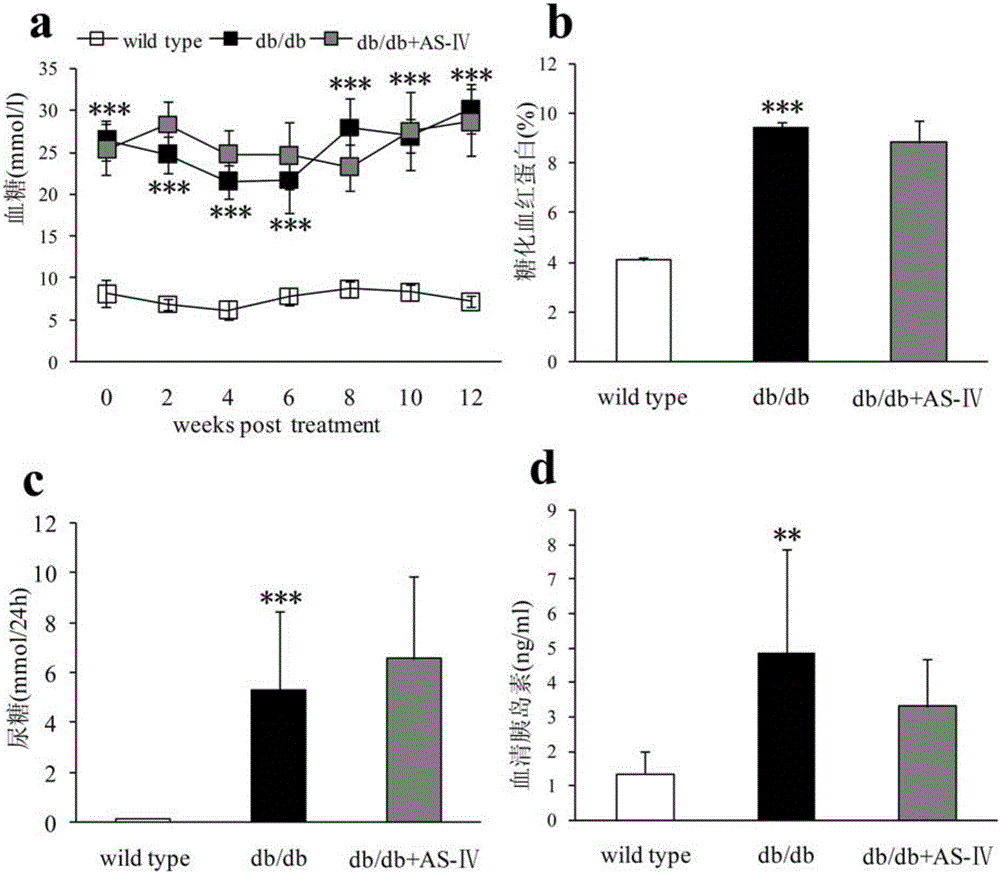
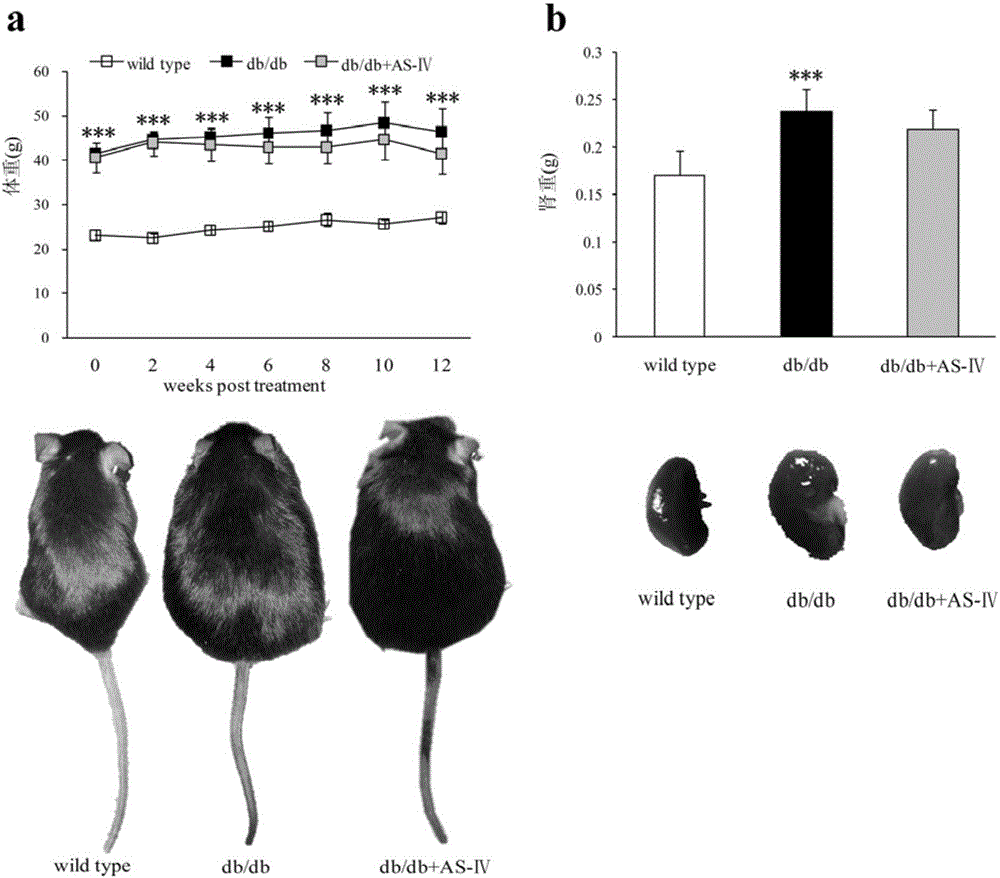
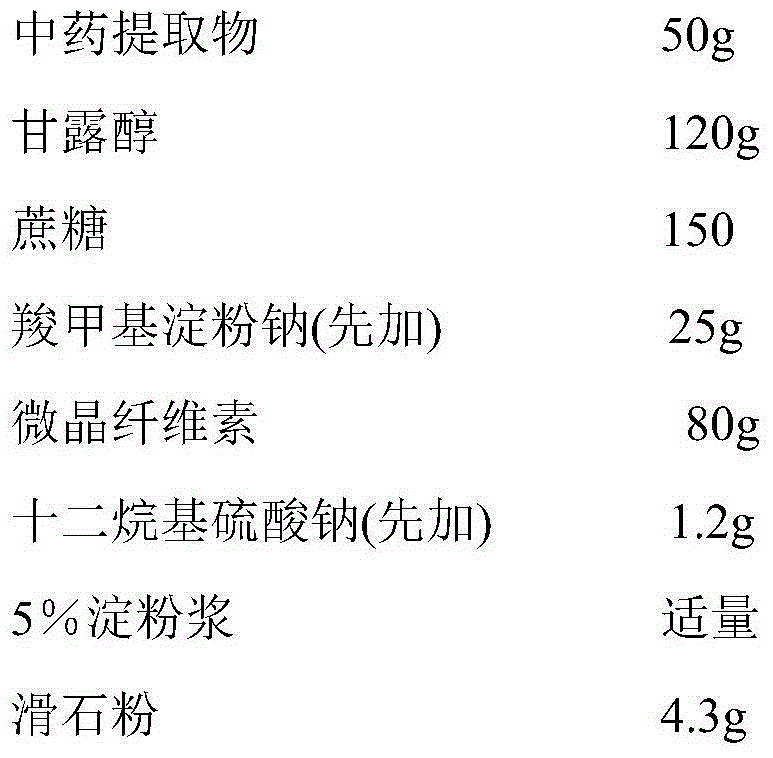
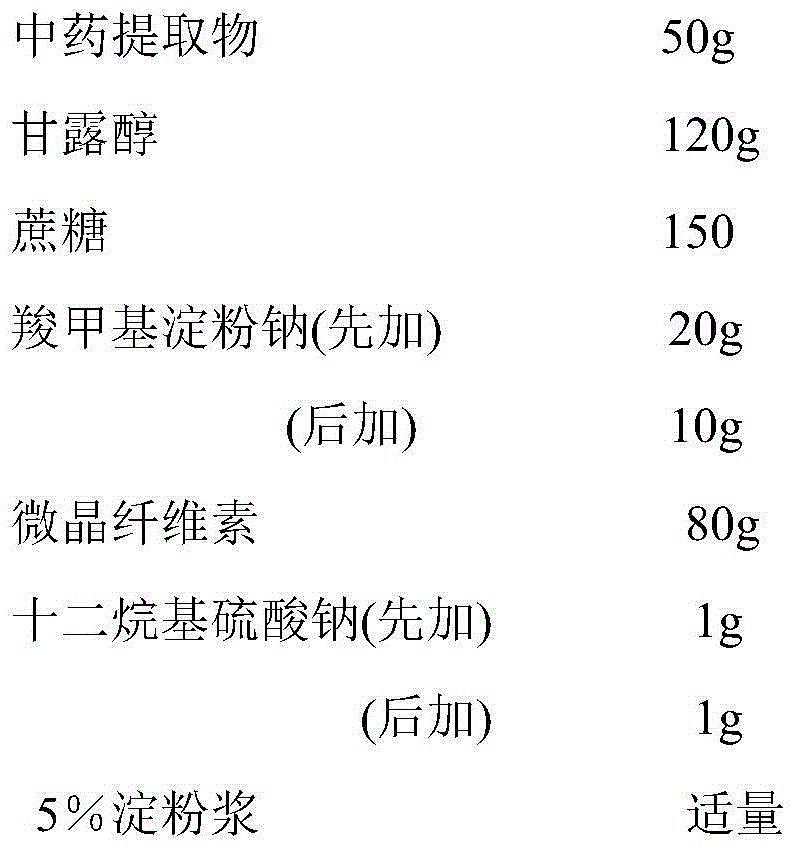
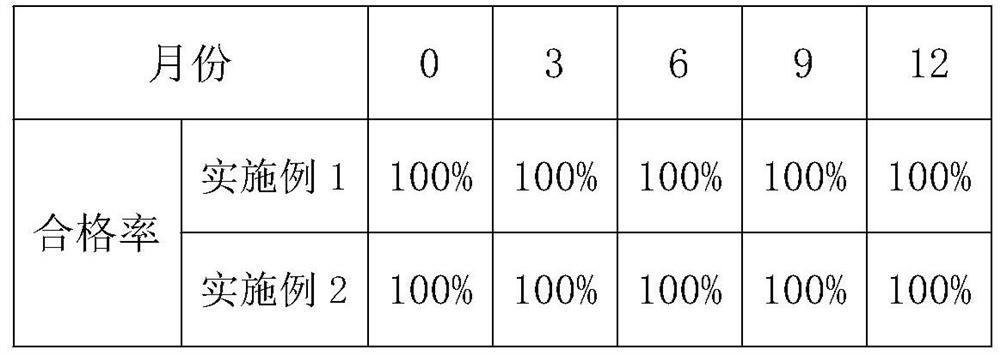
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Urinary albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Urinary albumin. the presence of albumin, a protein, in the urine. Normally protein is not found in the urine because the spaces in the glomerular membrane of the kidney are too small to allow escape of protein molecules.
