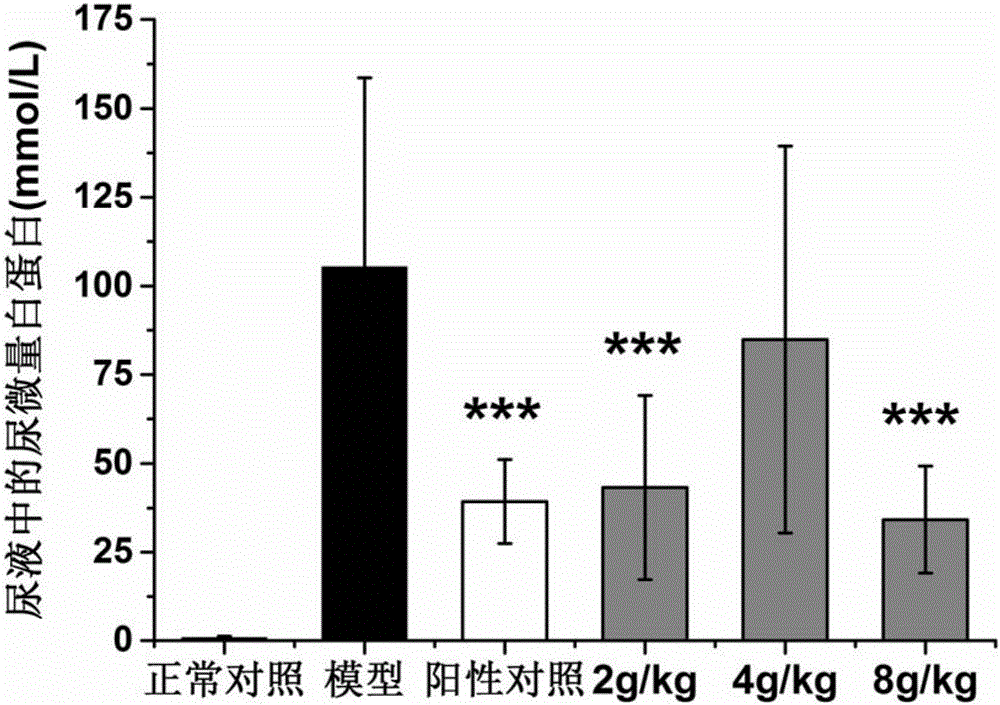
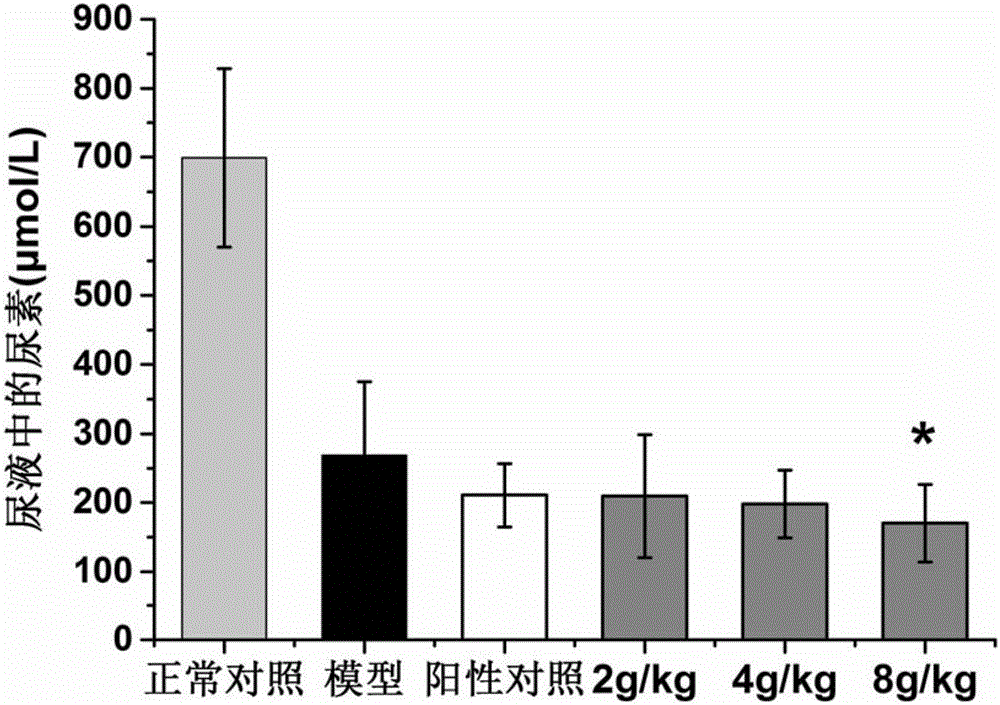
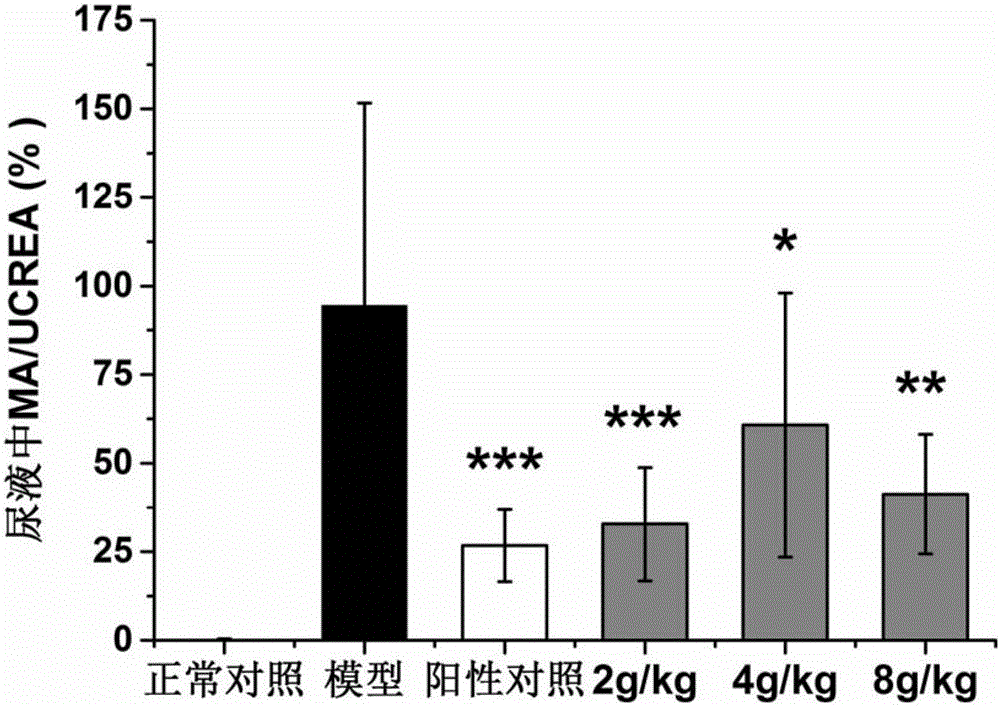
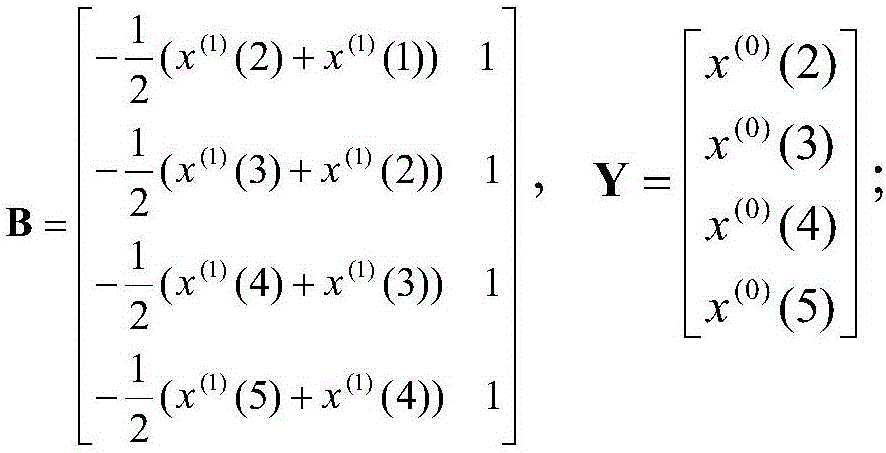Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Urine Creatinine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Renal monitor
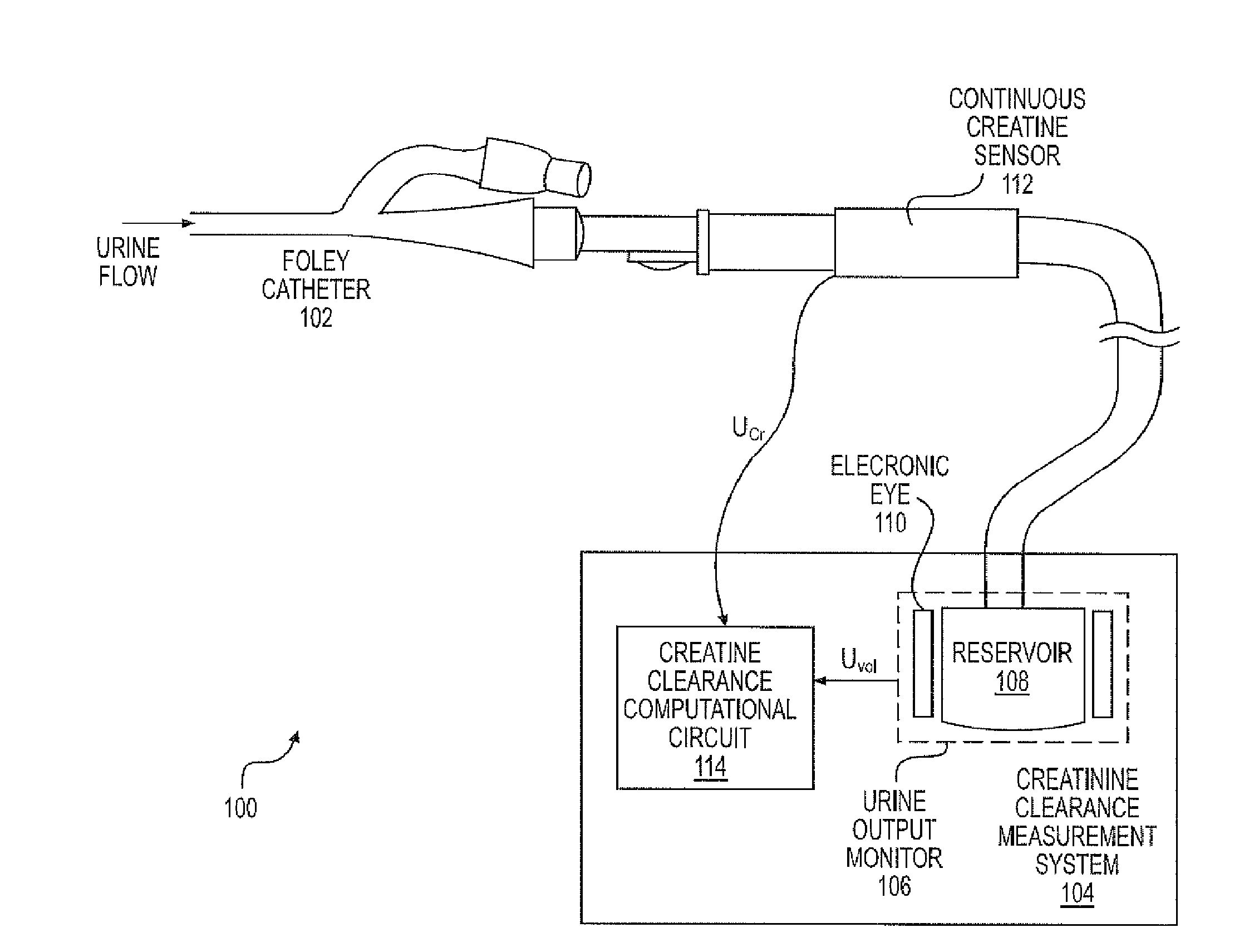
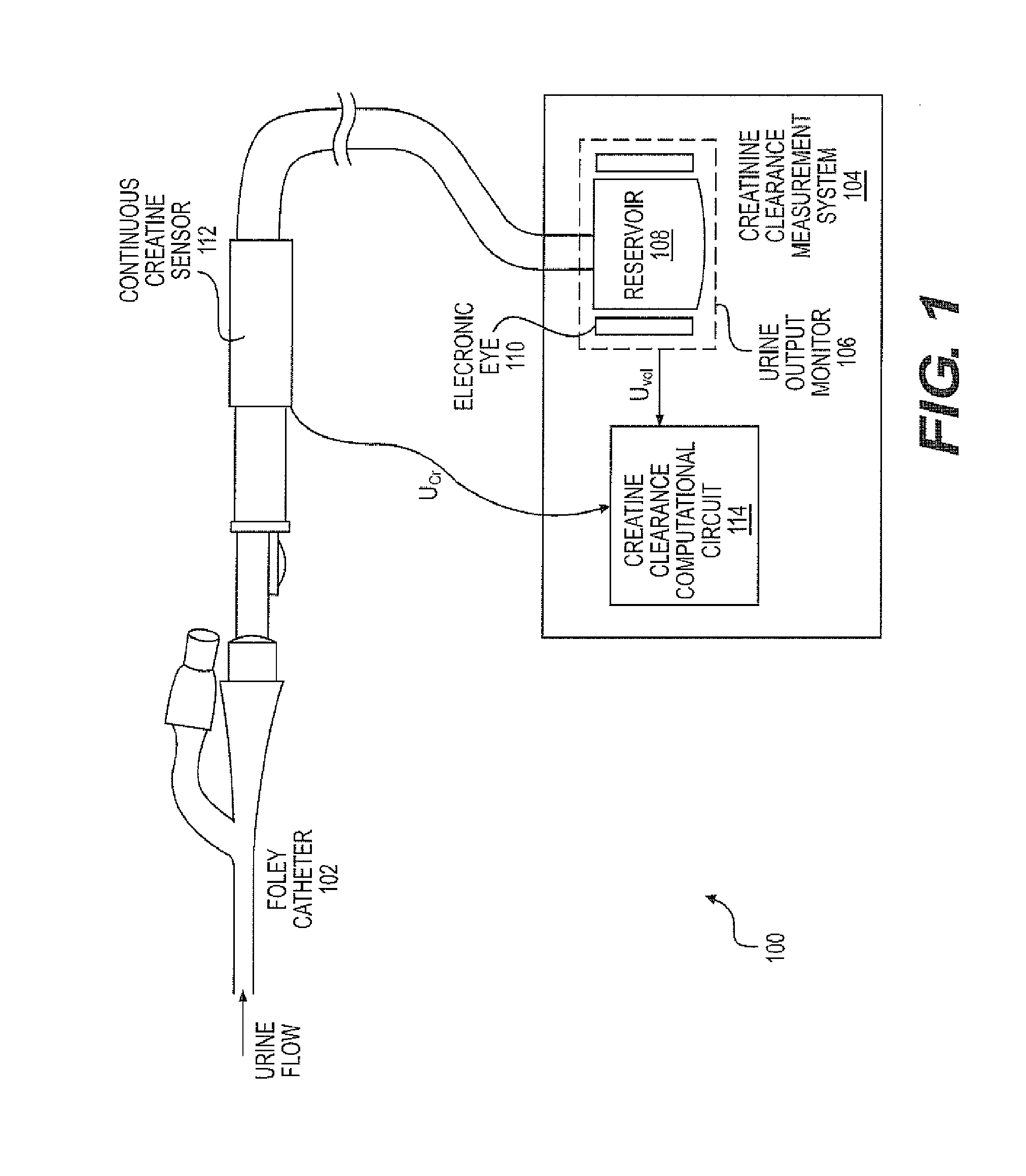
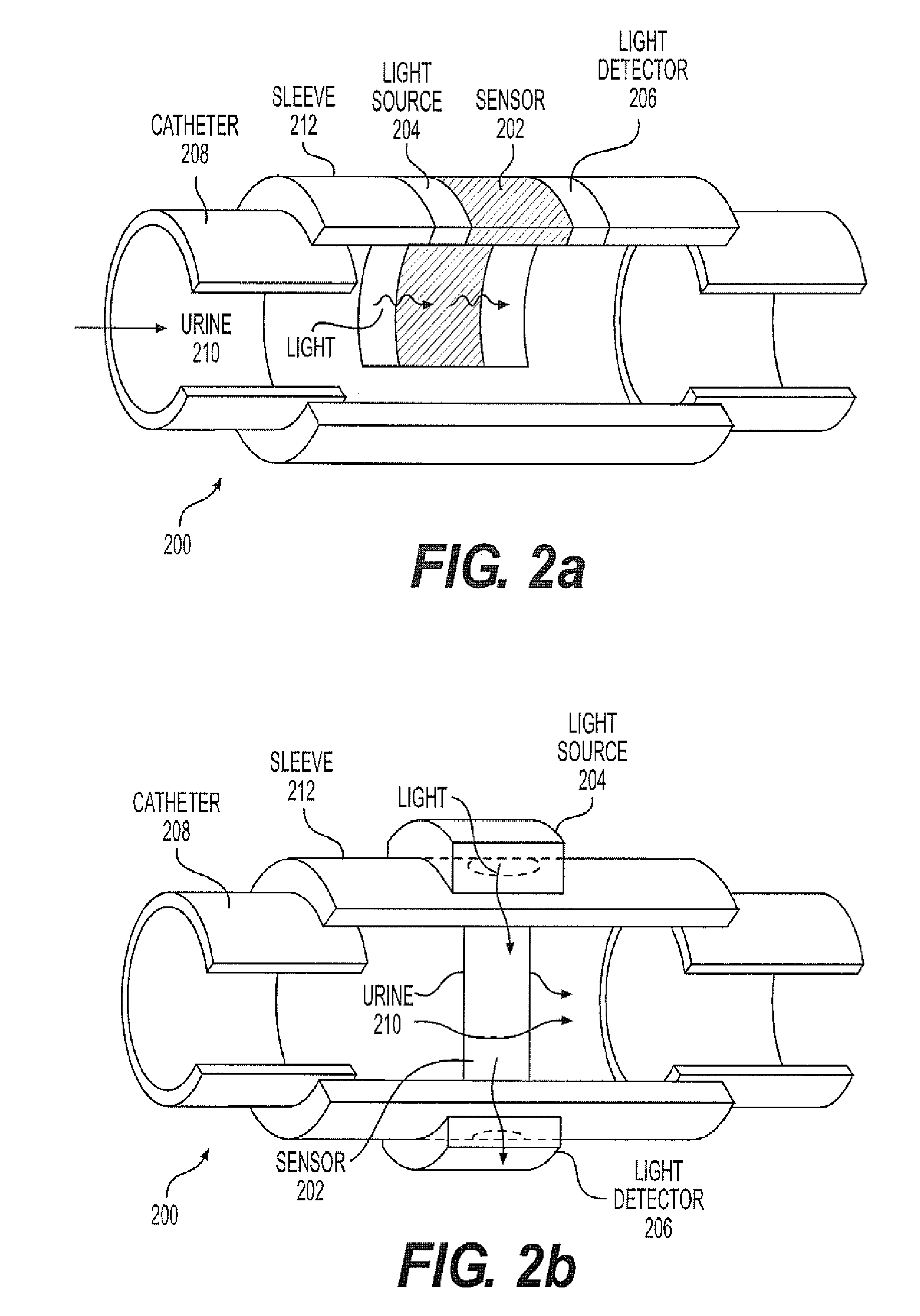
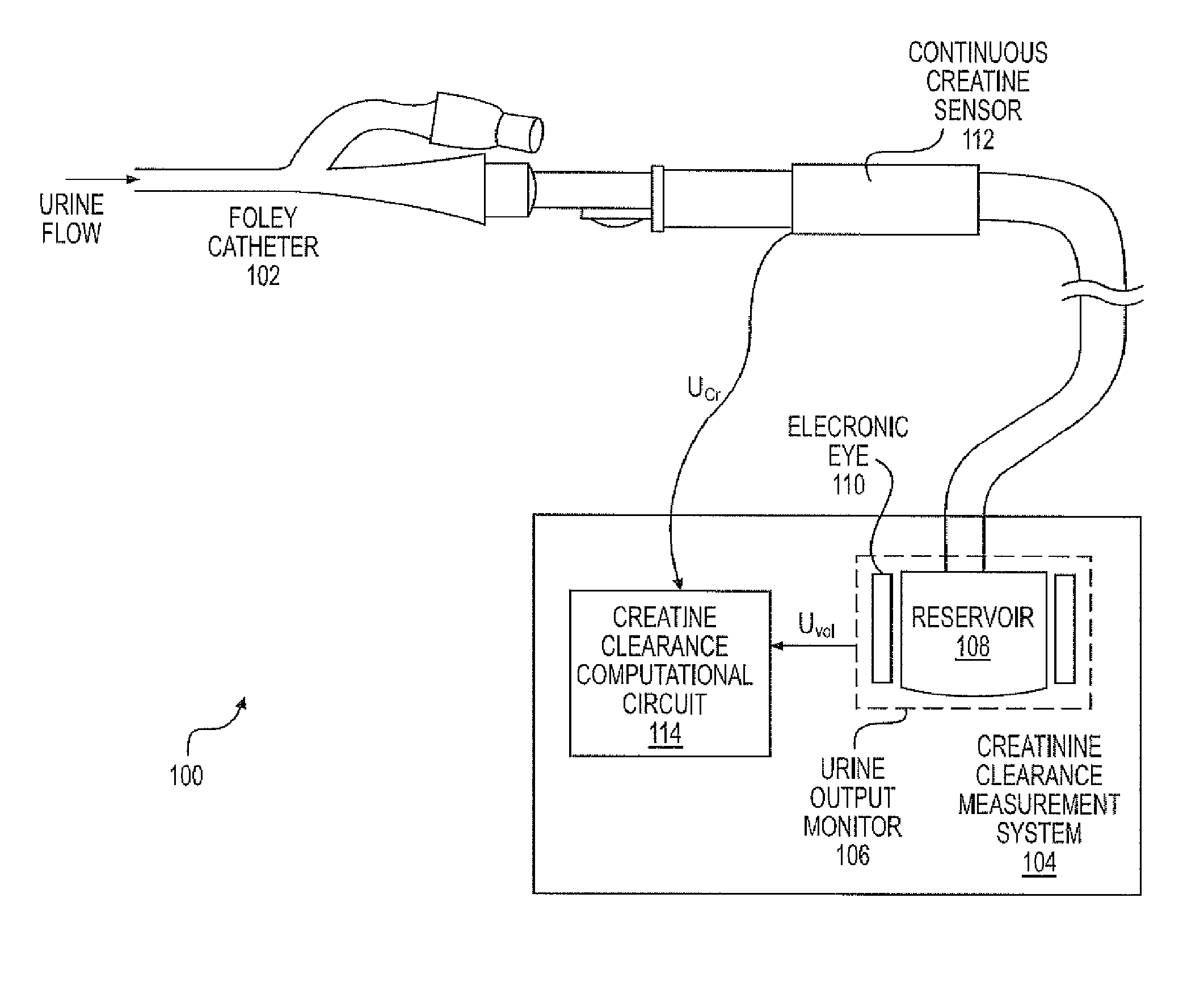
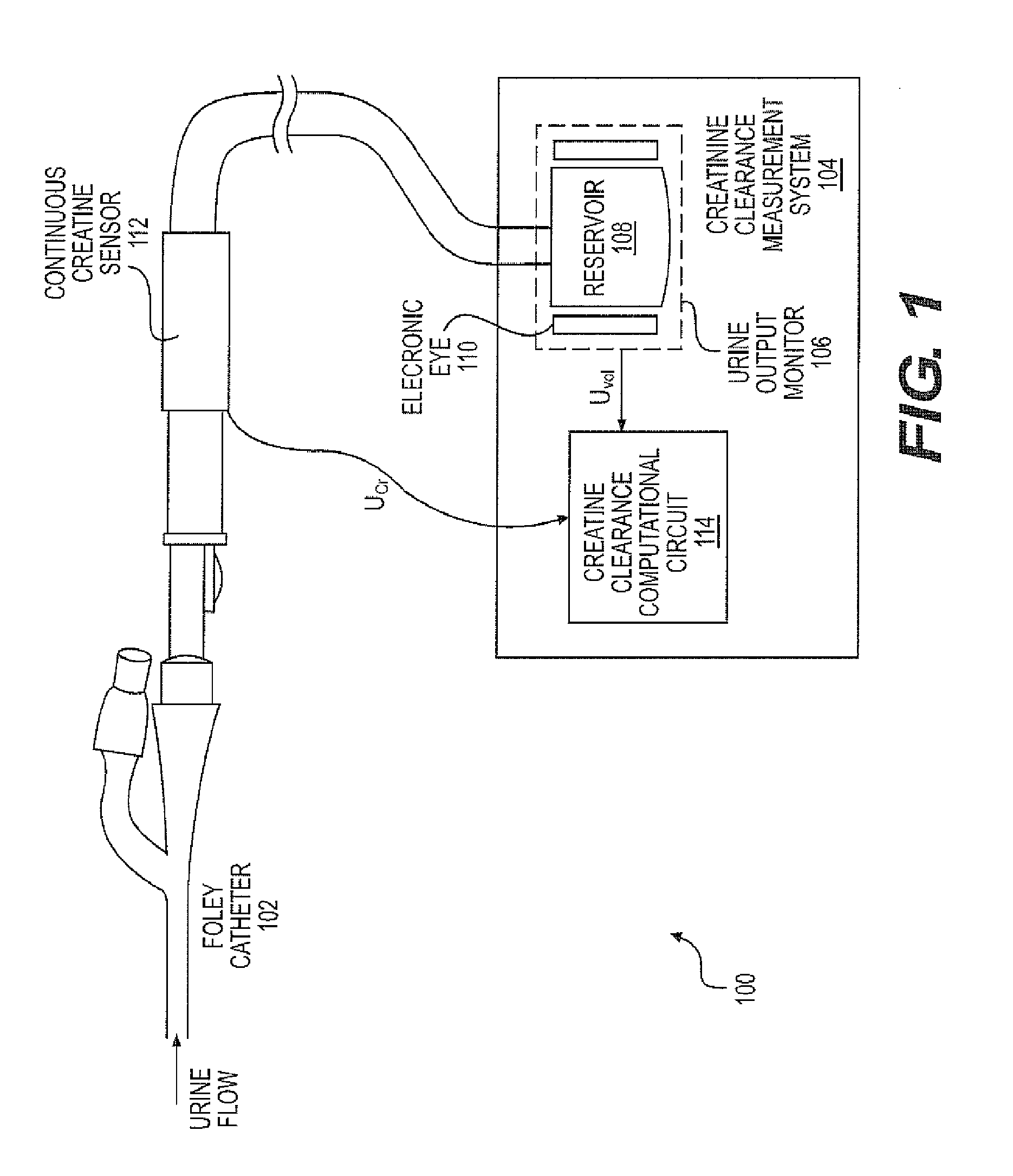
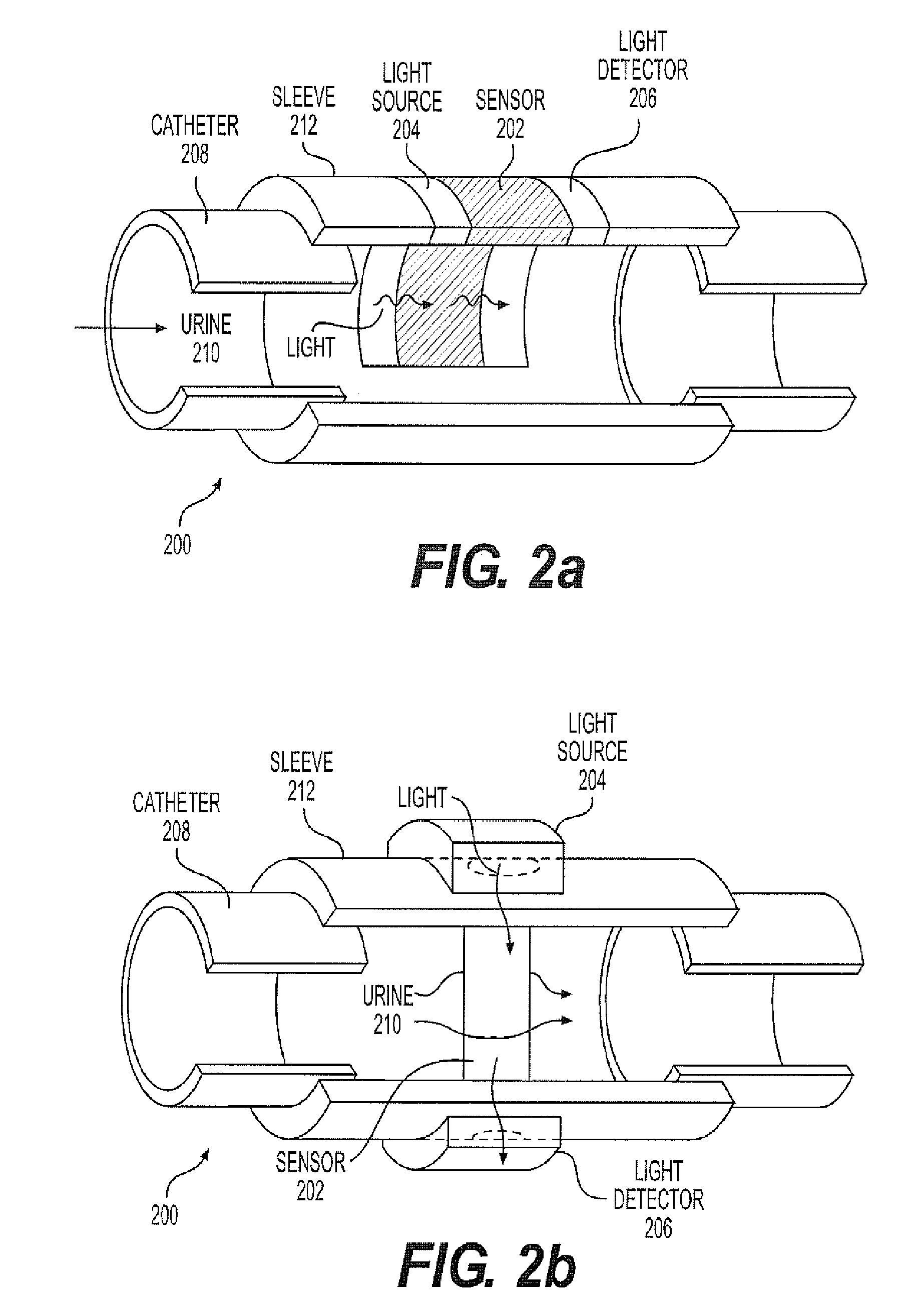
A continuous Glomerular Filtration Rate (GFR) estimation system may include a Foley catheter, a continuous urine creatinine sensor, and a urine output monitor. The continuous GFR estimation system computes creatinine clearance as CrCl=(Ucr×Uvol) / (Pcr×Imim), where Ucr is urine creatinine in mg / dL, Uvol is urine volume in mL, Pcr is plasma (serum) creatinine in mL, and Imin is time in minutes. A Foley catheter may be used to withdraw urine from the bladder. The urine may be delivered to a urine output monitor that provides the Uvol value over a time Imin. Attached to the catheter is the flow-through continuous urine creatinine sensor for providing the Ucr value. The remaining parameter is Pcr. Because serum creatinine levels do not change rapidly over time, a blood sample may be withdrawn prior to the start of the continuous GFR to obtain the PCr value.
Owner:CR BARD INC
Urine analysis and test card for urine microalbumin/urine creatinine
ActiveCN103575913ASolution rangeAddress operational complexityDisease diagnosisBiological testingAlbuminUrine creatinine test
