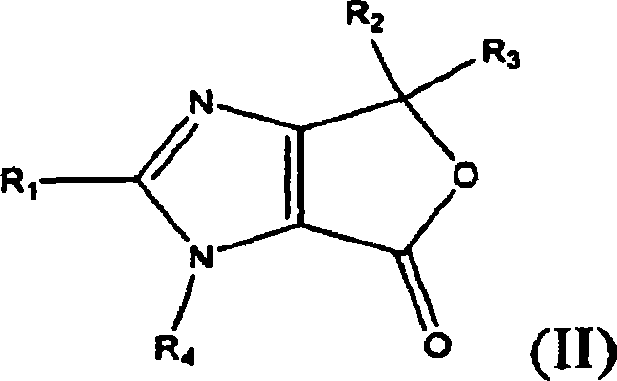
Process for the preparation of olmesartan medoxomil
A technology for olmesartan medoxomil and carboxylate is applied in the field of preparing olmesartan medoxomil, and can solve the problems of cumbersome process, reduced yield, removal of difficult pharmaceutical active compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
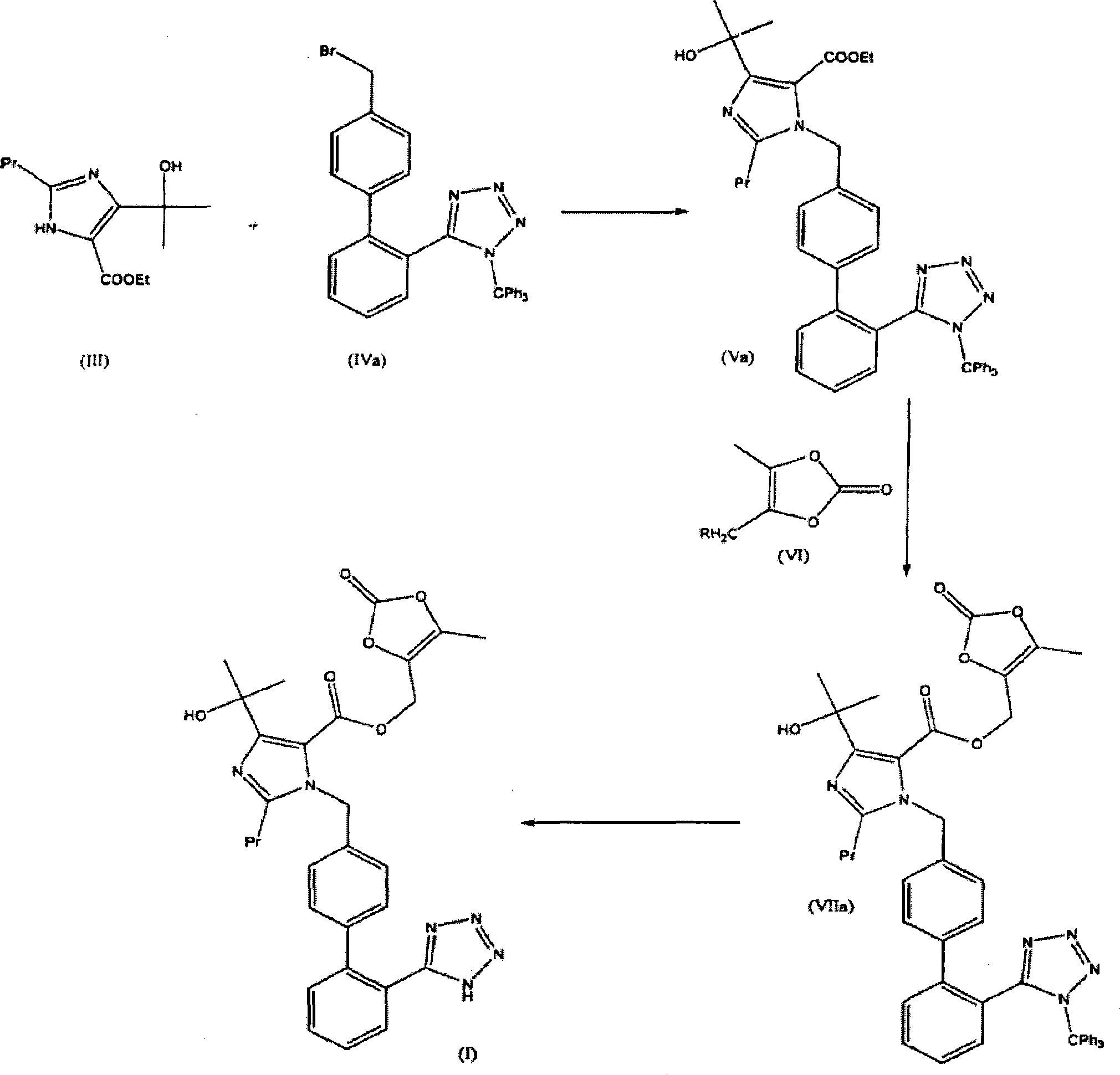
[0120] 17.3g (124.8mmol) potassium carbonate, 15g (62.4mmol) ethyl-4-(1-hydroxyl-1-methylethyl)-2-propylimidazole-5-carboxylate (III) and 38.3g ( 68.7 mmol) of 4-[2-(trityltetrazol-5-yl)phenyl]-benzyl bromide (IVa) was suspended in 750 ml of acetonitrile. The suspension was then heated under reflux until the reaction was complete (7 hours). 510ml of acetonitrile was distilled off, and the concentrate was cooled to 23-25°C. The mixture was stirred overnight at this temperature, then the suspension was cooled to 0°C and stirred at this temperature for 1 hour. The crude product (Va) was filtered off and washed 2x20ml with cold acetonitrile. The wet product was suspended in 450 ml of water, stirred for 1.5 hours, and filtered off. The mass of dry product (Va) was 39.5 g (89%).
