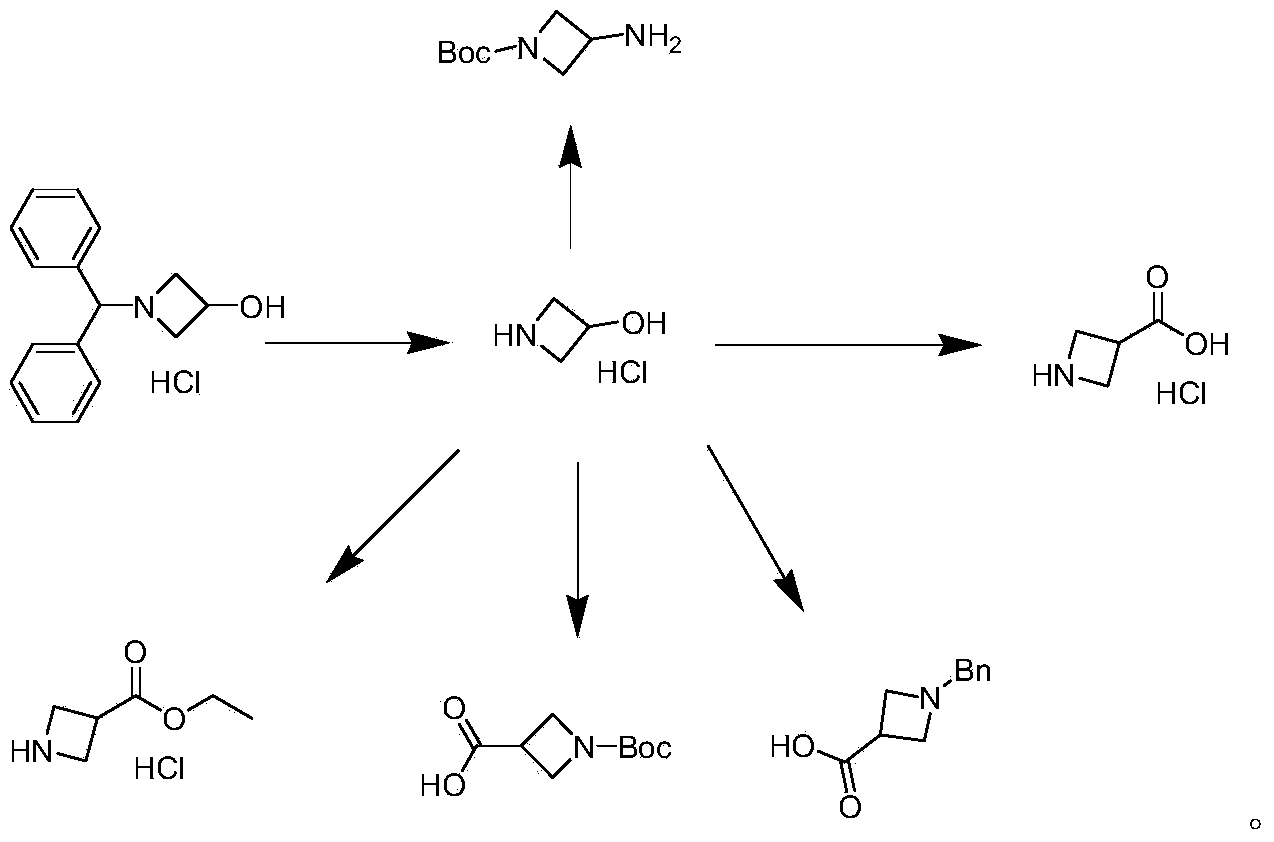
Preparation method of 1-benzhydryl-3-hydroxylazetidine hydrochloride
A technology of heterocyclobutane and benzhydryl, applied in the direction of organic chemistry, can solve the problems of increased cost and environmental pollution, long total reaction time, poor repeatability, etc., achieves no amplification effect, and the method is simple and easy to operate , the effect of easy temperature control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Preparation of reaction solution I: Take diphenylmethylamine (1500g, 8.19mol) and add it to a 5L four-neck flask, control the temperature at 20-25°C, add 1.5L methanol, and then add epichlorohydrin (984.52g, 10.64mol) , stir evenly, and the molar ratio of xylidine and epichlorohydrin is 1:1.3.
[0036] 2) Preparation of reaction solution II: React the reaction solution I at 27±2°C for 48 hours to prepare the reaction solution II, 99% of the benzhydrylamine is converted into the intermediate state B, and only a small amount of benzhydrylamine remains. No other impurities are formed. The reaction equation is:
[0037]
[0038] 3) Preparation of 1-benzhydryl-3-hydroxyazetidine hydrochloride: raise the temperature of the reactor to 230°C, set the pressure to 1.8MPa, and pump the reaction solution with a flow rate of 1-200ml / min Add II into the microreactor, and set the flow rate to 70ml / min. After 1.1 minutes, the reaction liquid flows out of the microreactor and is...
Embodiment 2
[0046] 1) Preparation of reaction solution Ⅰ: Add diphenylmethylamine (1500g, 8.19mol) into a 5L four-neck flask, control the temperature at 20-25°C, add 1.5L of ethanol, and then add epichlorohydrin (984.52g, 10.64mol) , stir evenly, and the molar ratio of xylidine and epichlorohydrin is 1:1.3.
[0047] 2) Preparation of reaction solution II: reaction solution I was reacted at 27±2° C. for 50 hours to prepare reaction solution II.
[0048] 3) Preparation of 1-benzhydryl-3-hydroxyazetidine hydrochloride: raise the temperature of the reactor to 230°C, set the pressure to 2MPa, and use a pump with a flow rate of 1-200ml / min to pump the reaction solution II Add in the microreactor, the flow rate is set to 60ml / min, after 1.2 minutes, the reaction solution flows out of the microreactor, is collected in the liquid storage tank, and continues to feed until all the materials are consumed.
[0049] Separation and purification: transfer the reaction solution to a rotary evaporator, ke...
Embodiment 3
[0055] 1) Preparation of reaction solution I: Take diphenylmethylamine (1000g, 5.46mol) and add it into a 5L four-neck flask, control the temperature in a water bath at 20-25°C, add 1.5L of n-butanol, and then add epichlorohydrin (656.35g, 7.09mol), stir evenly, and xylidine and epichlorohydrin are 1:1.3 according to the molar ratio.
[0056] 2) Preparation of reaction solution II: reaction solution I was reacted at 30° C. for 40 hours to prepare reaction solution II.
[0057] 3) Preparation of 1-benzhydryl-3-hydroxyazetidine hydrochloride: raise the temperature of the reactor to 230°C, set the pressure to 1.8MPa, and pump the reaction solution with a flow rate of 1-200ml / min Add II into the microreactor, and set the flow rate to 50ml / min. After 1.2 minutes, the reaction solution flows out of the microreactor and is collected in the liquid storage tank, and continues to feed until all the materials are consumed.
[0058] Separation and purification: transfer the reaction solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com