The invention relates to a urine analysis and test card for urine microalbumin / urine creatinine. The urine analysis and test card for the urine microalbumin / urine creatinine comprises a sample pad, wherein a loading hole is formed in the sample pad. The urine analysis and test card for the urine microalbumin / urine creatinine is characterized in that a urine microalbumin test card and a urine creatinine test card are arranged on the sample pad respectively; one end of the urine microalbumin test card and one end of the urine creatinine test card are connected with the loading hole respectively; an albumin detection line and a first quality control line are arranged on the urine microalbumin test card; the albumin detection line is positioned at the end near to the loading hole; a urine creatinine detection line and a second quality control line are arranged on the urine creatinine test card; the urine creatinine detection line is positioned at the end near to the loading hole; a first detection hole is formed in the back side of the urine microalbumin test card and at the position corresponding to the albumin detection line; a second detection hole is formed in the back side of the urine creatinine test card and at the position corresponding to the urine creatinine detection line. The urine analysis and test card for the urine microalbumin / urine creatinine can solve the problem of interference caused by the situation that urine protein is singly used as a detection index; a preparation method is simple, and easy to operate.
Owner:无锡博慧斯生物医药科技有限公司
Renal monitor
ActiveUS8715254B2Increase ratingsImprove filtration rateAnimal teeth treatmentCatheterFiltrationUrine volume
A continuous Glomerular Filtration Rate (GFR) estimation system may include a Foley catheter, a continuous urine creatinine sensor, and a urine output monitor. The continuous GFR estimation system computes creatinine clearance as CrCl=(Ucr×Uvol) / Pcr×Tmin), where Ucr is urine creatinine in mg / dL, Uvol is urine volume in mL, Pcr is plasma (serum) creatinine in mg / dL, and