[0121] T=165-169°C
[0122] IR: 1666, 1525, 1291, 1446, 1177, 881, 756, 669, 640
Embodiment 2
[0124] 36.0g (50.3mmol) ethyl-4-(1-hydroxyl-1-methylethyl)-2-propyl-1-{4-[2-(trityltetrazol-5-yl)benzene Base]phenyl}-methyl-imidazole-5-carboxylate (Va) and 3.0 g (75.4 mmol) of NaOH were suspended in 413 ml of dimethylacetamide. The suspension was then stirred at room temperature for 20 hours, and a further 6.9 g (50.3 mmol) of potassium carbonate were added. The mixture was cooled to 0°C, and slowly added in 39ml of dimethylacetamide containing 15.4g (70.4mmol) of 4-chloromethyl-5-methyl-2-oxo-1,3-dioxole The solution. The mixture was slowly heated to 50°C and stirred at this temperature for 2 hours. After the esterification was completed, the mixture was cooled to 10°C, poured into a mixture containing 625ml of ethyl acetate and 625ml of 10% NaCl, and stirred at 25°C for 15min. The liquid phases were separated and the organic phase was washed with 2x500ml 10% NaCl, dried over sodium sulfate and filtered. The filtrate was concentrated to 1 / 2 (about 270 g) under reduced ...
