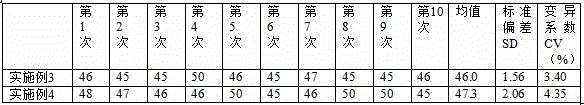
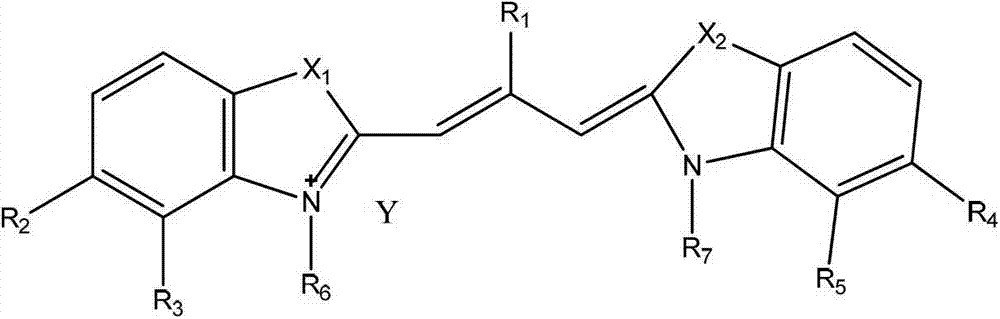
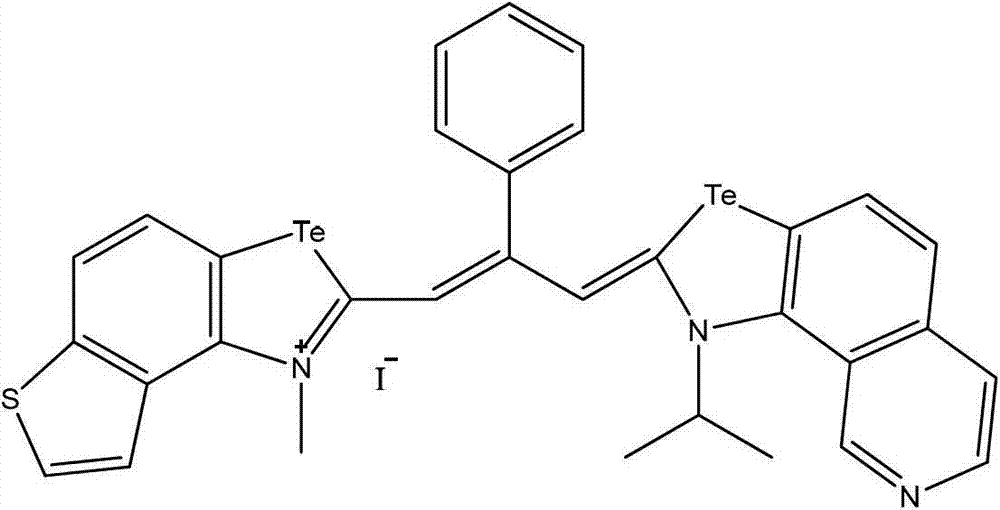
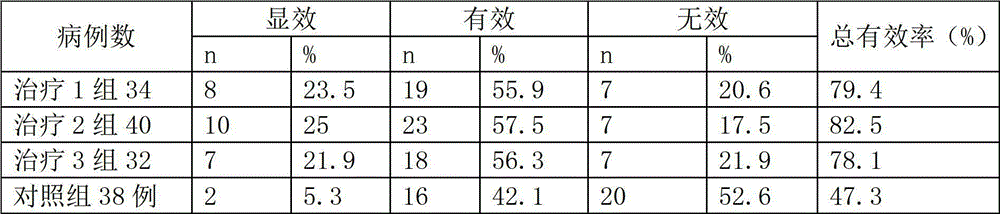
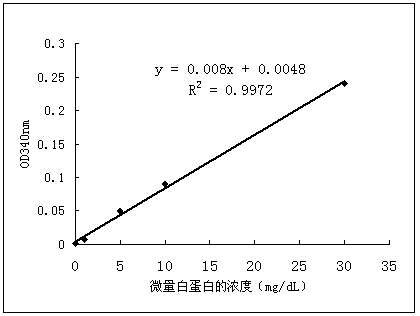
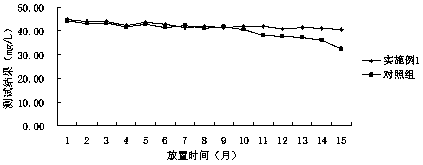
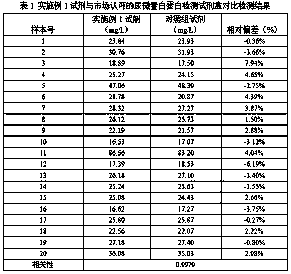
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
98 results about "Urine microalbumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kit and method for quantitatively detecting urine microalbumin through time-resolved fluorescence
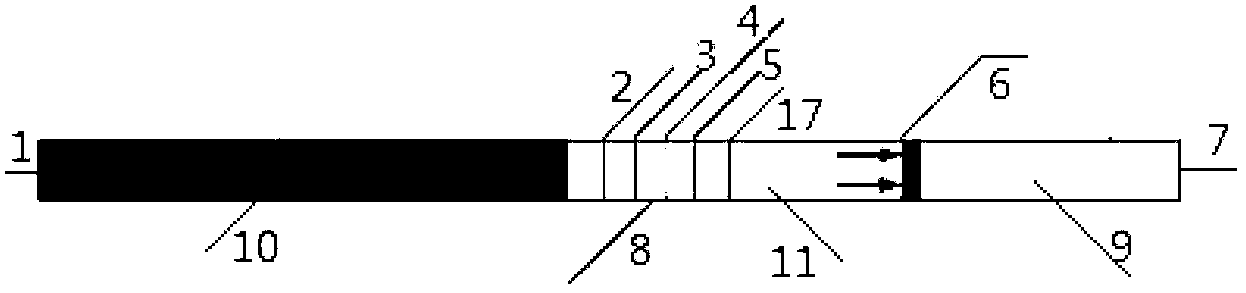