Tmin is time in minutes. A Foley catheter may be used to withdraw urine from the bladder. The urine may be delivered to a urine output monitor that provides the Uvol value over a time Tmin. Attached to the catheter is the flow-through continuous urine creatinine sensor for providing the Ucr value. The remaining parameter is Pcr. Because serum creatinine levels do not change rapidly over time, a blood sample may be withdrawn prior to the start of the continuous GFR to obtain the Pcr value.
Owner:CR BARD INC
Test paper for detecting urine creatinine and preparation method for test paper
InactiveCN102661949AAvoid hard to saveAvoid interferenceMaterial analysis by observing effect on chemical indicatorCopper sulfatePolyvinylpyrrolidone
The invention discloses a test paper for detecting urine creatinine and a preparation method for the test paper. The test paper belongs to in-vitro diagnosis test paper. The preparation method comprises the following steps: fully soaking a color sheet in a solution A, taking out the color sheet, drying at the temperature of between 70 and 100DEG C for 15 to 30 minutes, soaking the dried color sheet in a solution B, and drying at the temperature of between 70 and 100DEG C for 5 to 15 minutes, wherein each 1,000mL of solution A contains 2mol / L Tris buffer (with the pH of 6.0-8.0), 0.2 to 2.0g of copper sulfate, 1.5 to 5.0g of sodium citrate or potassium citrate, 100 to 200mg of orange G, and the rest of pure water; and each 1,000mL of solution B contains 10 to 20g of polyvinylpyrrolidone, 0.5 to 5.0g of reducing dyestuff and the rest of chloroform. The test paper is easy to prepare, stable in performance and easy to store, has obvious color gradation corresponding to different urine creatinine content, and is easily used by laboratory medicine of hospitals and nonprofessional inspectors.