Embodiment 3
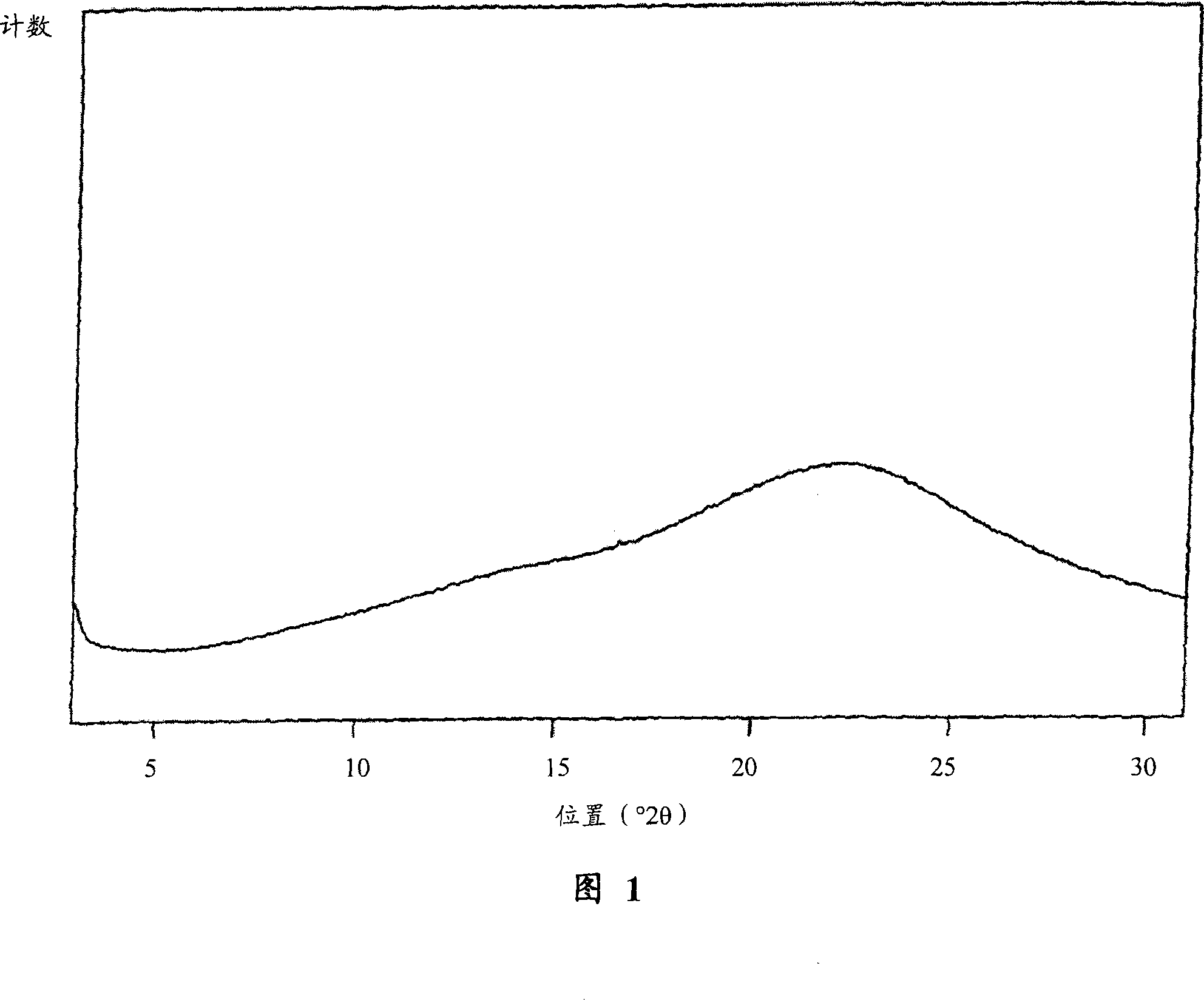
[0128] 1.11 g of olmesartan medoxomil was dissolved in 12.5 ml of 2-butanone under reflux conditions. The solution was slowly cooled to room temperature and stirred at this temperature for 20 hours. During this process, olmesartan medoxomil slowly crystallizes. The product was filtered and dried at room conditions for 18 hours. We obtain 0.98 g of olmesartan medoxomil.
[0129] The crystal form of the product was as described in Annual Report of Sankyo Research Laboratories Vol. 55 (2003).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com