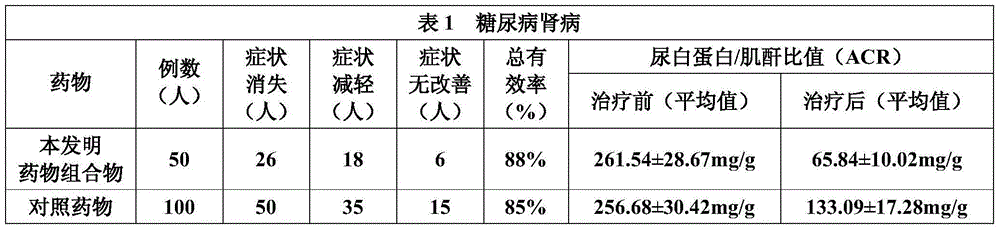
Chinese herbal extract for treating diabetic nephropathy and preparation method thereof
ActiveCN102228536AProtect kidney functionDefinite curative effectMetabolism disorderUrinary disorderRose hipCurative effect
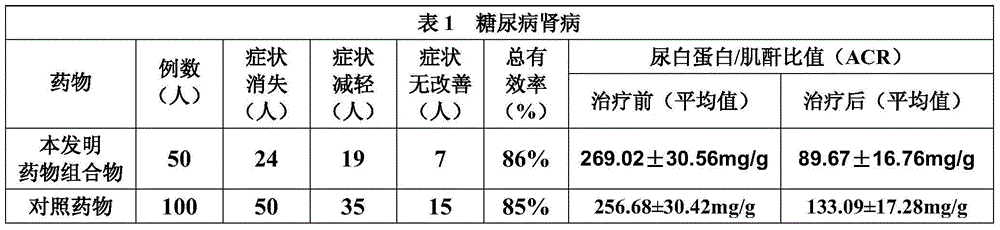
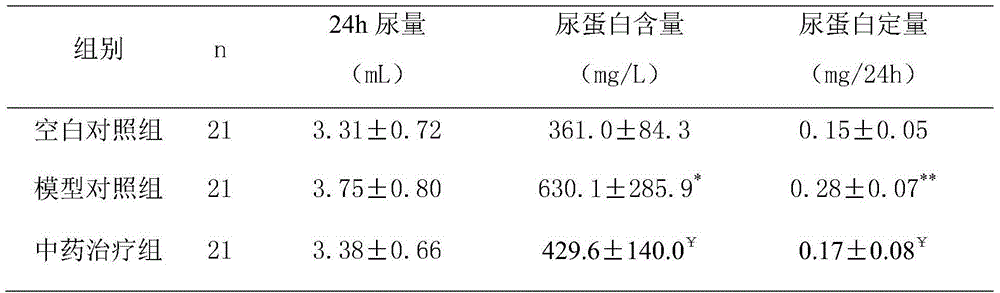
The invention discloses a Chinese herbal extract for treating diabetic nephropathy and a preparation method thereof. The Chinese herbal extract is prepared from 600 to 1000 parts by weight of Radix Astragali, 400 to 600 parts by weight of Gorgon fruit, 400 to 600 parts by weight of Cherokee rose-hip, 600 to 1000 parts by weight of Common achyranthes herb, 400 to 480 parts by weight of Ligustrum lucidum, 400 to 480 parts by weight of Ramulus Euonymi, and 320 to 480 parts by weight of Rheum officinale. Based on internationally unified clinical diagnostic criteria of diabetic nephropathy (DN), Compound particles of the Chinese herbal extract are carried out a comparison with Lotensin according to a randomized control principle through a contrast observation on 300 diabetic nephropathy patients taking respectively the compound particles and Lotensin, and a comparison result shows that 1) the compound particles can improve clinical symptoms of DN patients; and 2) the compound particles canreduce obviously urinary albumin in a DN early stage and urinary protein in a DN clinical stage, protect kidney functions and prevent effectively a development of early stage DN. Based on the prescribed curative effect evaluation criterion, a total effective rate of a Chinese herbal compound particle group is 86.7% and is obviously superior to that of a comparison group.
Owner:BEIJING HANDIAN PHARMA CO LTD +1
PH Sectional sequencing 13 item urine test paper
InactiveCN1888900AAvoid chemical changesAvoid interferenceBiological testingBilirubin+UrobilinogenCreatinine rise
A thirteen-item urine test paper with the pH value arranging in subsection relates to substrate and test paper mass fixed on it. From the top of the urine test paper to the handle ending, the arrangement sequence is white blood cell, acetone body, nitrite, urobilinogen, bilirubin, protein, dextrose, urine proportion, pH, occult blood, blank, uric creatinine, oxalate in urine, urinary albumin. It can avoid the disturb causing for great diversity of the pH value between each test paper mass by arranging in subsection according to different pH value. It reduces the disturb to the least causing for the flow and diffuse of the need test liquid, and prevents some test paper mass with high or low pH value changing for exposure in the air too much time. Its substrate length and effective test length are corresponding to the existing urine test paper and it matches to the existing test appearance.