Owner:NANJING UNIV OF TECH
Urine creatinine detection reagent strip and preparation method thereof
ActiveCN103630532AEasy to prepareEasy to operateMaterial analysis by observing effect on chemical indicatorReagent stripBromocresol green
The invention discloses a urine creatinine detection reagent strip and a preparation method thereof. The detection reagent strip comprises a substrate and a filter paper sheet, wherein the filter paper sheet is prepared by respectively sufficiently immersing filter paper in a solution A and a solution B, taking out and drying; the solution A is a 80-95v / v% ethanol solution comprising 3.2-4.8 wt% of buffer solution and 1-1.5 wt% of surfactant; and the solution B is a methanol solution comprising 0.8-1.2% of 3,5-dinitrobenzoic acid and 0.008-0.012% of bromocresol green. The urine creatinine detection reagent strip disclosed by the invention can quickly react with creatinine in the urine under alkaline conditions; the creatinine concentration is 0-26.4 mmol / 24h, and the test paper has an obvious color gradation from light blue to brown which can be easily visually distinguished and mechanically read; and the preparation method is simple and easy to operate, and has the advantages of stable performance and accurate detection result.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Urinary microalbumin (U-mALb)/urinary creatinine (U-Cr) integrated assay bigeminy strip and preparation method thereof
InactiveCN106353511AEasy to detectEasy to operateDisease diagnosisBiological testingCreatinine riseUrine volume
The invention relates to a urinary microalbumin (U-mALb) / urinary creatinine (U-Cr) integrated assay bigeminy strip, comprising a sample area and reaction areas. The U-mALb / U-Cr integrated assay bigeminy strip is characterized in that the sample area comprises a diffusion membrane and blood filtering membranes; the reaction areas comprise two detection windows, and the two detection windows can be used for detecting the ratios of the U-mALb and the U-Cr at the same time; the sample area for detecting the U-Cr by adopting a dry chemical method is arranged at the position where the reaction area is positioned, and the reaction area for detecting the U-mALb by using a colloidal gold dry type immune chromatography technology is arranged at a position which is 0.5cm away from the sample area. The different reaction areas contain different reagent pads, and the reagent pads respectively and independently react with corresponding components in urine. Synchronous detection can be realized only by dripping the random urine of a person to be tested into the sample area, so that the U-mALb / U-Cr integrated assay bigeminy strip is very suitable for medical institutions or individuals. By using the U-Cr for correction, the influence, caused by a sampling way and urine volume, on the excretory amount of urinary albumin can be eliminated, so that the U-mALb / U-Cr integrated assay bigeminy strip can provide more accurate evaluation for the albumin excretion rate and has a greater clinical significance for preventing diabetic nephropathy.
Owner:GETEIN BIOTECH
Kit for evaluating 24-hour urinary sodium value of woman
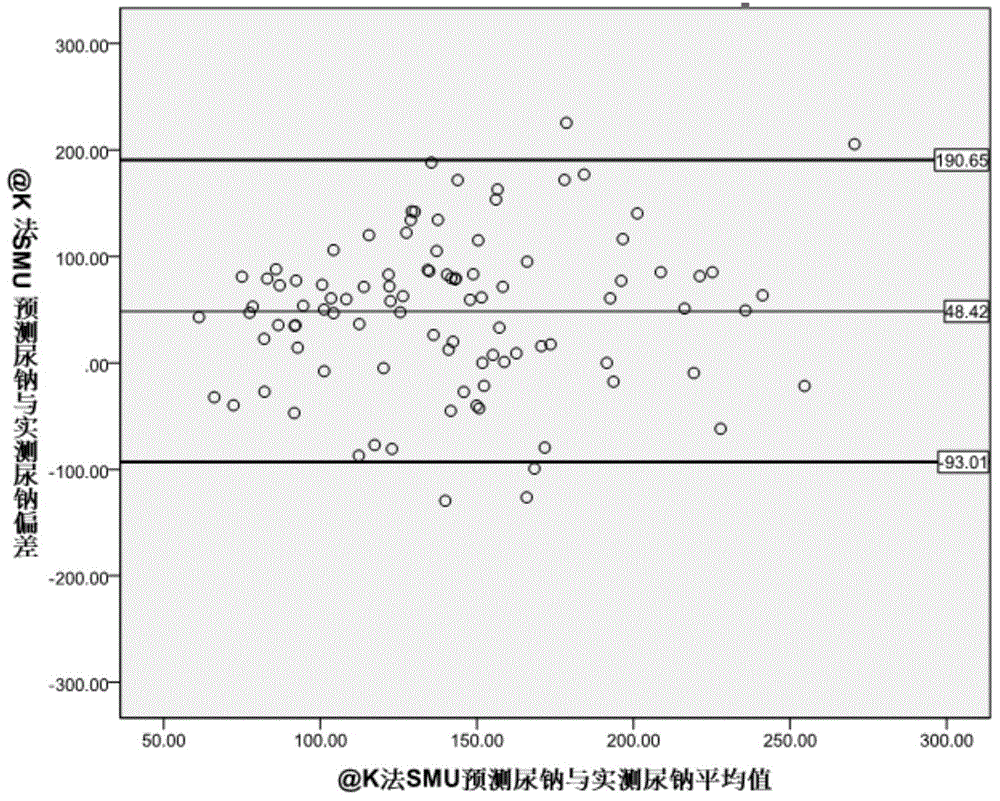
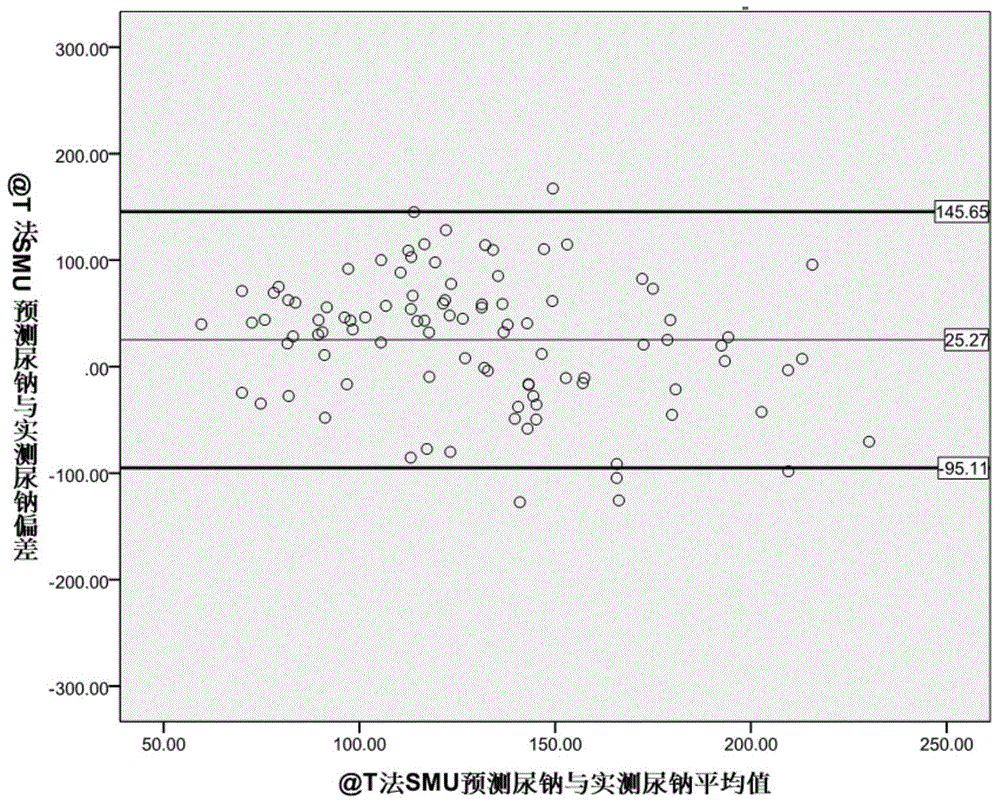
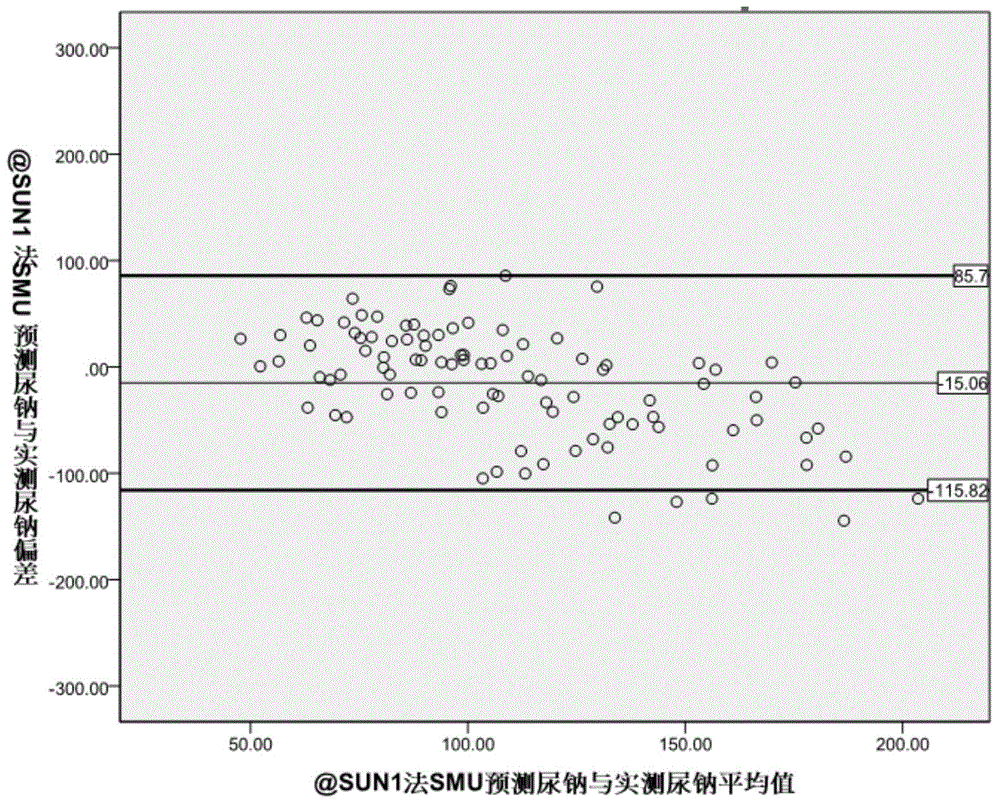
The invention provides a kit for evaluating the 24-hour urinary sodium value of a woman. The kit comprises a product for detecting a point urinary sodium value, a product for detecting a point urine creatinine value and a carrier with the following recorded formula: e-24HUNa=0.241*PRCr*(SUNa / SUCr)0.312, wherein PRCr represents for a predicted 24-hour urine creatinine value, and PRCr=e(7.210-0.013*age-0.002*height+0.010*weight); e-24HUNa represents for a predicted 24-hour urinary sodium value; SUNa represents for the point urinary sodium value; SUCr represents for the point urine creatinine value; and SUNa / SUCr represents for the ratio of the point urinary sodium value to the point urine creatinine value. The accuracy of the 24-hour urinary sodium value of the woman, predicted by using the kit provided by the invention, is equivalent to the 24-hour urinary sodium values of the woman, obtained by using the traditional Kawasaki method and the traditional Tanaka method, and the kit has an important role in guiding the lifestyle and drug therapy of a hypertensive patient and helping to judge whether the salt intake of the hypertensive patient exceeds the standard.
