Felodipine ramipril compound sustained-release preparation and preparation method thereof
A sustained-release preparation, the technology of felodipine, which is applied in the field of felodipine and ramipril compound sustained-release preparations and its preparation, can solve the problem that there is no production, marketing and registration application for felodipine and ramipril compound sustained-release preparations. problems, to achieve the effect of small differences in individual bioavailability, good fluidity, and reduction of drug toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
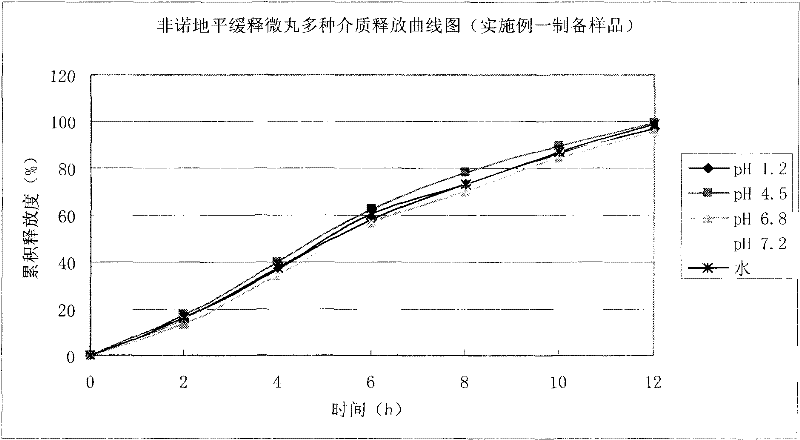
Examples
Embodiment 1
[0042] 1. Preparation of fenodipine sustained-release pellets:
[0043] Prescription: Fenordipine 15g, microcrystalline cellulose 180g, lactose 150g, sodium lauryl sulfate 6g, ethyl cellulose aqueous dispersion (Surelease) 140.4g, titanium dioxide 2g.
[0044] Preparation process: Mix fenodipine, microcrystalline cellulose and lactose uniformly in an equal increasing method, and mix the powder for later use. Dissolve sodium lauryl sulfate in 35% ethanol solution, add to the above mixed powder to make soft material, use extrusion spheronization method to make pill core, dry, sieve and select 20-30 mesh pill core for coating . The prescribed amount of Surelease and titanium dioxide was prepared with purified water to form a slow-release coating solution with a solid content of 15%, dissolved and stirred for 45 minutes to make it fully dispersed. The 300g drug-containing pill core is placed in a fluidized bed, the process parameters are adjusted to ensure a fluidized state, the...
Embodiment 2
[0055] 1. Preparation of fenodipine sustained-release pellets:
[0056] Prescription: Fenordipine 15g, microcrystalline cellulose 180g, lactose 150g, sodium lauryl sulfate 6g, ethylcellulose aqueous dispersion (Aquacoat) 182.5g, dibutyl phthalate 4.5g, titanium dioxide 2g.
[0057] Preparation process: Mix fenodipine, microcrystalline cellulose and lactose uniformly in an equal increasing method, and mix the powder for later use. Dissolve sodium lauryl sulfate in 35% ethanol solution, add to the above mixed powder to make soft material, use extrusion spheronization method to make pill core, dry, sieve and select 20-30 mesh pill core for coating . Prepare the prescribed amount of Aquacoat, dibutyl phthalate and titanium dioxide with purified water to prepare a slow-release coating solution with a solid content of 15%, dissolve and stir for 45 minutes to fully disperse. 300g of drug-containing pill cores are placed in a fluidized bed, and the process parameters are adjusted to...
Embodiment 3
[0068] 1. Preparation of fenodipine sustained-release pellets:
[0069] Prescription: Fenordipine 15g, microcrystalline cellulose blank core 300g, PVP-K30 10g, Tween 5g, Eudragit NE30D 65.2g, talcum powder 15.6g, titanium dioxide 2g.
[0070] Preparation process: Dissolve fenodipine, PVP-K30 and Tween in 50% ethanol solution, stir and use to dissolve completely, and prepare the drug-containing solution. Put the microcrystalline cellulose blank pellet core in a fluidized bed, spray the above-mentioned drug-containing solution on the surface of the blank pellet core, and prepare the drug-containing pellet core by using the method of adding medicine, dry, sieve and select 20-30 mesh drug-containing pellet cores, and coat use. Dissolve and dilute the prescribed amount of Eudragit NE30D, talcum powder and titanium dioxide with purified water to prepare a slow-release coating solution with a solid content of 20%, and stir for 45 minutes to make it fully dispersed. 300g of drug-con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com