Owner:URIT MEDICAL ELECTRONICS CO LTD
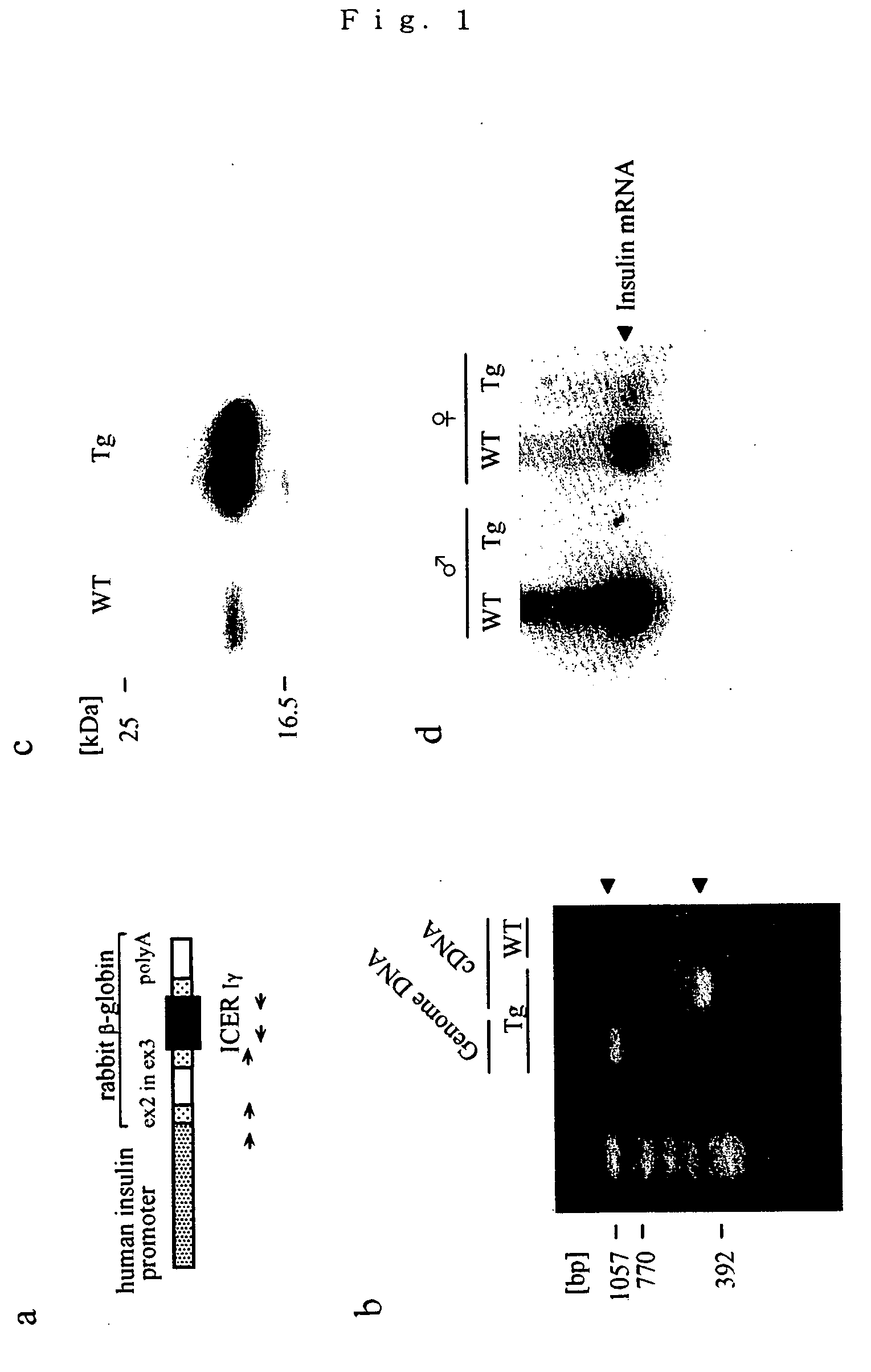
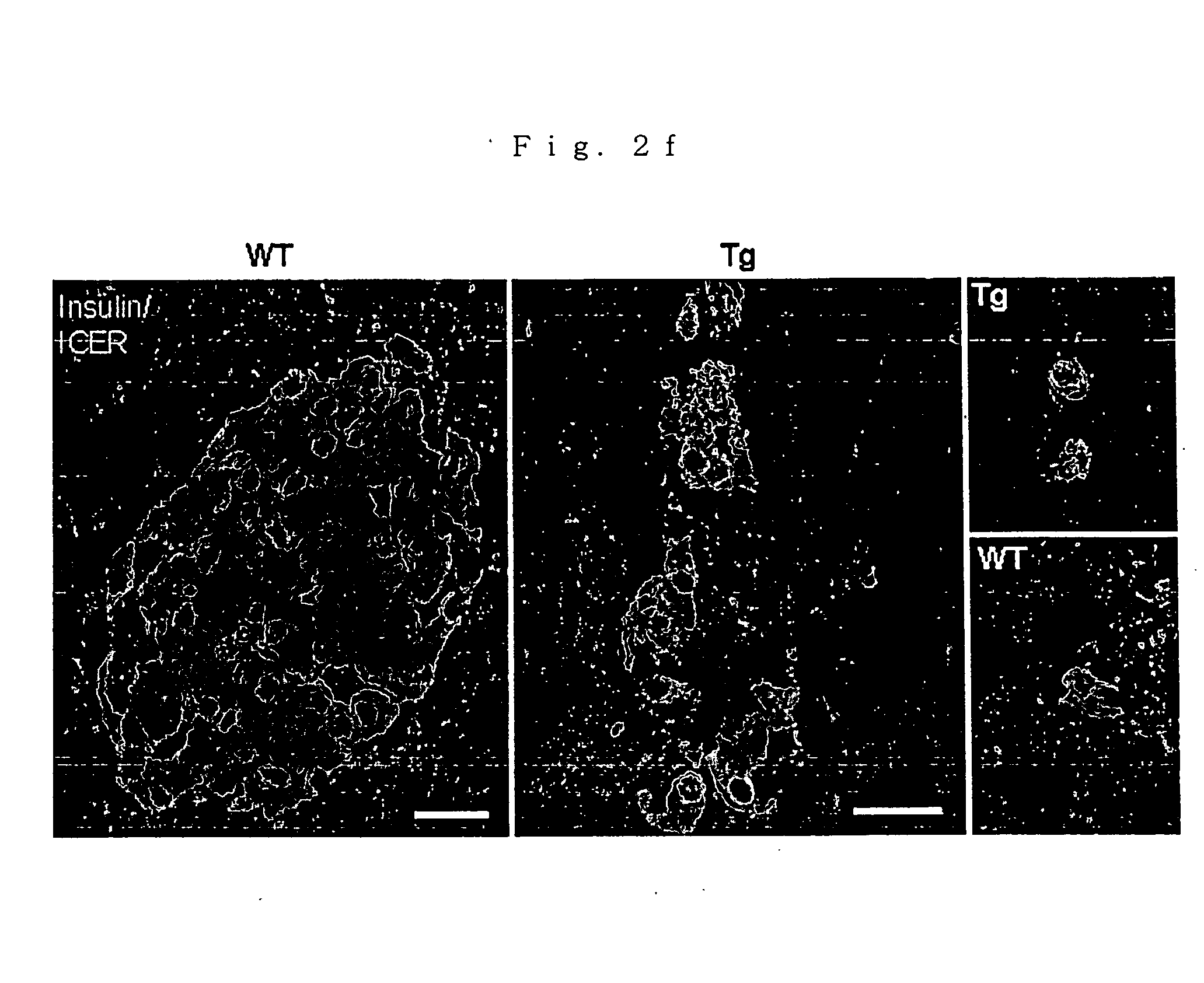
Nephropathy-associated gene
A nephropathy-associated gene which encodes a transcription repressor; and a nonhuman transgenic animal suffering from nephropathy which is constructed by transferring the above gene and allows the observation of increases in urinary volume, urinary albumin and urinary NAG, pyelectasis, enlargement in kidney tubule and glomerular swelling at the early stage and the following sclerosis.
Owner:INADA AKARI +1
Telmisartan medicinal composition, telmisartan medicinal composition tablets and preparation method for telmisartan medicinal composition tablets
ActiveCN102526037AGood water solubilityHas a diuretic effectOrganic chemistryHydroxy compound active ingredientsTherapeutic effectMagnesium stearate
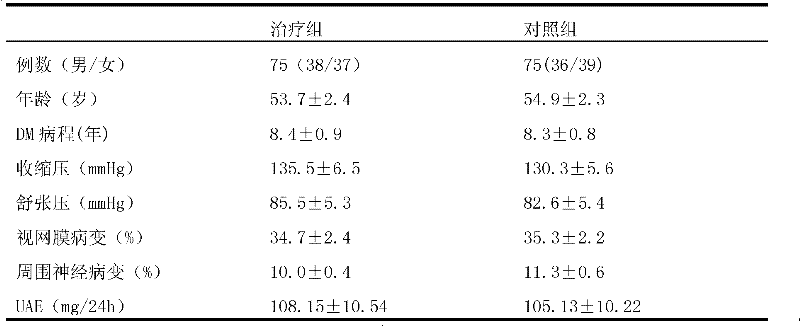
The invention provides a telmisartan medicinal composition, telmisartan medicinal composition tablets and a preparation method for the telmisartan medicinal composition tablets. The composition consists of the following components: telmisartan sodium salt, sorbitol, polacrilin potassium, anhydrous calcium phosphate, lactose, microcrystalline cellulose and magnesium stearate. The invention provides a preparation method for the telmisartan sodium salt, reaction conditions are mild, few side reactions are performed, and the generated sodium salt has higher water solubility; meanwhile, the sorbitol in the medicine has a diuresis effect, and has a synergistic antihypertensive effect with telmisartan in human bodies, so that the therapeutic effect of the product is strengthened; and after the telmisartan is taken, the urinary albumin excretion rate (UAER) of patients with early diabetic nephropathy is remarkably reduced, so the telmisartan has a kidney protection effect.