Owner:PEOPLES HOSPITAL PEKING UNIV
Kit for evaluating male urine sodium value within 24 hours
The invention provides a kit for evaluating a male urine sodium value within 24 hours. The kit provided by the invention comprises a product for detecting a point urine sodium value, a product for detecting a point urine creatinine value and a carrier with the following formula: e-24HUNa=0.225*PRC*(SUNa / SUCr)<0.310>, wherein PRCr represents a predicted 24h urine creatinine value and is equal to e<6.916-0.007*age-0.002*height+0.013*weight)>; e-24HUNa represents a predicted 24h urine sodium value; SUNa represents the point urine sodium value; SUCr represents the point urine creatinine value; SUNa / SUCr represents the ratio of the point urine sodium value to the point urine creatinine value. Compared with that determined by a conventional Kawasaki method and Tanaka method, the male urine sodium value within 24 hours predicted by using the kit is equivalent in accuracy, and the kit plays an important role in helping to judge whether the salt intake of a hypertensive exceeds the standard or not and guiding the living style and drug therapy of the hypertensive.
Owner:PEOPLES HOSPITAL PEKING UNIV
Kit for evaluating 24-hour urinary sodium value of woman
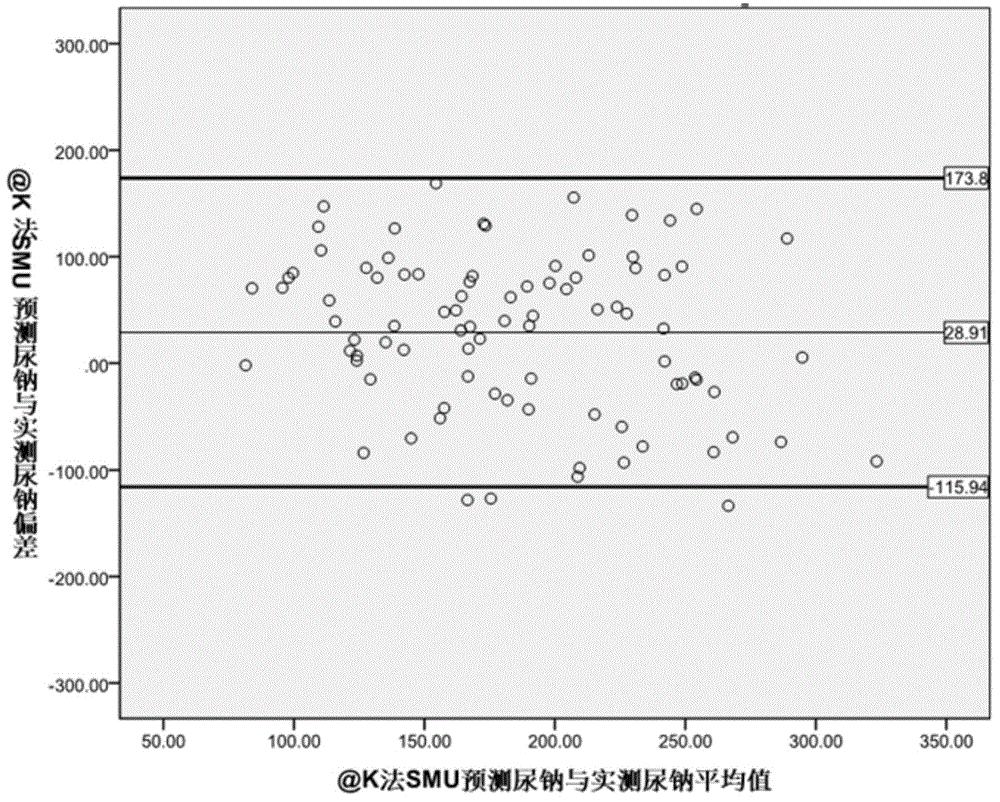
The invention provides a kit for evaluating the 24-hour urinary sodium value of a woman. The kit comprises a product for detecting a point urinary sodium value, a product for detecting a point urine creatinine value and a carrier with the following recorded formula: e-24HUNa=0.329*PRCr*(SUNa / SUCr)0.463, wherein PRCr represents for a predicted 24-hour urine creatinine value, and PRCr=e(7.210-0.013*age-0.002*height+0.010*weight); e-24HUNa represents for a predicted 24-hour urinary sodium value; SUNa represents for the point urinary sodium value; SUCr represents for the point urine creatinine value; and SUNa / SUCr represents for the ratio of the point urinary sodium value to the point urine creatinine value. The accuracy of the 24-hour urinary sodium value of the woman, predicted by using the kit provided by the invention, is equivalent to the 24-hour urinary sodium value of the woman, obtained by using the traditional Tanaka method, and the kit has an important role in guiding the lifestyle and drug therapy of a hypertensive patient and helping to judge whether the salt intake of the hypertensive patient exceeds the standard.
Owner:PEOPLES HOSPITAL PEKING UNIV
Kit for evaluating 24-hour urinary sodium value of man
The invention provides a kit for evaluating the 24-hour urinary sodium value of a man. The kit comprises a product for detecting a point urinary sodium value, a product for detecting a point urine creatinine value and a carrier with the following recorded formula: e-24HUNa=0.208*PRCr*(SUNa / SUCr)0.300, wherein PRCr represents for a predicted 24-hour urine creatinine value, and PRCr=e6.916-0.007*age-0.002*height+0.013*weight); e-24HUNa represents for a predicted 24-hour urinary sodium value; SUNa represents for the point urinary sodium value; SUCr represents for the point urine creatinine value; and SUNa / SUCr represents for the ratio of the point urinary sodium value to the point urine creatinine value. The accuracy of the 24-hour urinary sodium value of the man, predicted by using the kit provided by the invention, is equivalent to the 24-hour urinary sodium values of the man, obtained by using the traditional Tanaka method, and the kit has an important role in guiding the lifestyle and drug therapy of a hypertensive patient and helping to judge whether the salt intake of the hypertensive patient exceeds the standard.
Owner:PEOPLES HOSPITAL PEKING UNIV
Urine microalbumin/urine creatinine detection kit
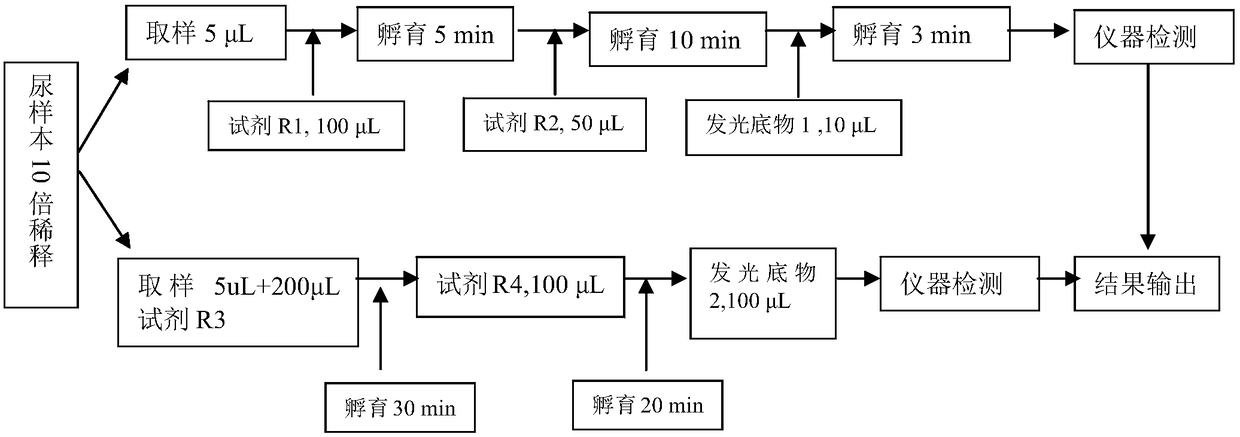
ActiveCN108088839AEasy to operateImprove automationChemiluminescene/bioluminescenceCreatinine riseSarcosine
The invention discloses a urine microalbumin / urine creatinine detection kit. The kit comprises a urine creatinine detection reagent and a urine microalbumin detection reagent, wherein the urine creatinine detection reagent comprises a reagent R1 used for eliminating endogenous creatine and sarcosine, a reagent R2 used for converting creatinine into creatine and a luminescent substrate 1 used for detecting hydrogen peroxide; the urine microalbumin detection reagent comprises an immobilized rabbit-anti-human serum albumin polyclonal antibody, a reagent R3 used for binding urine microalbumin andthe immobilized rabbit-anti-human serum albumin polyclonal antibody, an alkaline phosphatase labelled reagent R4 used for binding with urine microalbumin and a luminescent substrate 2 used for detecting alkaline phosphatase. Urine microalbumin and urine creatinine are detected with a glow-type chemiluminiscence method; the detection method can detect two indexes on a chemiluminescence instrument and enables measurement to be more accurate, more convenient and rapider.
Owner:GUANGZHOU JINDE BIOTECH
Microalbuminuria and urine creatinine integrated test card
The invention relates to a microalbuminuria and urine creatinine integrated test card, which comprises a backboard. A microalbuminuria detecting part and a urine creatinine detecting part are arrangedon the backboard and share one sample pad. Reacting areas and detecting areas of the microalbuminuria detecting part and the urine creatinine detecting part are located at the two ends of the samplepad correspondingly. The microalbuminuria and urine creatinine integrated test card has the advantages of being simple in preparation method, convenient to operate, quick and accurate in detecting andthe like.
Owner:BEIJING YICHENG BIOELECTRONICS TECHNOLOGY COMPANY
Application of fungus polysaccharide sulfate in the preparation of chronic nephritis and kidney failure treating medicine
InactiveCN1557332ASignificant effectSmall side effectsOrganic active ingredientsUrinary disorderSide effectUrine Creatinine
The present invention is new use of mycose sulfate in pharmaceutical field. The medicine has obvious effects of obviously lowering kidney coefficient, serum inosine and urea nitrogen level, obviously increasing total serum protein and albumin, increasing urine creatinine exhaust amount, obviously decreasing exhausted urine protein, etc. It is used in treating nephritis and kidney failure disease and has the advantages of obvious curative effect, less toxic side effect, etc.
