Olmesartan medoxomil tablets and preparation method thereof
A technology of olmesartan medoxomil and tablet core, applied in the field of olmesartan medoxomil tablet and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
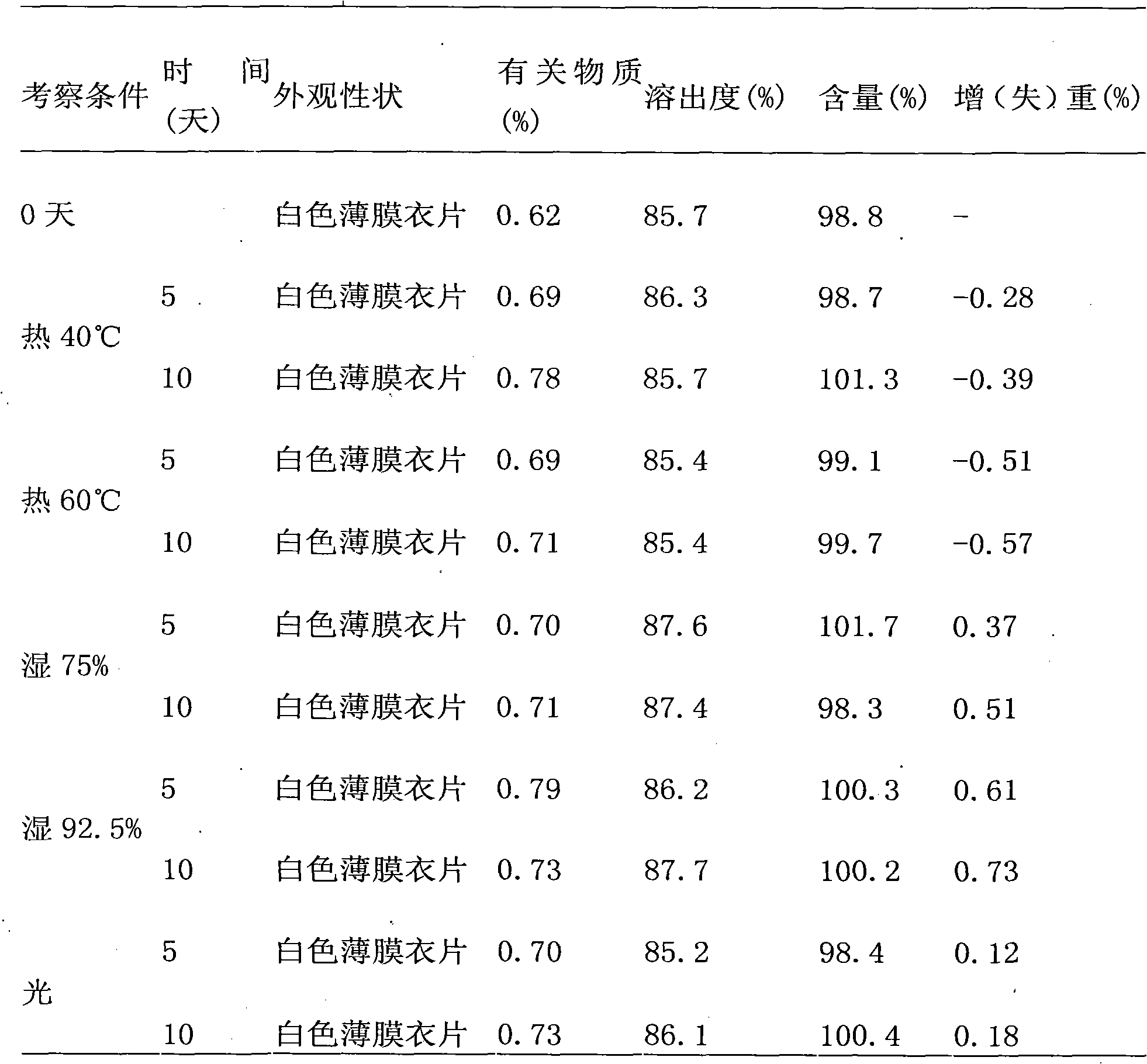
[0019] It should be noted that, in the case of no conflict, the embodiments in the present application and the features in the embodiments can be combined with each other. The present invention will be described in detail below with reference to the accompanying drawings and examples.