Owner:CHONGQING CONQUER PHARML
Microscale urinary albumin colloidal gold detection kit and preparation technology thereof
The invention relates to the field of colloidal gold immunization chromatography, and discloses a microscale urinary albumin colloidal gold detection kit. The detection kit comprises a reagent strip, wherein the reagent strip comprises a substrate, filtering sample paper, an immunization colloidal gold paper sheet, an immunization cellulose nitrate film and absorbent paper, wherein the filtering sample paper, the immunization colloidal gold paper sheet, the immunization cellulose nitrate film and the absorbent paper are successively connected with the each other end to end and are fixed on the substrate; and the immunization colloidal gold paper sheet is coated with a human albumin resistant monoclonal antibody I labeled by colloidal gold, and the immunization cellulose nitrate film is provided with a detection line which is coated with a human albumin resistant monoclonal antibody II and a quality control line which is coated with goat anti mouse IgG. The microscale urinary albumin colloidal gold detection kit provided by the invention has the advantages of rapid response, convenience in detection, stable detection result and cost-saving property.
Owner:SHANGHAI CHEMTRON BIOTECH
Micro fluidic chip apparatus used for immunization analysis
InactiveCN103170378ARapid determinationEasy to measureLaboratory glasswaresBiological testingReaction zoneUrinary albumin
The invention relates to a micro fluidic chip apparatus used for immunization analysis, which comprises a sample introduction part, a micro fluidic chip and a detector having light source; the micro fluidic chip comprises a sample introduction zone, a reaction zone and a detection zone, the sample introduction zone provides a mixing micro channel for mixing a sample to be measured and a reaction solution, a bending micro channel with a specific microstructure on the inner wall is provided in the reaction zone for fully mixing the sample to be measured and the reaction solution, a detection micro channel as a detection optical path optical path is provided in the detection zone, a detector light source irradiates a reaction mixed liquor in the detection micro channel for determining the sample to be measured; and the mixing micro channel of the sample introduction zone, the bending micro channel of the reaction zone and the detection micro channel of the detection zone are communicated in order. The invention also provides the above mentioned micro fluidic chip used for determining urinary albumin.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI +1
Method for test on diabetic nephropathy
InactiveUS20120164667A1Inhibit progressEarly detectionImmunoglobulins against animals/humansTransmissivity measurementsPodocalyxinUrinary albumin
Provided is a test method for the detection of diabetic nephropathy at an early stage as compared to a conventional method. Specifically provided are: a test method for diabetic nephropathy, including detecting urinary podocalyxin; the test method, further including assessing diabetic nephropathy at least Stage I; a test reagent for use in the test method; and a test reagent kit for use in the test method. The present invention is based on a finding that urinary podocalyxin reflects the development and condition of diabetic nephropathy with high sensitivity at an early stage as compared to urinary albumin.
Owner:NIIGATA UNIVERSITY +3
Application of medicinal composition for treating diabetes mellitus
The invention provides application of a Tibetan medicinal composition in preparing a medicament for treating diabetes mellitus. The Tibetan medicinal composition consists of the following raw material drugs in parts by weight: 60 to 600 parts of gymnadenia conopsea, 30 to 300 parts of radix pleurospermi tibetanici, 40 to 400 parts of sealwort, 30 to 300 parts of radix mirabilis himalaicae, 40 to 400 parts of asparagus fern, 10 to 100 parts of cordyceps sinensis, 30 to 300 parts of cynomorium songaricum, 10 to 100 parts of caltrop, 4 to 45 parts of przewalskia tangutica, and 50 to 500 parts of myrobalan. Tests prove that the medicinal composition can remarkably improve the physical sign of a patient, and has remarkable improvement to fasting plasma-glucose (FPG), 2 hour postprandial blood glucose (PBG), urinary albumin excretion rate (UAE), microalbuminuria (MAV) and glycosylated hemoglobin (HbAlc).
Owner:SHANDONG JINHE DRUG RES DEV
Urinary microalbumin (U-mALb)/urinary creatinine (U-Cr) integrated assay bigeminy strip and preparation method thereof
InactiveCN106353511AEasy to detectEasy to operateDisease diagnosisBiological testingCreatinine riseUrine volume
The invention relates to a urinary microalbumin (U-mALb) / urinary creatinine (U-Cr) integrated assay bigeminy strip, comprising a sample area and reaction areas. The U-mALb / U-Cr integrated assay bigeminy strip is characterized in that the sample area comprises a diffusion membrane and blood filtering membranes; the reaction areas comprise two detection windows, and the two detection windows can be used for detecting the ratios of the U-mALb and the U-Cr at the same time; the sample area for detecting the U-Cr by adopting a dry chemical method is arranged at the position where the reaction area is positioned, and the reaction area for detecting the U-mALb by using a colloidal gold dry type immune chromatography technology is arranged at a position which is 0.5cm away from the sample area. The different reaction areas contain different reagent pads, and the reagent pads respectively and independently react with corresponding components in urine. Synchronous detection can be realized only by dripping the random urine of a person to be tested into the sample area, so that the U-mALb / U-Cr integrated assay bigeminy strip is very suitable for medical institutions or individuals. By using the U-Cr for correction, the influence, caused by a sampling way and urine volume, on the excretory amount of urinary albumin can be eliminated, so that the U-mALb / U-Cr integrated assay bigeminy strip can provide more accurate evaluation for the albumin excretion rate and has a greater clinical significance for preventing diabetic nephropathy.
Owner:GETEIN BIOTECH
Application of diacerein to prepare medicine for treating diabetic nephropathy
InactiveCN103110617AImprove pathological lesionsInhibition formationOrganic active ingredientsMetabolism disorderRenal cortexAdjuvant
The invention discloses an application of diacerein to prepare medicines for preventing and treating diabetic nephropathy. Diacerein is taken as a raw material of an active pharmaceutical ingredient and combined with a pharmaceutically-acceptable adjuvant to prepare a pharmaceutical composition for preventing and treating diabetic nephropathy, wherein the composition contains an effective treatment dose of the diacerein and a pharmaceutically-acceptable carrier. A research on studying the diacerein capability of protecting a diabetic rat kidney through adopting streptozotocin to induce an experimental diabetic rat model accidentally discovers that the diacerein can reduce the glycated hemoglobin level and urinary albumin excretion rate of the blood of the diabetic rat, inhibits renal cortex protein non-enzyme advanced glycation end products from forming and improves renal pathology and lesion.
Owner:KIDNEY DISEASES INST P L A
Drug composition for treating diabetic nephropathy, preparation method and uses thereof
ActiveCN104971102AClear effectGood curative effectMetabolism disorderUrinary disorderTreatment effectCreatinine rise
The invention belongs to the field of medicine, and particularly relates to a drug composition for treating diabetic nephropathy, a preparation method and uses thereof. The technical problem solved by the present invention is to provide a completely-new drug composition for treating diabetic nephropathy. According to the drug composition of the present invention, caulis sinomenii, astragalus complanatus, milkvetch root and coptis chinensis are adopted as main bulk drugs to achieve the diabetic nephropathy treating purpose; and rosa laevigata michx and euryale ferox can further be added so as to further improve the treatment effect of the drug composition. According to the present invention, the most typical symptom of the diabetic nephropathy is nodular glomerulosclerosis and mainly the excretion amount of the urinary albumin is adopted as the diagnostic indicator, and after patients take the drug composition, the proteinuria excretion is significantly reduced, the 24 h urine total protein level and the urinary albumin excretion rate are reduced, the urinary albumin / creatinine ratio is reduced, the serum creatinine level is lowered, the life quality of patients is significantly improved, and the progress of the diabetic nephropathy disease is delayed.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
Application of artemisinin derivative to preparation of medicine for preventing and treating 2-type diabetes mellitus and complications of diabetes mellitus and medicine composition
InactiveCN108403686AIncrease respiratory quotientLower fasting blood sugarOrganic active ingredientsMetabolism disorderAlbumin excretionUrinary albumin
The invention relates to an application of an artemisinin derivative to preparation of a medicine for preventing and treating 2-type diabetes mellitus and complications of the 2-type diabetes mellitusand a medicine composition containing the artemisinin derivative. According to the application, experiments confirm that the artemisinin derivative such as artemether can increase the respiration quotient of the 2-type diabetes mellitus, oxidation of metabolic substrate glucose is facilitated, blood glucose of the 2-type diabetes mellitus is reduced, polydipsia and polyuria symptoms are improved,and urinary albumin excretion is decreased. The artemisinin derivative has an obvious protection effect on the 2-type diabetes mellitus and the complications thereof.