Owner:NANJING UNIV
Method for evaluating daily salt content of hypertensive patient by random point urine
ActiveCN111508584AReduce the difficulty of operationIncrease the difficulty of clinical detectionHealth-index calculationMaterial analysis by electric/magnetic meansUrine sodiumHuman body
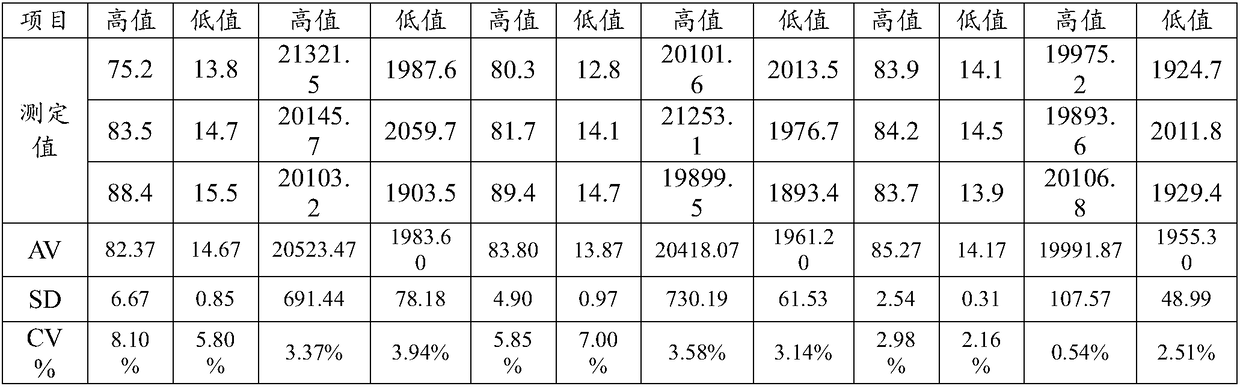
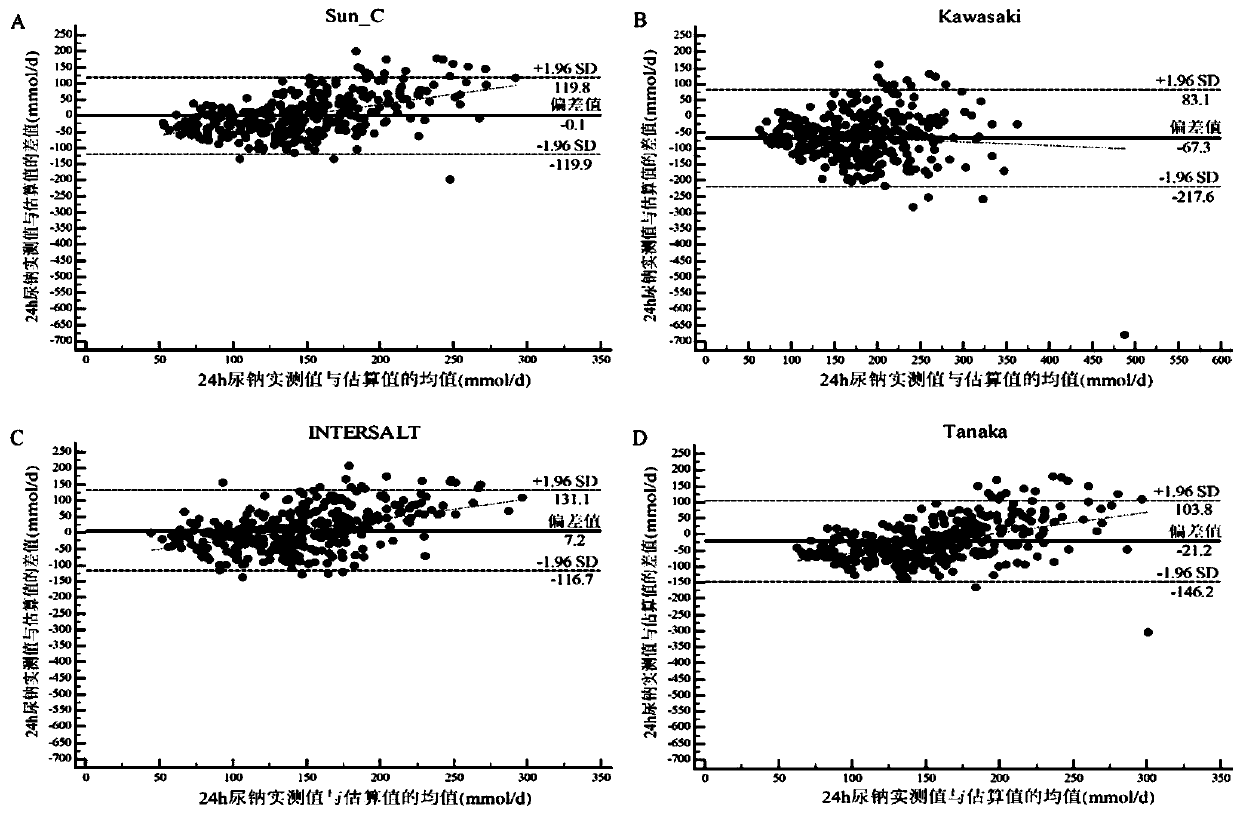
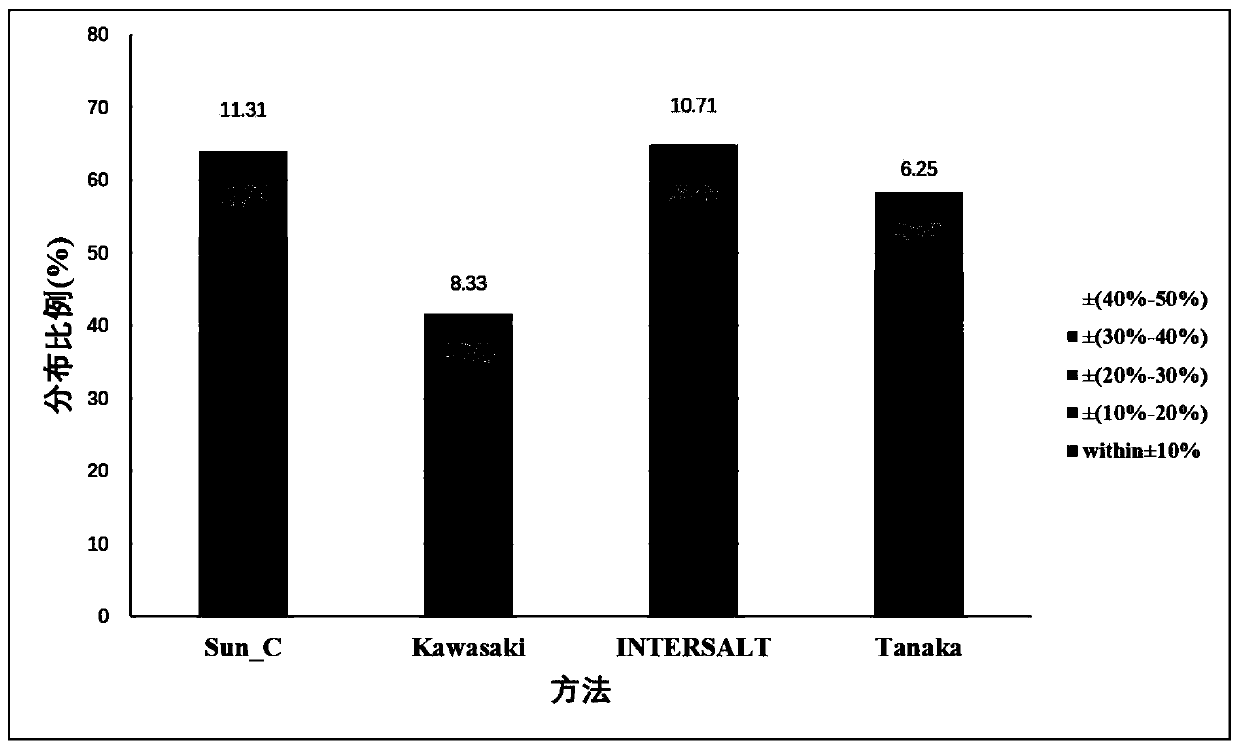
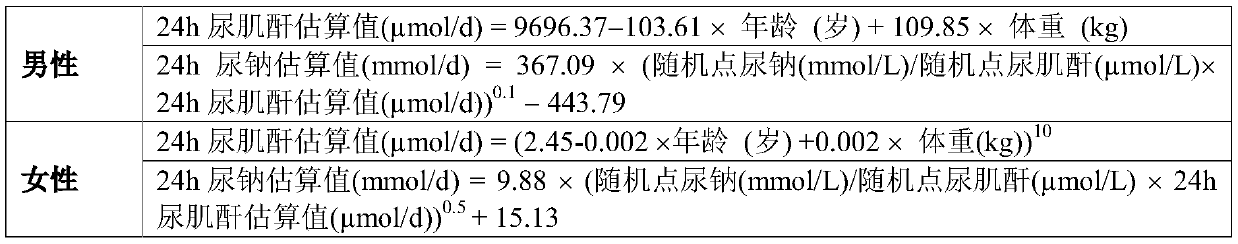
The invention discloses a method for evaluating daily salt content of a hypertensive patient by using random point urine, which comprises the following steps: 1, acquiring basic information of a hypertensive patient, the basic information comprising gender, age and weight; 2, measuring random point urine sodium and point urine creatinine values; 3, substituting the data into the following calculation formula according to the gender, obtaining a 24-hour urine creatinine estimated value according to age and weight data and then obtaining the 24-hour urine sodium estimated value of the hypertension patient by combining the random point urine sodium and point urine creatinine data; and 4, assuming that the table salt contains 100% of sodium chloride, and calculating the daily table salt intake(g / d) according to 96-99% of sodium chloride excretion of the kidney of the human body and the 24-hour urine sodium estimated value. According to the invention, the problem that the existing common formula has many clinical indexes is solved, the clinical detection difficulty is increased, the clinical applicability is reduced, and the application is relatively tedious.
Owner:孙宁玲 +1
New use of traditional Chinese medicinal material cordyceps cicadae
InactiveCN106138115AGood effectAnthropod material medical ingredientsSkeletal disorderATPaseCreatinine rise
The invention relates to use of cordyceps cicadae in preparation of drugs for preventing or treating gout. According to the use, due to cordyceps cicada preparation products, a novel gout preventing or treating method is provided and is obvious in effect. The cordyceps cicadae have the functions of reducing blood and urine creatinine, increasing endogenous creatinine clearance rate, increasing the content of serum protein, reducing the discharge of urinary protein, and the like. Therefore, the cordyceps cicadae have definite treatment effect on early- and middle-stage chronic renal insufficiency patients. Confirmed by further researches: the cordyceps cicadae have relatively good treatment effect on renal tubulointerstitial lesions and can be used for protecting Na<+>-K<+>-ATPase of renal tubule cells, alleviating cytolysosome and cell lipid peroxidation injury, improving renal hemodynamics and alleviating endothelial cell injury and blood coagulation property.
Owner:湖州新驰医药科技有限公司
Early warning system for chronic renal failure
InactiveCN106361289ATimely detection of non-obvious damageDiagnostic recording/measuringSensorsCreatinine riseClearance rate
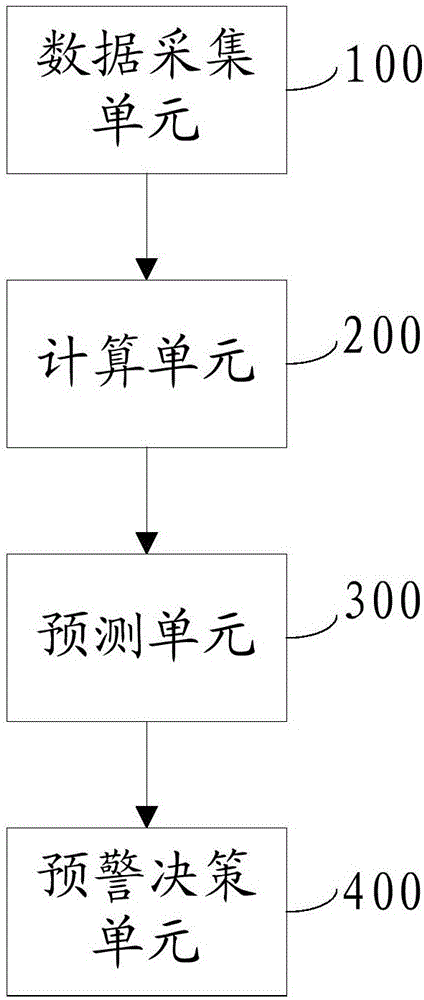
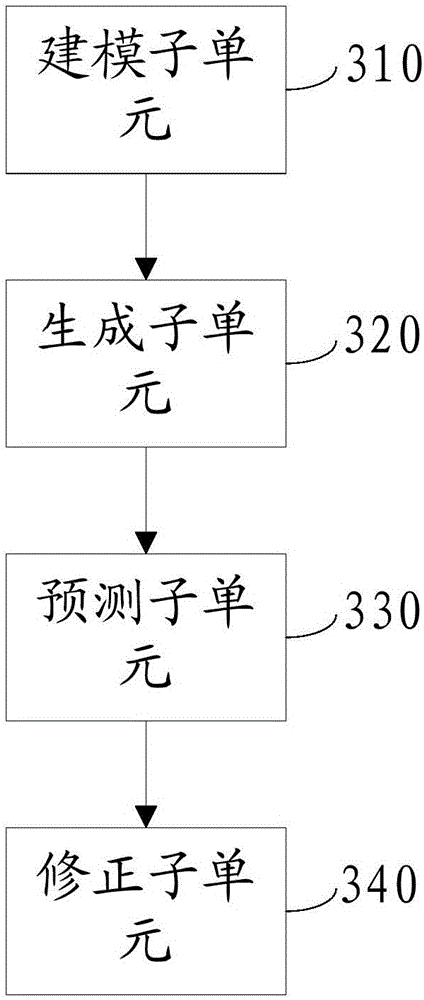
The invention discloses an early warning system for a chronic renal failure, and belongs to the field of medical detection. The early warning system for the chronic renal failure comprises a data acquisition unit used for collecting the urine volume, urine creatinine and serum creatinine of a patient to generate detection values, a calculation unit used for calculating creatinine clearance rates according to the detection values, a prediction unit used for establishing a Markov-grey model, and calculating predicted values of the creatinine clearance rates in future time periods according to the N creatinine clearance rates calculated through the detection values of N detection time periods, wherein N is an integer greater than 3, and an early warning decision unit used for determining the early warning level of the chronic renal failure according to the predicted values of the creatinine clearance rates. According to the invention, modeling analysis is carried out on the creatinine clearance rates to predict the probability that a kidney has the chronic renal failure and timely discover non-overt renal injuries in early kidney asymptomatic lesions so as to provide effective help in clinical early confirmed diagnosis and later treatment.