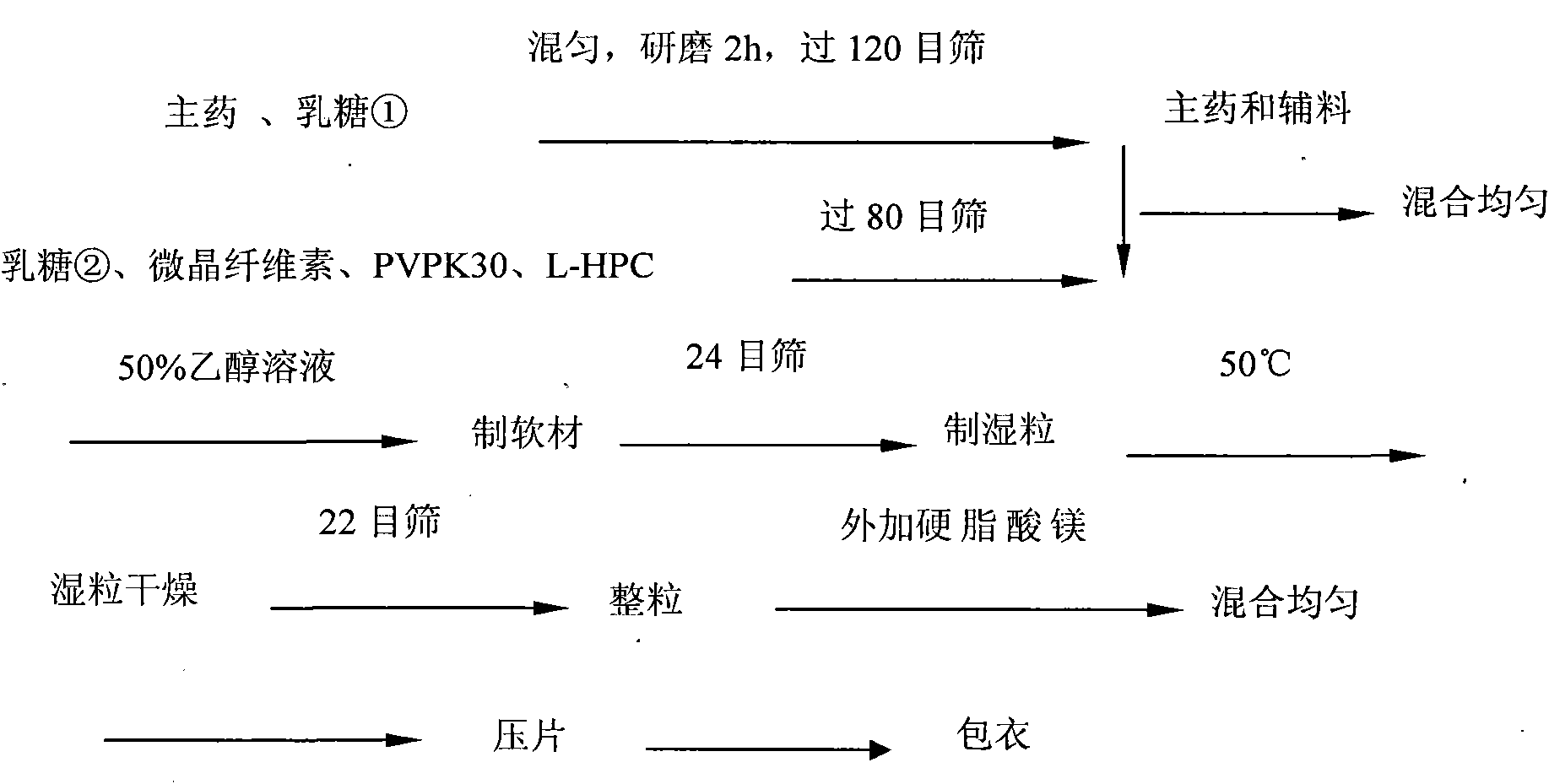
[0020] Olmesartan medoxomil tablets can be available in three sizes of 5 mg, 20 mg, and 40 mg to facilitate individualized administration according to the patient's condition. In the following implementation with
[0021] 1. Prescription
[0022] 20mg is selected as the specification for research. Through research, it is determined that the prescription of this product is:
[0023] 1. Tablet core prescription:
[0024] Olmesartan Medoxomil 20g
[0025] Lactose① 60g
[0026] Lactose② 30g
[0027] Microcrystalline Cellulose 45g
[0028] PVPK30 5g
[0029] Low-substituted hydroxypropyl cellulose (L-HPC) 8g
[0030] 50% ethanol aqueous solution appropriate amount
[0031] Magnesium Stear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com