Owner:SHENZHEN TRADITIONAL CHINESE MEDICINE HOSPITAL
Application of niclosamide ethanolamine salt in preparing diabetes type 1 treating medicines
ActiveCN106420684AImprove drinkingLower levelOrganic active ingredientsMetabolism disorderMuscle functionsKidney
The invention relates to a novel application of niclosamide ethanolamine salt, and in particular to the application of the niclosamide ethanolamine salt in preparing medicines for preventing and treating diabetes type 1 and complications thereof; and the invention also conducts studies on the protective effect and the action mechanism of the niclosamide ethanolamine salt on the diabetes type 1 and the complications thereof. Results show that the niclosamide ethanolamine salt can improve symptoms of polyuria, polydipsia and polyphagia in a mouse with the diabetes type 1, reduce blood glucose, glycosylated hemoglobin and urine glucose levels, increase a serum insulin level and improve pancreas pathological damage. In the aspect of protecting a kidney target organ, the niclosamide ethanolamine salt can reduce a urinary albumin excretion rate, lower a creatinine clearance rate, diminish the area of glomerular vascular loops, reduce NAG and NGAL discharge in urine and inhibit activation of Akt / mTOR / 4E-BP1 signaling pathways in kidney tissues. In addition, the niclosamide ethanolamine salt can also take an obvious protecting effect on liver and achieve a significant improving effect on muscle functions. Therefore, the niclosamide ethanolamine salt has a significant protective effect on the diabetes type 1 and the complications thereof.
Owner:SHENZHEN TRADITIONAL CHINESE MEDICINE HOSPITAL
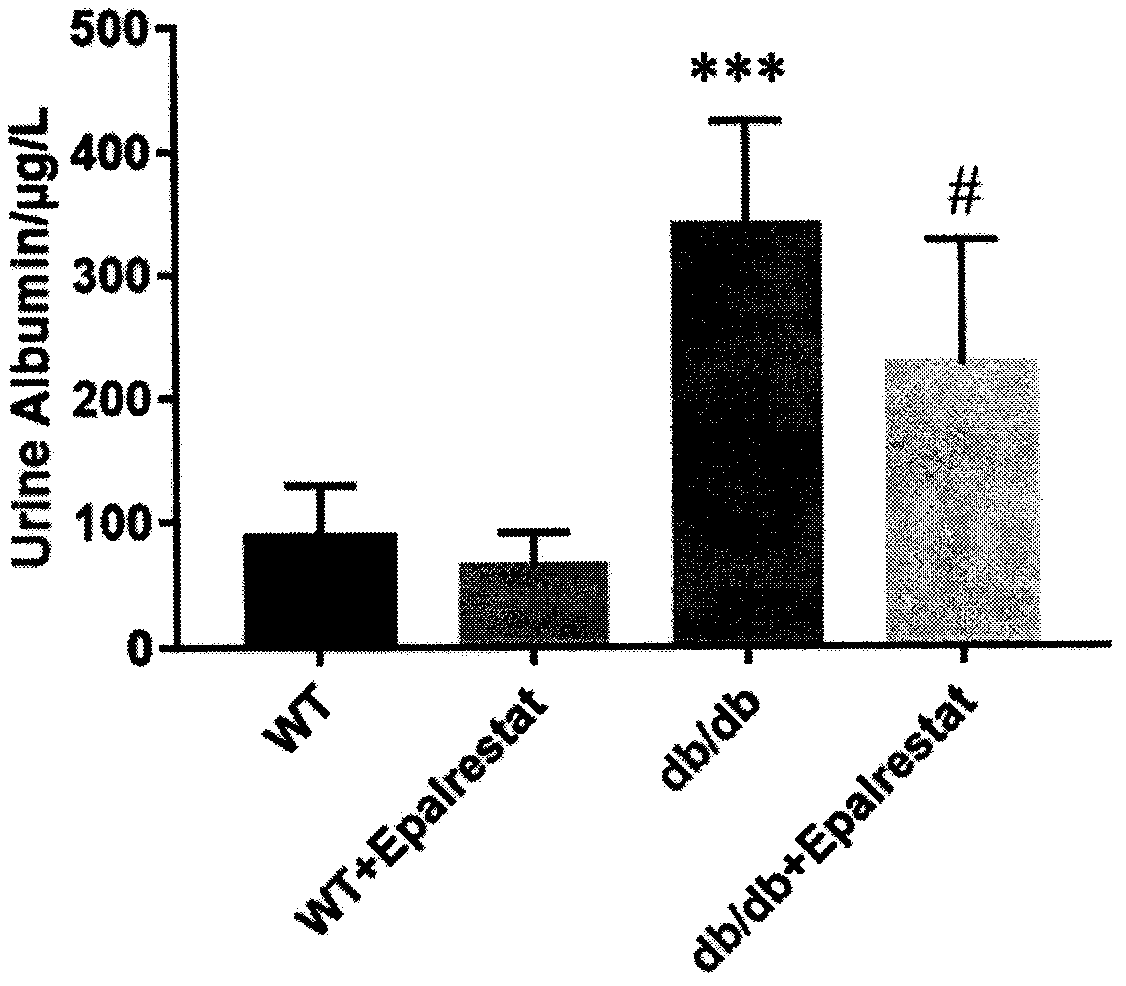
Application of epalrestat in preparation of drug for treating diabetic nephropathy
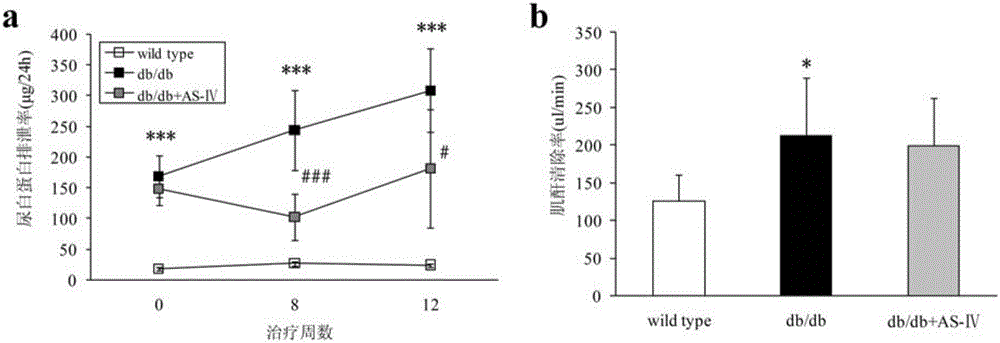
The invention discloses an application of epalrestat in the preparation of a drug for treating diabetic nephropathy, relating o the technical field of biological medicine. The epalrestat is an only on-sale aldose reductase inhibitor in China and is administered to a db / db mouse through gavage, so that the urinary albumin excretion of the db / db mouse is remarkably reduced, and the pathological injury of the kidney is improved. The orally administered epalrestat disclosed by the invention has a good kidney protection effect, and a candidate drug is provided for the kidney protection of crowds with clinic diabetic nephropathy.
Owner:CHINA PHARM UNIV
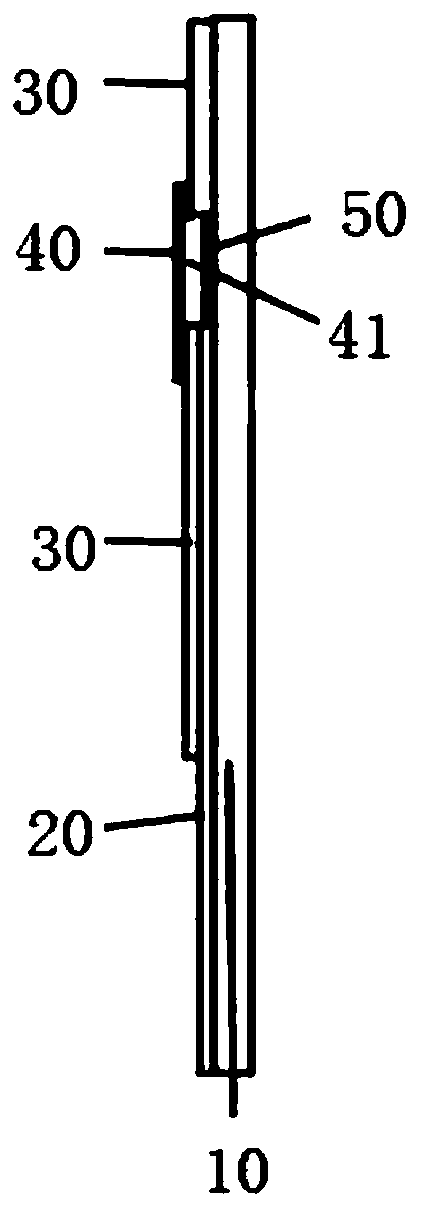
Manufacturing method of urinary albumin test electrode
InactiveCN110487865AGuaranteed accuracyImprove accuracyMaterial electrochemical variablesCross-linkProper time
The invention discloses a manufacturing method of a urinary albumin test electrode. The manufacturing method comprises the steps of preparing a PET substrate; manufacturing a graphene working electrode, a reference electrode and a counter electrode; printing high-molecular insulating paint on the PET substrate except an insertion end and a reaction area by adopting a printing method to form an insulating layer; uniformly coating a cross-linking agent and a urinary albumin selective reactant solution on a sample reaction area taking graphene as a working electrode, silver / silver chloride as a reference electrode and carbon as a counter electrode by a spin-coating method, drying at a temperature of 39-42 DEG C, covering the reaction area with a layer of PVC insulating film to form a sample inlet, and thus obtaining the urinary albumin test electrode. The urinary albumin test electrode has the advantages of short consumed time and high working efficiency during detection; in addition, thecontent of the urinary albumin can be detected at proper time, and the difficulty of collecting a 24-hour urine sample during detection in the prior art is avoided.