Owner:CHONGQING TECH & BUSINESS UNIV
Reagent for detecting urine creatinine and application of reagent
ActiveCN108152282AHigh precisionImprove accuracyMaterial analysis by observing effect on chemical indicatorLinear relationshipInterference factor
The invention relates to the technical field of medical detection and discloses a reagent for detecting urine creatinine and application of the reagent. The reagent disclosed by the invention is prepared from a liquid A and a liquid B, wherein the liquid A takes a buffer solution as a base solution and containsis prepared from phytic acid, copper sulfate, EDTA (ethylene diamine tetraacetic acid) and sodium sulfonated phthaleinsulfonamide; and the liquid B takes methanol or ethanol as a solvent and containsis prepared from TMB (tetramethylbenzidine), PVP (polyvinyl pyrrolidone) and peroxide. Byadjusting and optimizing the components in the reagent, the influence of each interference factor to detection in a urine sample is reduced, higher accuracy and precision are achieved, the linear relationship is good in a large range, the reliability is high, and the defects of poor accuracy and precision of existing likesimilar products are made up.
Owner:吉林省汇酉生物技术股份有限公司
Urinary albumin/urinary creatinine composite quality control product and production method thereof
PendingCN112858690AReduce degradationPrevent oxidation precipitationBiological testingFlufenamic acidDextran
The invention provides a urinary albumin / urinary creatinine composite quality control product and a production method thereof. The urinary albumin / urinary creatinine composite quality control product comprises the following components: 50-100 mmol / L of a buffer solution, 5-15 g / L of salt, 5-15 g / L of flufenamic acid, 5-15 g / L of dimethyl sulfoxide, 5-15 g / L of a reducing agent, 5-15 g / L of dextran, 5-15 g / L of a defoaming agent; 0.5-1 g / L of a tackifier, 100-200 mg / L of human serum albumin, 10-20 mmol / L of creatinine, and 1 g / L of a preservative. A pH value of the quality control product is 7.2-7.4. The product can be used for evaluating the accuracy of the urinary albumin / urinary creatinine detection reagent based on a microfluidic technology, and plays an important role in guaranteeing the laboratory detection result quality of the urinary albumin / urinary creatinine detection reagent and reducing errors.
Owner:NINGBO POLYTECHNIC
Use of carnosic acid and rosmarinic acid in joint preparation of medicine for preventing and treating type Ⅱ diabetic nephropathy
ActiveCN110384692BLower fasting blood sugarLow excretion rateOrganic active ingredientsMetabolism disorderPharmacologyGlucose blood
The invention discloses application of carnosic acid and rosemary acid to jointly prepare a drug for prevention and treatment of type II diabetes nephropathy. According to the application, it is foundthat the carnosic acid and the rosemary acid with the quality ratio of 6:1 can significantly and synergistically reduce fasting blood glucose of a type II diabetic nephropathy mouse, can significantly and synergistically decrease the urinary protein excretion rate and increase the urinary creatinine content and has significant and synergetic improvement effect on the kidney function of the diabetes nephropathy mouse, and the synergetic effect is unexpected by the technicians in the field.
Owner:CHINA PHARM UNIV
Nephrosis dialysis index calculation method based on calculator form
InactiveCN107016232AMedical automated diagnosisSpecial data processing applicationsCreatinine riseClearance rate
The invention relates to a nephrosis dialysis index calculation method based on a calculator form. The solute clearance status after dialysis is judged according to index calculation of a nephrosis dialysis patient, and the method simultaneously integrates three functions of the glomerular filtration rate (GFR), the body mass index (BMI) and the urea clearance index (Kt / V). The glomerular filtration rate is measured through the creatinine clearance rate (Ccr), the urine creatinine value, the serum creatinine value i, the urine volume and other indexes of the patient are input in a calculator, and the total Ccr of every week is obtained; according to the body mass index, the body weight and the body height of the patient are input to obtain basic standards; according to the urea clearance index, the dialysis time, the urea clearance amount and other indexes are input to judge the solute clearance status. The three calculation formulas are integrated, a doctor can be simply and conveniently helped to rapidly and comprehensively judge the physical condition of the nephrosis dialysis patient, preparation is made for next dialysis and following treatment, or when the doctor is not available, the patient can judge the condition of himself / herself.
Owner:SICHUAN UNIV
Stem cell preparation modified by long-acting GLP-1 gene, preparation method and application of stem cell preparation
InactiveCN112138025APromote regenerationFunction increaseOrganic active ingredientsSenses disorderBlood sugarUrinary albumin
The invention discloses a stem cell preparation modified by a long-acting GLP-1 gene, a preparation method and application of the stem cell preparation. An in vivo experiment proves that the stem cellpreparation containing a mesenchymal stem cell modified by the GLP-1 gene and ipriflavone has a good function of regulating blood sugar, meanwhile, a urea nitrogen, urine creatinine and urinary albumin excretion rate can be lowered, and a diabetic lower limb ischemia condition and a limb necrosis degree can be obviously lowered. The stem cell preparation disclosed by the invention has a good effect when the stem cell preparation is used for treating diabetes mellitus and complications thereof.
Owner:NORTHEAST NORMAL UNIVERSITY
Measurement device and method of female urine sodium predicted value
ActiveCN107918006AHigh precisionTime-saving measurementBiological testingMeasurement deviceUrine potassium
The invention provides a measurement device and method of 24h female urine sodium predicted value. The measurement device comprises a urine component amount detection unit and a 24h urine sodium predicted value calculation unit, wherein the urine component amount detection unit is used for detecting a component amount in point urine in the point urine urinated within a specified period and the component amount comprises point urine sodium value, point urine potassium value and point urine creatinine value; the 24h urine sodium predicted value calculation unit is used for calculating the 24h urine sodium predicted value of the female in people according to the component amount in the point urine. By adopting the device provided by the invention, the 24h urine sodium value of the female canbe measured in a high-precision, time-saving and convenient manner.
Owner:PEOPLES HOSPITAL PEKING UNIV +1
Urine analysis reagent combination
The embodiment of the invention discloses a urine analysis reagent combination. The combination comprises a first reagent, a second reagent, a third reagent, a fourth reagent, a fifth reagent, a sixthreagent, a seventh reagent, an eighth reagent and a ninth reagent, wherein the first reagent is a ketone body single quality control solution, the second reagent is a protein single quality control solution, the third reagent is an occult blood single quality control solution, the fourth reagent is a urine glucose single quality control solution, the fifth reagent is a urinary bile original single quality control solution. the sixth reagent is bilirubin single-phase quality control liquid, the seventh reagent is leukocyte single-phase quality control liquid, the eighth reagent is urine creatinine single-phase quality control liquid, and the ninth reagent is urine VC single-phase quality control liquid. Compared with the existing urine analysis quality control liquid, the urine analysis reagent combination disclosed by the embodiment of the invention can be stored at normal temperature, is low in toxicity and even non-toxic, and is beneficial to popularization and application in commonfamily self-inspection.
Owner:成都恩普瑞生物工程有限公司
Quantitative immune colloidal gold detection card and kit for urocystatin C, urine microalbumin and urine creatinine
PendingCN110988365AImprove the detection rateImprove detection accuracyDisease diagnosisBiological testingElevated creatinineColloidal au
The invention discloses a quantitative immune colloidal gold detection card for urine cystatin C, urine microalbumin and urine creatinine. The detection card comprises a buckle box, a bottom plate, and a sample pad, a combination pad, a chromatographic membrane and a sample absorption pad which are sequentially adhered to the bottom plate. The combination pad is coated with a colloidal gold labeled cystatin C antibody I, a urine microalbumin antibody I, a urine creatinine antibody I and a chicken IgY. A first detection line coated with a cystatin C antibody II, a second detection line coated with a urine microalbumin antibody II, a third detection line coated with a urine creatinine antigen modified by BSA, and a quality control line coated with goat anti-chicken IgY are arranged on the chromatographic membrane. Compared with single detection of U-mALb and U-Cr values, the U-mAlb / U-Cr ratio obtained in the invention can more sensitively reflect the degree of renal function impairment,and the accuracy of the detection result is improved.
Owner:HUNAN UNIV OF TECH
A urine microalbumin/urine creatinine detection kit
ActiveCN108088839BEasy to operateImprove automationChemiluminescene/bioluminescenceCreatinine riseSarcosine
The invention discloses a urine microalbumin / urine creatinine detection kit. The kit comprises a urine creatinine detection reagent and a urine microalbumin detection reagent, wherein the urine creatinine detection reagent comprises a reagent R1 used for eliminating endogenous creatine and sarcosine, a reagent R2 used for converting creatinine into creatine and a luminescent substrate 1 used for detecting hydrogen peroxide; the urine microalbumin detection reagent comprises an immobilized rabbit-anti-human serum albumin polyclonal antibody, a reagent R3 used for binding urine microalbumin andthe immobilized rabbit-anti-human serum albumin polyclonal antibody, an alkaline phosphatase labelled reagent R4 used for binding with urine microalbumin and a luminescent substrate 2 used for detecting alkaline phosphatase. Urine microalbumin and urine creatinine are detected with a glow-type chemiluminiscence method; the detection method can detect two indexes on a chemiluminescence instrument and enables measurement to be more accurate, more convenient and rapider.
