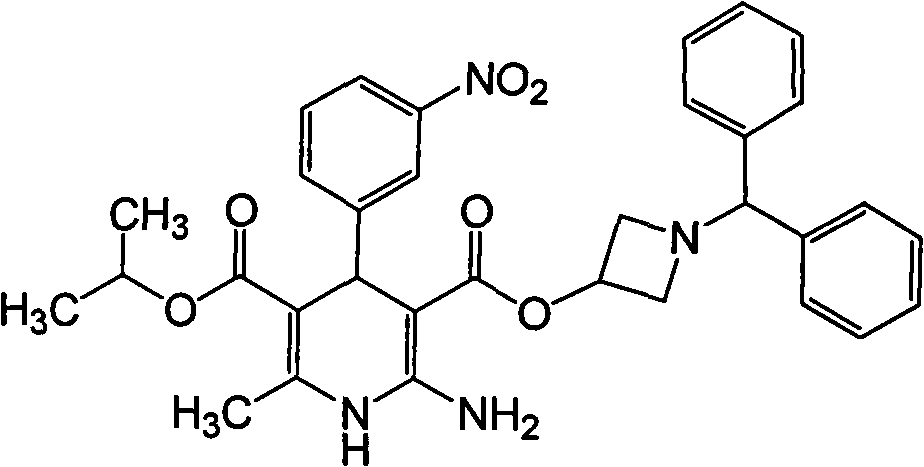
[0043] Preparation of Azedipine:
[0044] Acetate of 1-benzhydryl-3-azetidinylamidinoacetate
[0045]
[0046] In a 2000ml reaction flask, add 1500ml of dichloromethane, 153g (0.5mol) of 1-benzhydryl-3-azetidinyl cyanoacetate, 35ml (0.6mol) of absolute ethanol, and ice-salt bath Cool at -5°C, pass through dry hydrogen chloride gas (prepared by 140g of sodium chloride) for 2h, stir the reaction solution below -5°C for 3h, overnight at 0°C, pass through nitrogen at -5°C for 2h, and pass through at -5°C Add ammonia gas for 3 hours, add 1000ml of cold 10% ammonia water under stirring, separate the organic layer, dry over anhydrous sodium sulfate, concentrate to dryness at room temperature in vacuo, add 500ml of acetonitrile and 46g (0.6mol) of ammonium acetate to the residue, and react the mixture at 55°C for 2h , cooled, filtered, the mother liquor was concentrated to dryness in vacuum, crystallized by adding toluene, filtered to obtain 117 g of acetate, with a yield of 61%. ...
Embodiment 2
[0051] Preparation of Rosuvastatin:
[0052]
[0053] In a 500ml reaction bottle, add rosuvastatin calcium 30g, water 150ml, ethanol 150ml, add dropwise 10% sodium carbonate aqueous solution 50ml under stirring, add, filter, wash calcium carbonate with 50% ethanol aqueous solution 100ml, combine filtrate, use 10% hydrochloric acid aqueous solution neutralized the pH5, extracted 3 times with 300 ml of dichloromethane, combined the dichloromethane layers, washed with saturated saline, recovered the dichloromethane to obtain 24 g of rosuvastatin.
Embodiment 3
[0055] Preparation of Rosuvastatin Azeldipine Salt:
[0056]
[0057] Add an organic solvent such as 600ml of dichloromethane into a 1000ml reaction flask, add 58.3g of azedipine and 48.2g of rosuvastatin under stirring, stir at 45°C until completely dissolved, concentrate in vacuo to dryness, add 200ml of methanol, and heat to 55 ℃, add 2g of activated carbon, heat filter, cool to crystallize, and filter to obtain rosuvastatin azeldipine salt 98g, mp76℃, FAB-MS: m / z 1087[M+Na] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com