Owner:安徽信灵检验医学科技股份有限公司
Application of oxymatrine in preparation of medicine for prevention and treatment of diabetic nephropathy
ActiveCN103432124AGood hypoglycemic effectGood treatment effectOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaSide effect
The invention discloses an application of oxymatrine in preparation of a medicine for prevention and treatment of diabetic nephropathy. The oxymatrine with different purity has remarkable hypoglycemic activity and high sugar-induced renal injury repair effect, and can lower the kidney hypertrophy degree caused by diabetes mellitus. The oxymatrine with different purity can remarkably inhibit the increase of urine protein and urinary albumin excretion caused by hyperglycemia, and can delay formation of proteinuria, thereby having the effect on treating the diabetic nephropathy. The oxymatrine with different purity can remarkably inhibit the oxidative stress increase and inflammatory reaction caused by hyperglycemia, and the therapy is one way to treat the diabetic nephropathy with the oxymatrine. In comparison with the traditional Chinese medicine positive Xiaoke pill, the oxymatrine has a more remarkable treatment effect when used for preventing and treating the diabetic nephropathy; and in comparison with the chemical drug positive metformin hydrochloride, the oxymatrine has a better treatment effect and side effects are reduced obviously.
Owner:杨中林
Application of astragaloside in preventing and treating type 2 diabetic nephropathy
InactiveCN106309465AReduce excretionLow excretion rateOrganic active ingredientsMetabolism disorderAstragalosideBacteriuria
The invention relates to novel application of astragaloside, in particular to application of astragaloside in preventing and treating type 2 diabetic nephropathy, and provides a study on the protective effect and acting mechanism of astragaloside for type 2 diabetic nephropathy. The results indicate that the astragaloside is capable of reducing urinary albumin excretion rate in type 2 diabetic db / db mouse, improving glomerulus and renal tubule pathological injury, and reducing excretion of urine NAG, NGAL and TGF-Beta1. The astragaloside is also capable of inhibiting activation of Akt / mTOR, NFkB and Erk1 / 2 signal channels, with no significant hepatotoxicity present. In total, the astragaloside can protect type 2 diabetic nephropathy, and its acting mechanism is associated with the inhibition of Akt and its related signal channels.
Owner:SHENZHEN TRADITIONAL CHINESE MEDICINE HOSPITAL
Pharmaceutical composition for treating anaphylactoid purpura and preparation method of pharmaceutical composition
ActiveCN104667020AIncrease contentReduce contentImmunological disordersCardiovascular disorderLycopus lucidusActive component
The invention discloses a pharmaceutical composition for treating anaphylactoid purpura and a preparation method of the pharmaceutical composition. The pharmaceutical composition comprises an active component and a pharmaceutically acceptable auxiliary material, wherein the active component is prepared from lysimachia christinae hance, semen plantaginis, lygodium japonicum, corn stigma, oldenlandia diffusa, houttuynia cordata, lalang grass rhizome, crotalaria albida, salviae miltiorrhizae, flos carthami, peach kernel, lycopus lucidus, dianthus superbus, herba leonuri, caulis spatholobi, China rose, rhizoma panacis majoris, cortex moutan, radix scrophulariae, red peony root, radices lithospermi, cynanchum atratum, dried rehmannia root, eclipta, rhizoma anemarrhenae, scutellaria baicalensis, fructus forsythia and polygonum bistorta. The pharmaceutical composition is capable of lowering the quantity of urinary albumin of a patient and the content of serum circulating immune complex, so that the target of curing anaphylactoid purpura is reached.
Owner:北京古楼疑难病医学研究院有限公司
Drug composition for treating diabetic nephropathy, preparation method and uses thereof
ActiveCN104971101AClear effectGood curative effectMetabolism disorderUrinary disorderDiseaseTherapeutic effect
The invention belongs to the field of medicine, and particularly relates to a drug composition for treating diabetic nephropathy, a preparation method and uses thereof. The technical problem solved by the present invention is to provide a completely-new drug composition for treating diabetic nephropathy. According to the drug composition of the present invention, caulis sinomenii, astragalus complanatus, milkvetch root, coptis chinensis and safflower are adopted as main bulk drugs to achieve the diabetic nephropathy treating purpose; and rosa laevigata michx and euryale ferox can further be added so as to further improve the treatment effect of the drug composition. According to the present invention, the most typical symptom of the diabetic nephropathy is nodular glomerulosclerosis and mainly the excretion amount of the urinary albumin is adopted as the diagnostic indicator, and after patients take the drug composition, the proteinuria excretion is significantly reduced, the 24 h urine total protein level and the urinary albumin excretion rate are reduced, the urinary albumin / creatinine ratio is reduced, the serum creatinine level is lowered, the life quality of patients is significantly improved, and the progress of the diabetic nephropathy disease is delayed.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
Experiment analysis method for blood glucose reducing efficacy of Shenlian decoction rapidly disintegrating tablets for reducing blood glucose
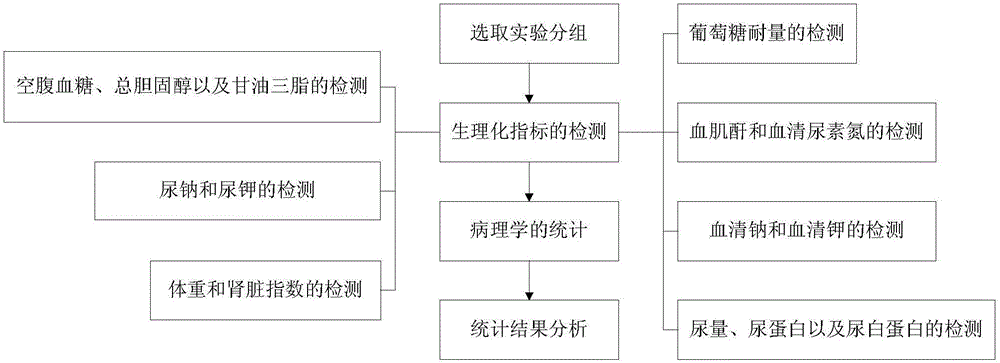
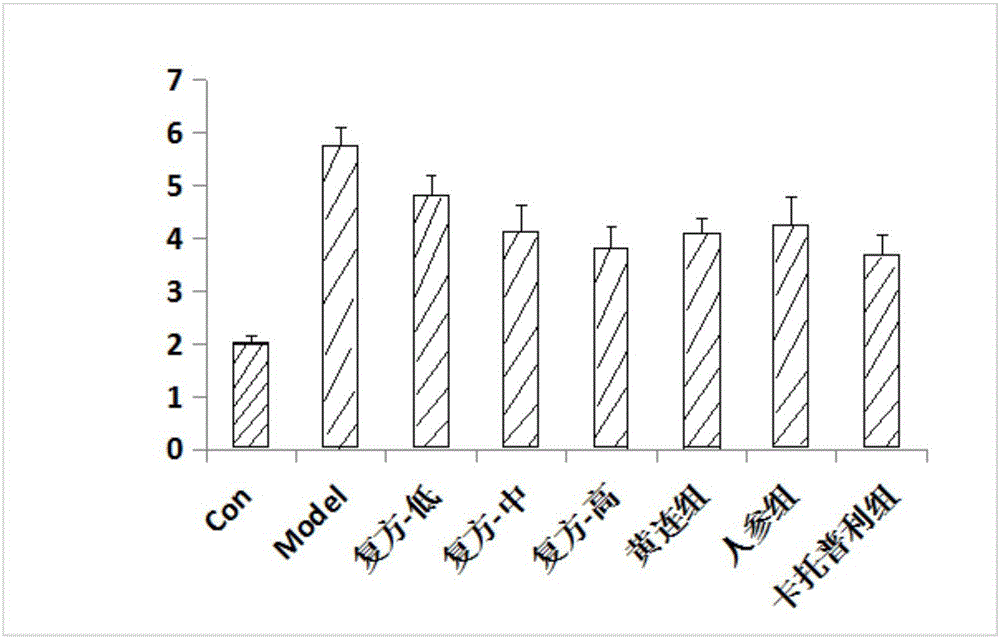
The invention provides an experiment analysis method for blood glucose reducing efficacy of Shenlian decoction rapidly disintegrating tablets for reducing blood glucose and belongs to the technical field of traditional Chinese medicine research. The method comprises the following steps of step one, selection of experiment grouping; step two, detection of physiological indicators, including detection of mice fasting blood-glucose, total cholesterol and triglyceride, detection of glucose tolerance, detection of urine volume, urine protein and urinary albumin, detection of serum creatinine and serum urea nitrogen, detection of serum sodium and serum potassium, detection of urine sodium and urine potassium and detection of weight and kidney indexes; step three, statistics of pathology; step four, analysis of statistical results; according to the experiment analysis method, an effective constituent ginseng total saponin and coptis total alkaloid compound (RH for short) of the Shenlian decoction rapidly disintegrating tablets for reducing blood glucose can effectively reduce the blood glucose level of a diabetic patient and has a certain therapeutic effects for early diabetic nephropathy.