Owner:GUANGZHOU JINDE BIOTECH
Effective medicine part compatibility composition for preventing and treating diabetics and vascular diseases of diabetics
InactiveCN104173568ASignificant effectImprove pathological changesMetabolism disorderCardiovascular disorderDiseaseKidney
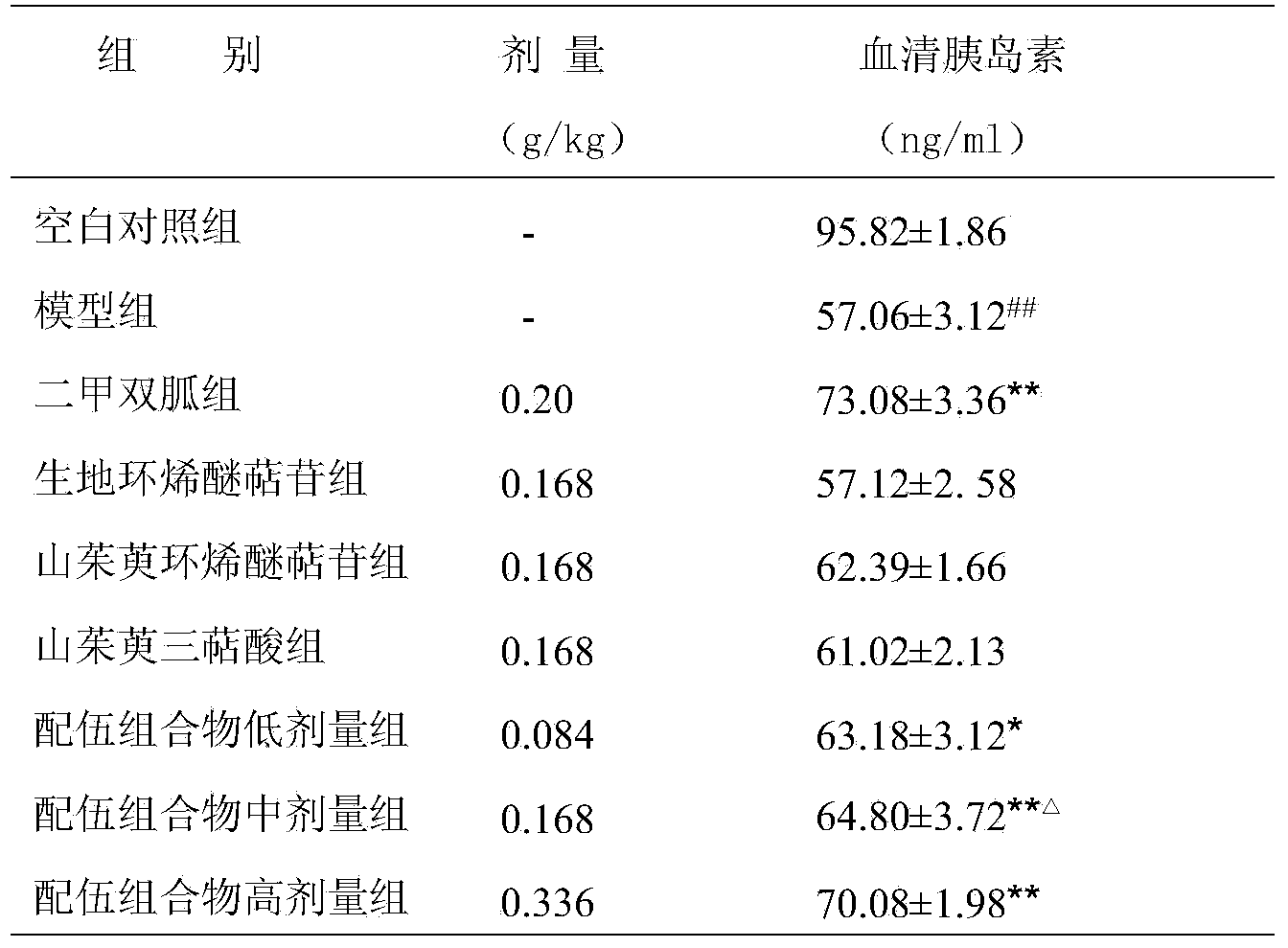
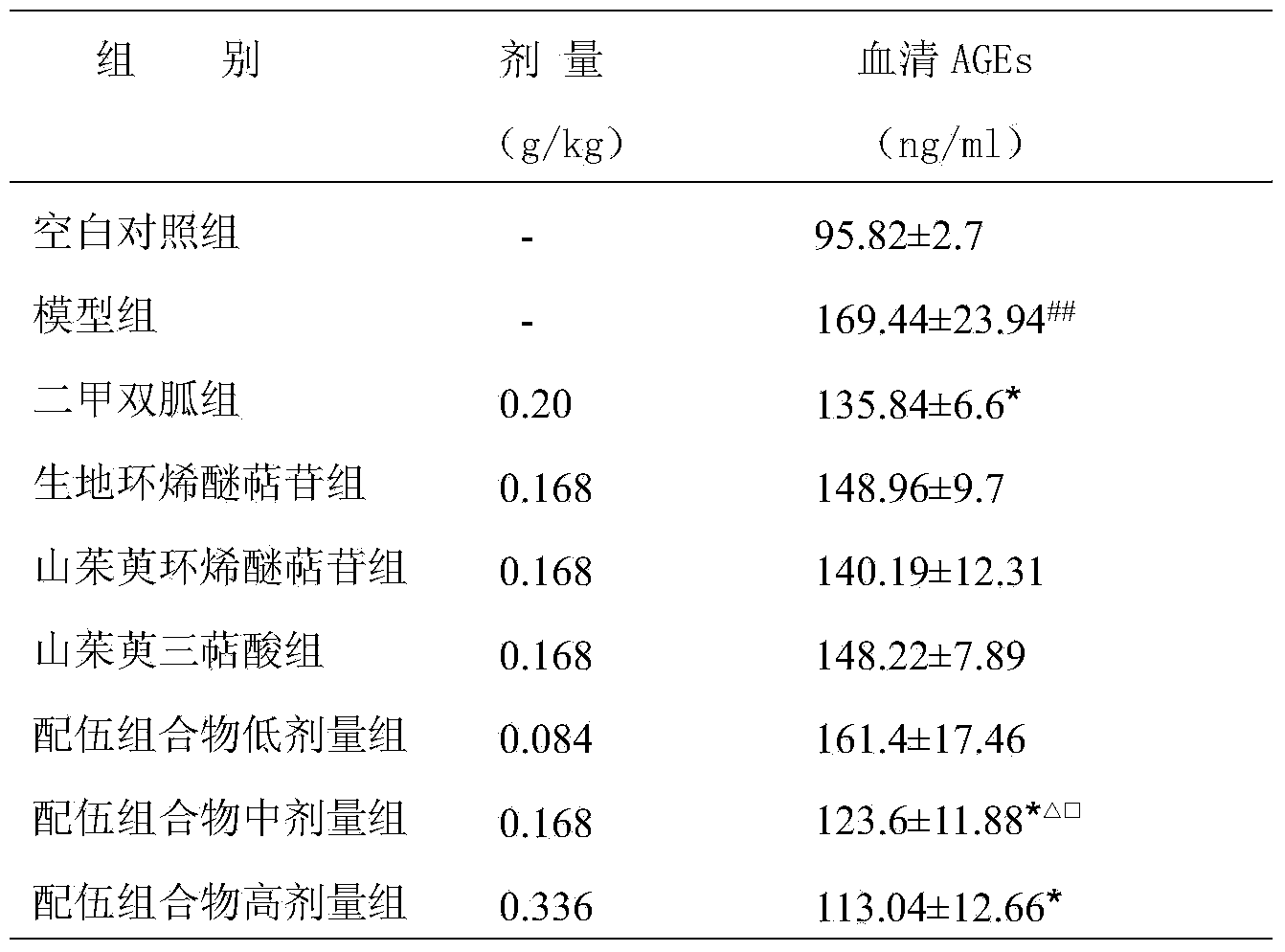
The invention provides an effective medicine part compatibility composition for preventing and treating diabetics and vascular diseases of diabetics. The effective medicine part compatibility composition is prepared from the following effective parts of rehmannia glutinosa and dogwood in weight proportions: 2-3 parts of total iridoid glycoside extract of rehmannia glutinosa, 3-5 parts of total iridoid glycoside of dogwood and, 0.5-1.5 parts of total triterpenes acid extract of dogwood. The research result of an STZ-induced C57BL / 6 mouse diabetic model shows that the composition can remarkably improve the symptoms of 'three excessiveness and one insufficiency' of a diabetic mouse, can reduce the blood sugar value, can improve secretion of insulin, can reduce serum creatinine, urea nitrogen and AGEs levels, can improve discharge of urine creatinine, can reduce discharge of urine protein, and can improve pathological change of pancreas, kidneys, testis and hearts. The composition has the function of reducing blood sugar and ascular diseases of diabetics.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Application of zanamivir in preparation of medicine for treating or preventing preeclampsia
PendingCN114533721AImprove high blood pressureGood treatment effectOrganic active ingredientsSenses disorderPregnanetriolonePharmacology
The invention provides application of zanamivir in preparation of a medicine for treating or preventing preeclampsia, a corresponding medicine and a treatment method. Zanamivir can effectively improve the symptoms of hypertension, proteinuria, creatinine, neuraminidase and sialic acid rise of preeclampsia and increase the NO level; the pregnancy outcome is improved. The combination of zanamivir and hydroxyprogesterone hexanoate shows a better effect.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
Urinalysis Test Card for Urine Microalbumin/Urine Creatinine
ActiveCN103575913BHigh sensitivityEasy to prepareDisease diagnosisBiological testingBacteriuriaUrinary albumin
The invention relates to a urine analysis and test card for urine microalbumin / urine creatinine. The urine analysis and test card for the urine microalbumin / urine creatinine comprises a sample pad, wherein a loading hole is formed in the sample pad. The urine analysis and test card for the urine microalbumin / urine creatinine is characterized in that a urine microalbumin test card and a urine creatinine test card are arranged on the sample pad respectively; one end of the urine microalbumin test card and one end of the urine creatinine test card are connected with the loading hole respectively; an albumin detection line and a first quality control line are arranged on the urine microalbumin test card; the albumin detection line is positioned at the end near to the loading hole; a urine creatinine detection line and a second quality control line are arranged on the urine creatinine test card; the urine creatinine detection line is positioned at the end near to the loading hole; a first detection hole is formed in the back side of the urine microalbumin test card and at the position corresponding to the albumin detection line; a second detection hole is formed in the back side of the urine creatinine test card and at the position corresponding to the urine creatinine detection line. The urine analysis and test card for the urine microalbumin / urine creatinine can solve the problem of interference caused by the situation that urine protein is singly used as a detection index; a preparation method is simple, and easy to operate.
Owner:无锡博慧斯生物医药科技有限公司
Traditional Chinese medicine alcohol extract for treating diabetic nephropathy and preparation method and application thereof
InactiveCN105943819AReduce urinary protein excretion rateReduce contentMetabolism disorderUrinary disorderBlood sugarAstragalus mongholicus
The invention discloses a traditional Chinese medicine alcohol extract for treating diabetic nephropathy and a preparation method and application thereof. The preparation method comprises the following steps of: extracting a traditional Chinese medicine composition with an ethanol aqueous solution, filtering to obtain filtrate, and concentrating and drying to obtain the traditional Chinese medicine extract, wherein the traditional Chinese medicine composition comprises humifuse euphorbia herb, radix salviae miltiorrhizae, raw astragalus mongholicus, rhizoma anemarrhenae and coptis chinensis in a mass ratio of (1.32-5.34) to (1.76-7.12) to (0.88-6) to (0.88-3.56) to 1. The traditional Chinese medicine extract is obtained by carrying out alcohol extraction on the traditional Chinese medicine composition which comprises humifuse euphorbia herb, radix salviae miltiorrhizae, raw astragalus mongholicus, rhizoma anemarrhenae and coptis chinensis, and the method is simple. The traditional Chinese medicine extract is capable of lowering blood sugar concentration, lowering the content of urine micro albumin, lowering the content of urine urea, and lowering the ratio of the content of urine micro albumin to the content of the urine creatinine, and thus the traditional Chinese medicine extract can be used for preventing and / or treating diabetic nephropathy.
Owner:PEKING UNIV FIRST HOSPITAL
A kit for simultaneously detecting sodium and creatinine in urine
ActiveCN105334211BImprove accuracySolve instabilityMaterial analysis by observing effect on chemical indicatorUrine sodiumCreatinine rise
The invention provides a kit simultaneously detecting sodium and creatinine in urine. The kit comprises a reaction base plate possessing a sodium reactive hole and a creatinine reactive hole, a sodium diluent, a creatinine diluent and a standard shade guide; a substrate pad and an enzyme pad are placed in the sodium reactive hole from bottom to top, the substrate pad is prepared by dropwise adding a substrate solution on a carrier, and the enzyme pad contains a beta-galactosidase and an enzyme stabilizing agent; an upper pad and a lower pad are placed in the creatinine reactive hole from bottom to top, the lower pad is prepared by dropwise adding an enzyme solution and a color developing agent on a carrier, and the lower pad contains an peroxidase, a creatinine amide hydrolase, a color developing agent, a stabilizing agent and the like; and the standard shade guide comprises standard color development of different concentration sodium ion and standard color development of different concentration creatinine. The kit preparation method is simple, the kit is stable in performance and easy to store, the color intervals corresponding to different concentration urine sodium and creatinine are obvious, whether the salt content of a detected person exceeds standard is convenient to determine, and also the kit is applicable to hospital clinical laboratory and non-professional examination personnel.
Owner:北京中生金域诊断技术股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com