Owner:CHANGCHUN UNIV OF CHINESE MEDICINE
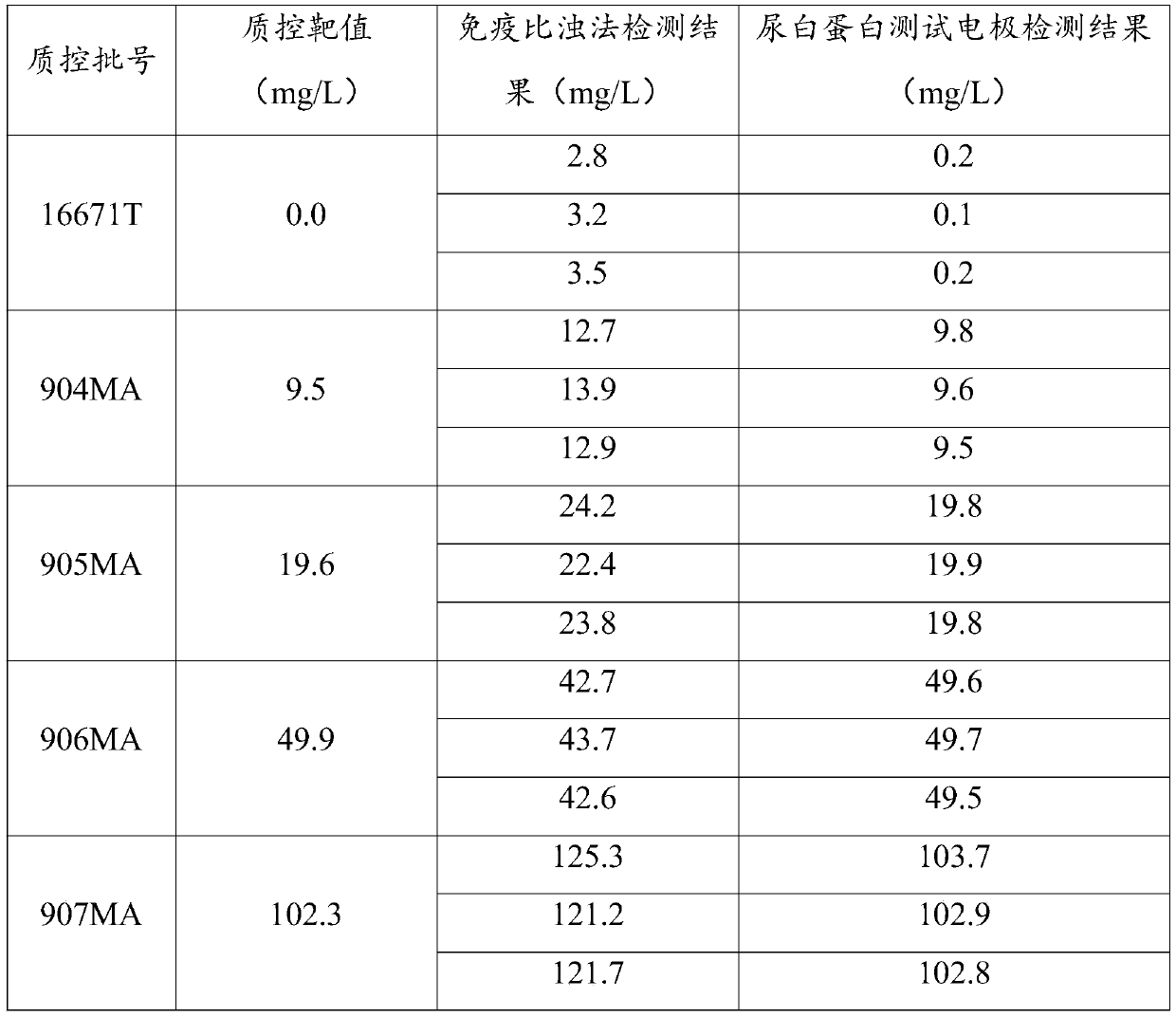
Urinary albumin/urinary creatinine composite quality control product and production method thereof
PendingCN112858690AReduce degradationPrevent oxidation precipitationBiological testingFlufenamic acidDextran
The invention provides a urinary albumin / urinary creatinine composite quality control product and a production method thereof. The urinary albumin / urinary creatinine composite quality control product comprises the following components: 50-100 mmol / L of a buffer solution, 5-15 g / L of salt, 5-15 g / L of flufenamic acid, 5-15 g / L of dimethyl sulfoxide, 5-15 g / L of a reducing agent, 5-15 g / L of dextran, 5-15 g / L of a defoaming agent; 0.5-1 g / L of a tackifier, 100-200 mg / L of human serum albumin, 10-20 mmol / L of creatinine, and 1 g / L of a preservative. A pH value of the quality control product is 7.2-7.4. The product can be used for evaluating the accuracy of the urinary albumin / urinary creatinine detection reagent based on a microfluidic technology, and plays an important role in guaranteeing the laboratory detection result quality of the urinary albumin / urinary creatinine detection reagent and reducing errors.
Owner:NINGBO POLYTECHNIC
Traditional Chinese medicine compound preparation for treating diabetes and nephrosis and preparing method thereof
The invention relates to a traditional Chinese medicine compound medicament for treating diabetic nephropathy and process for preparation, belonging to the traditional Chinese medicine field. The invention is prepared into various solid dosage forms which are suitable to oral administration and is made from the following traditional Chinese medicine, raw glutinous rehmannia, salvia miltiorrhizae, epimedium, prepared rhubarb, and raspberry and appropriate amount of excipient. The invention is showed through animal experiments that fasting plasma glucose of diabetic nephropathy model rat is significantly reduced, plasma glucagons is lowered, the contents of urinary albumin and urine beta 2 microglobulin are decreased, and the level of pituitary growth hormone is reduced. The invention has the effects of lowering hyperglycemia, reducing urinary albumin, and protecting kidney functions, which has important significance and applicable value for the treatment of diabetic nephropathy.
Owner:中国人民解放军第八十五医院
Applications of Shenkang injection in preparation of medicine used for treatment of hypertensive nephropathy
ActiveCN103285096APharmaceutical delivery mechanismUrinary disorderSalvia miltiorrhizaCreatinine rise
The invention provides applications of Shenkang injection in medicine used for treatment of hypertensive nephropathy. The injection comprises following bulk drugs, by weight: 7 to 15 portions of rheum officinale, 6 to 15 portions of red sage root, 8 to 15 portions of carthamus tinctorius and 15 to 45 portions of radix astragali. It is confirmed by experiments that: the Shenkang injection may help to decrease blood pressure, and the content of urinary albumin microspheres, serum creatinine and urea nitrogen of rats with hypertensive nephropathy substantially; and may also help to improve renal blood flow of rats with hypertensive nephropathy, and adjust nitric oxide and endothelin levels. Pathologic results show that the Shenkang injection may improve and suppress damages of kidneys caused by hypertension. The Shenkang injection has substantial effects for treatment of hypertensive nephropathy.
Owner:XIAN SHIJISHENGKANG PHARMA IND
Stem cell preparation modified by long-acting GLP-1 gene, preparation method and application of stem cell preparation
InactiveCN112138025APromote regenerationFunction increaseOrganic active ingredientsSenses disorderBlood sugarUrinary albumin
The invention discloses a stem cell preparation modified by a long-acting GLP-1 gene, a preparation method and application of the stem cell preparation. An in vivo experiment proves that the stem cellpreparation containing a mesenchymal stem cell modified by the GLP-1 gene and ipriflavone has a good function of regulating blood sugar, meanwhile, a urea nitrogen, urine creatinine and urinary albumin excretion rate can be lowered, and a diabetic lower limb ischemia condition and a limb necrosis degree can be obviously lowered. The stem cell preparation disclosed by the invention has a good effect when the stem cell preparation is used for treating diabetes mellitus and complications thereof.
Owner:NORTHEAST NORMAL UNIVERSITY
Human urinary albumin latex enhanced secondary-antibody competitive immunity turbidity detection kit as well as production method and use method thereof
The invention discloses a human urinary albumin latex enhanced secondary-antibody competitive immunity turbidity detection kit as well as a production method and a use method thereof. By utilizing a secondary-antibody competitive immunity turbidity method, the defects of existing enhanced immunity turbidity methods are overcome from immune-reaction principles; when measuring that no antigen existsin a reaction system, a secondary antibody linked to a latex particle reacts with a primary antibody in the system to generate agglutination reaction, and the agglutination effect reaches the maximum; and when measuring that the antigen exists in the reaction system, the antigen is bound with the primary antibody to form an antigen-antibody (primary antibody) immune compound, a binding site between the primary antibody and the secondary antibody is closed by through the binding, the competition is formed with immune agglutination reaction of the primary antibody and the secondary antibody, and if the amount of the primary antibody is limited, the agglutination effect of a primary antibody-secondary antibody immune compound in a sample is competitively inhibited by the antigen, so that theagglutination effect of the immune compound generated through the reaction between the primary antibody and secondary antibody-latex is reduced along the increase of free antibodies in the reaction system.
Owner:长沙文瀚生物技术有限责任公司
A traditional Chinese medicine preparation for treating diabetic nephropathy, and preparation method and application thereof
InactiveCN101675950AReduce clearanceLower indexMetabolism disorderUrinary disorderAlkaloidDiabetic nephropathy syndrome
The present invention discloses a traditional Chinese medicine preparation for treating diabetic nephropathy, which is prepared from rhubarb polysaccharides, the total flavone of scutellaria root, total alkaloids of rhizoma coptis, and pharmaceutically acceptable auxiliary materials. Pharmacodynamic trials proved that the present invention has the effects of decreasing the blood glucose of the normal mouse, reducing the area under blood glucose concentration curve, and improving the sugar tolerance of the mouse; the present invention can improve the multi-food, the will drink, and the polyuriasymptoms of diabetic nephropathy model rats, reduce urinary protein and urinary albumin, decrease glycosylated hemoglobin, serum cholesterol and serum triglyceride, decrease overhigh creatinine clearance rate, reducing the renal index, alleviate the renal lesion, and have the effect for antagonizing early diabetic nephropathy. In addition, the present invention also discloses a preparation methodof the traditional Chinese medicine preparation and application in preparing a medicament for preventing and controlling diabetic nephropathy.
Owner:SHANGHAI UNIV OF T C M +1
Application of cyclic erythropoietin-derived peptide in protection of renal injury and cyclosporine A injury
PendingCN112076309AImprove survival rateCompounds screening/testingMicroencapsulation basedApoptosisThelial cell
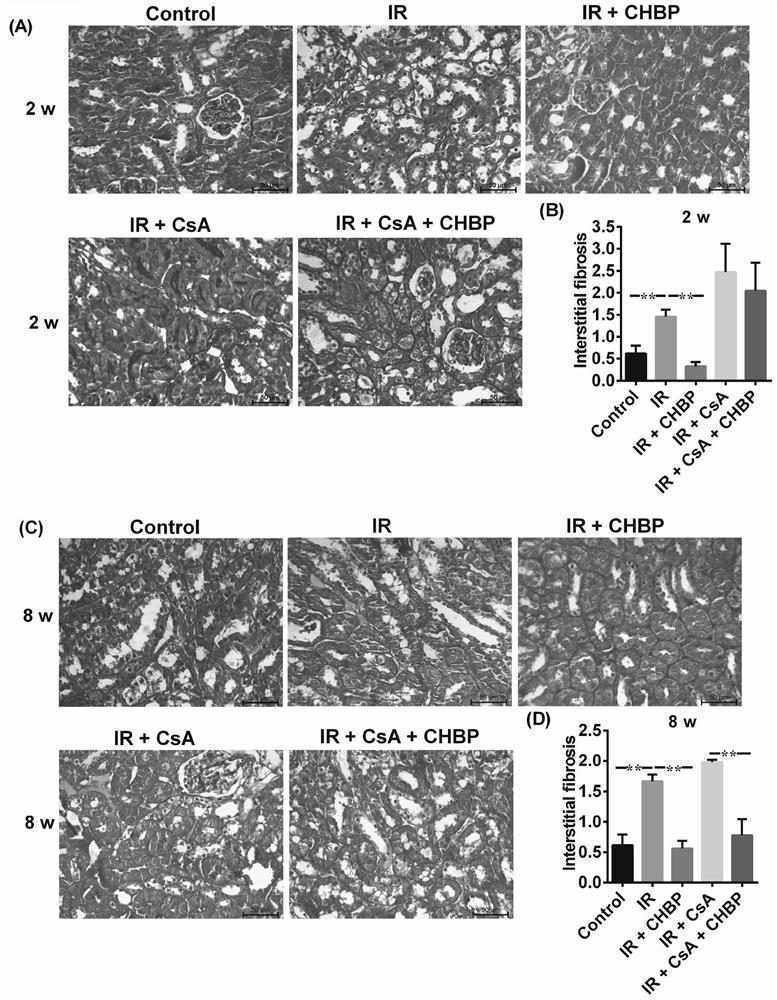
The invention discloses application of a cyclic erythropoietin-derived peptide in protection of renal injury and cyclosporine A injury. In a control group, the abdominal cavity and renal pedicle are exposed; in IR, two renal pedicles are separated, and are clipped with a vessel clamp for 30 minutes to enable color of the kidneys to be changed, and refilling is performed for 2 or 8 weeks; in IR plus CsA, CsA is dissolved in olive oil, and IR mice are subjected to intragastric administration every day; in IR plus CHBP, CHBP is dissolved in saline water, and the IR mice are subjected to intraperitoneal injection every three days; in IR plus CsA plus CHBP, CsA and CHBP are used for treating the IR mice at the same time; urine albumin / creatinine, serum creatinine, histology, apoptosis, caspase-3 and HMGB1 are evaluated, and intracellular signal channels are screened through a protein chip; and a renal epithelial cell model is established, renal injury is simulated, and the influence of CsA,CHBP and / or caspase-3 siRNA on TCMK1 is studied.
Owner:NANTONG UNIVERSITY +1
Urinalysis Test Card for Urine Microalbumin/Urine Creatinine
ActiveCN103575913BHigh sensitivityEasy to prepareDisease diagnosisBiological testingBacteriuriaUrinary albumin
The invention relates to a urine analysis and test card for urine microalbumin / urine creatinine. The urine analysis and test card for the urine microalbumin / urine creatinine comprises a sample pad, wherein a loading hole is formed in the sample pad. The urine analysis and test card for the urine microalbumin / urine creatinine is characterized in that a urine microalbumin test card and a urine creatinine test card are arranged on the sample pad respectively; one end of the urine microalbumin test card and one end of the urine creatinine test card are connected with the loading hole respectively; an albumin detection line and a first quality control line are arranged on the urine microalbumin test card; the albumin detection line is positioned at the end near to the loading hole; a urine creatinine detection line and a second quality control line are arranged on the urine creatinine test card; the urine creatinine detection line is positioned at the end near to the loading hole; a first detection hole is formed in the back side of the urine microalbumin test card and at the position corresponding to the albumin detection line; a second detection hole is formed in the back side of the urine creatinine test card and at the position corresponding to the urine creatinine detection line. The urine analysis and test card for the urine microalbumin / urine creatinine can solve the problem of interference caused by the situation that urine protein is singly used as a detection index; a preparation method is simple, and easy to operate.
Owner:无锡博慧斯生物医药科技有限公司
New use of Flatstem Milkvetch Seed
InactiveCN104127475AEasy dischargeImprove the quality of lifeMetabolism disorderUrinary disorderCreatinine riseSide effect
The invention belongs to the field of medicines, concretely relates to a new use of Flatstem Milkvetch Seed, and especially relates to a use of Flatstem Milkvetch Seed in the preparation of diabetic nephropathy treatment medicines. A technical scheme to be solved is providing the new use of Flatstem Milkvetch Seed, and concretely the use of Flatstem Milkvetch Seed in the diabetic nephropathy treatment medicines, and the use of Flatstem Milkvetch Seed in the preparation of diabetic nephropathy treatment medicines as the only active component. The adoption of Flatstem Milkvetch Seed to treat the diabetic nephropathy reduces the proteinuria discharge amount, reduces the urinary albumin / creatinine ratio, reduces the serum creatinine level, improves the survival quality of patients, delays the diabetic nephropathy development, and has the advantages of definite curative effect, no side effects and the like.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
Kidney-protecting probiotic composition
PendingCN112007052AReduce congestionInhibit the inflammatory responseOrganic active ingredientsUnknown materialsBiotechnologyDisease
The invention discloses a kidney-protecting probiotic composition, and belongs to the field of all related food industries of probiotics. The composition comprises lactobacillus acidophilus, bifidobacterium longum, bifidobacterium bifidum and xylooligosaccharide powder. The kidney-protecting probiotic composition disclosed by the invention is a composition of probiotics and prebiotics, and can beused for effectively regulating blood pressure and relieving kidney blockage. Meanwhile, the yield of short-chain fatty acid is increased, the levels of blood urea nitrogen, serum creatinine and urinary albumin are reduced, the condition of renal fibrosis is improved, the inflammatory reaction of the kidney is inhibited, and the progress of kidney diseases is further relieved. Through the combination of the probiotics and the prebiotics, the composition can help a human body to regulate intestinal flora, and is a food supplement or medicine which can be safely taken for a long time and can effectively improve chronic kidney diseases.
Owner:上海泓商生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com