ActiveCN106872420AAvoid fluorescence interferenceMark stableFluorescence/phosphorescenceFluorescenceMicrosphere
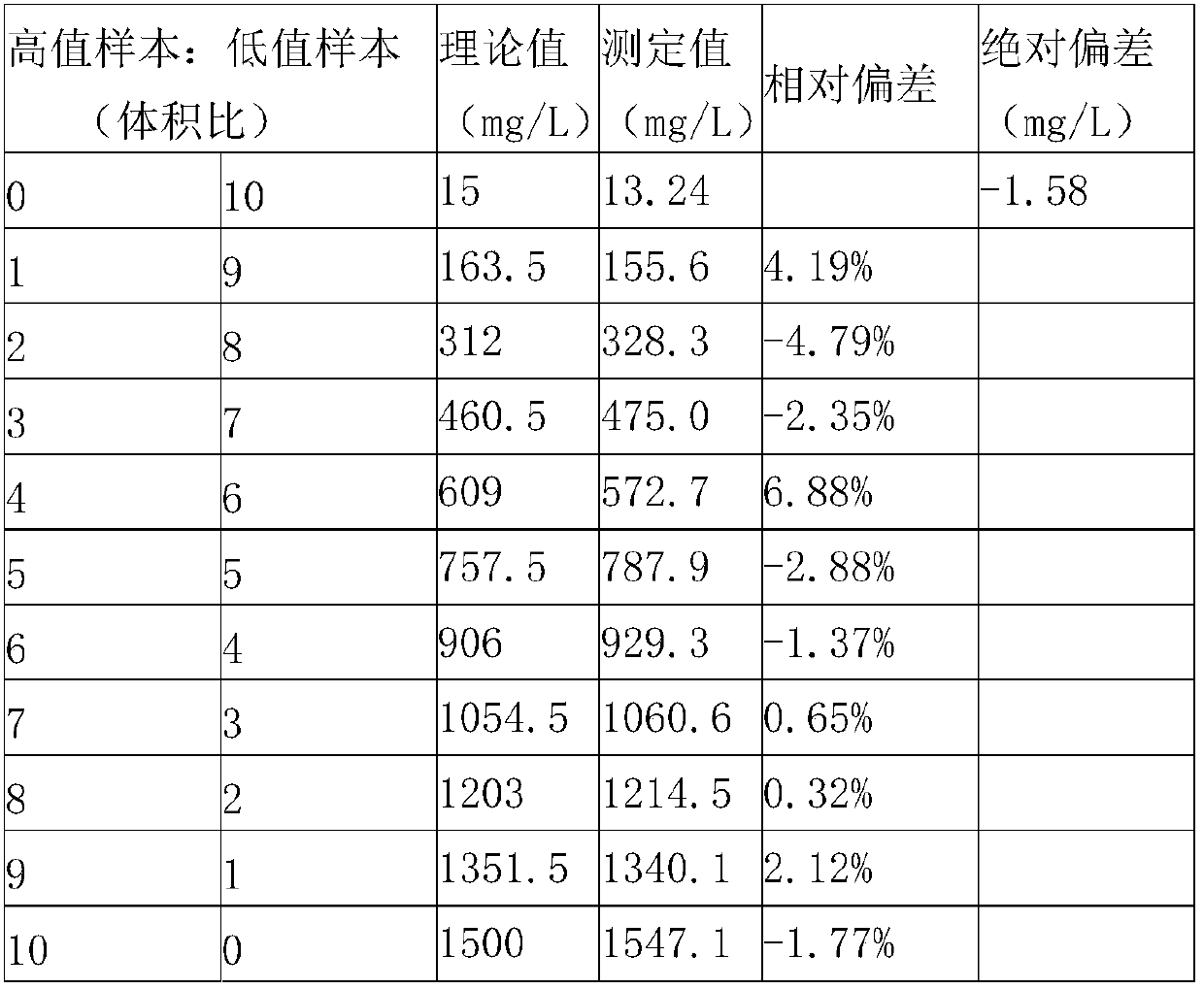
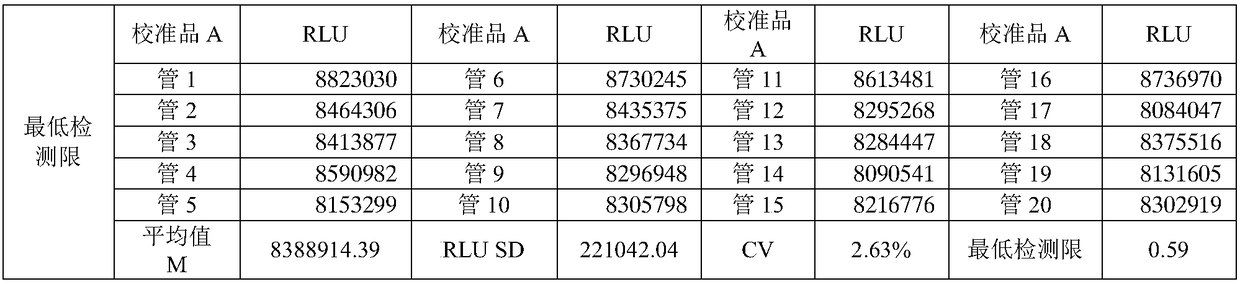
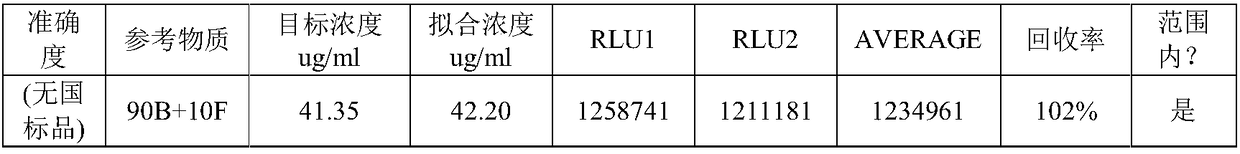
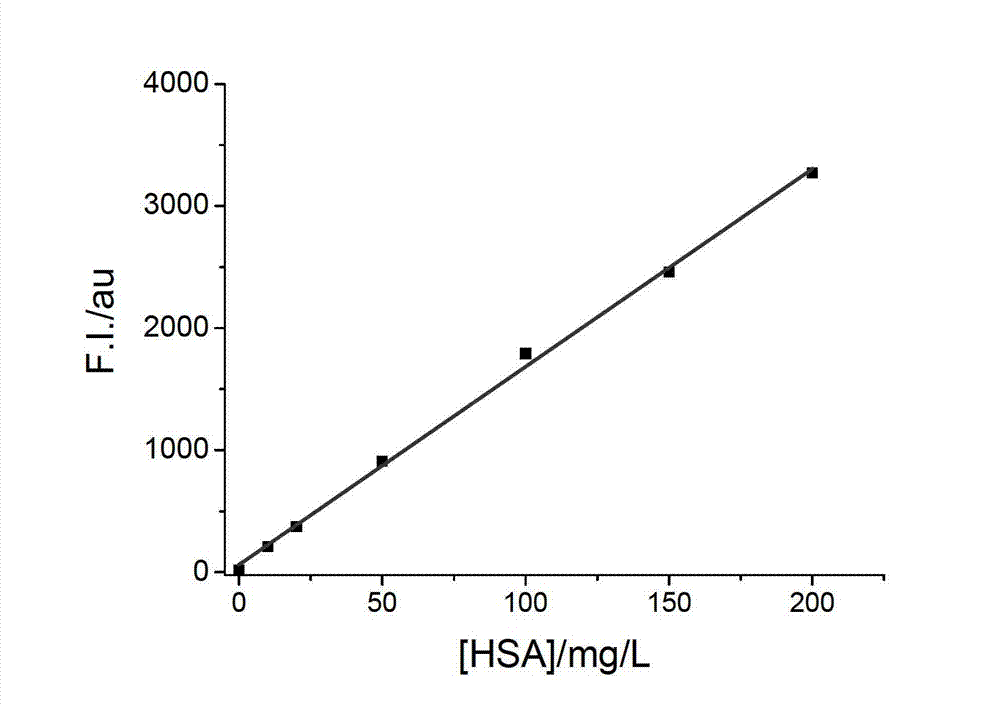
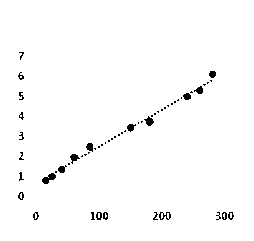
The invention relates to a kit and a method for quantitatively detecting urine microalbumin through time-resolved fluorescence. The kit comprises a time-resolved fluorescence test paper card, sample diluent and a DS card containing a calibration curve, wherein the time-resolved fluorescence test paper card comprises a detection test paper strip which consists of a bottom liner, and a sample pad, a nitrocellulose membrane and absorbent paper which are pasted on the bottom liner; the nitrocellulose membrane is successively provided with a microsphere line, a detection line and a quality control line; the microsphere line consists of time-resolved fluorescence microspheres marked with human seroalbumin monoclonal antibodies; the detection line is coated with human seroalbumin recombinant antigens; the quality control line is coated with goat anti-mouse IgG. The kit provided by the invention can accurately and quantitatively detect the content of microalbumin in human urine; markers are used for connecting the microspheres with antibodies through covalent bonds; and the marked products are stable. The kit has the characteristics of wide detection range (2 to 300mg / ml), high sensitivity (the detection limit is 2300mg / ml), high accuracy, fast and easy detection and the like.
Owner:厦门奥德生物科技有限公司
Test strip for semi-quantitative detection of microalbuminuria
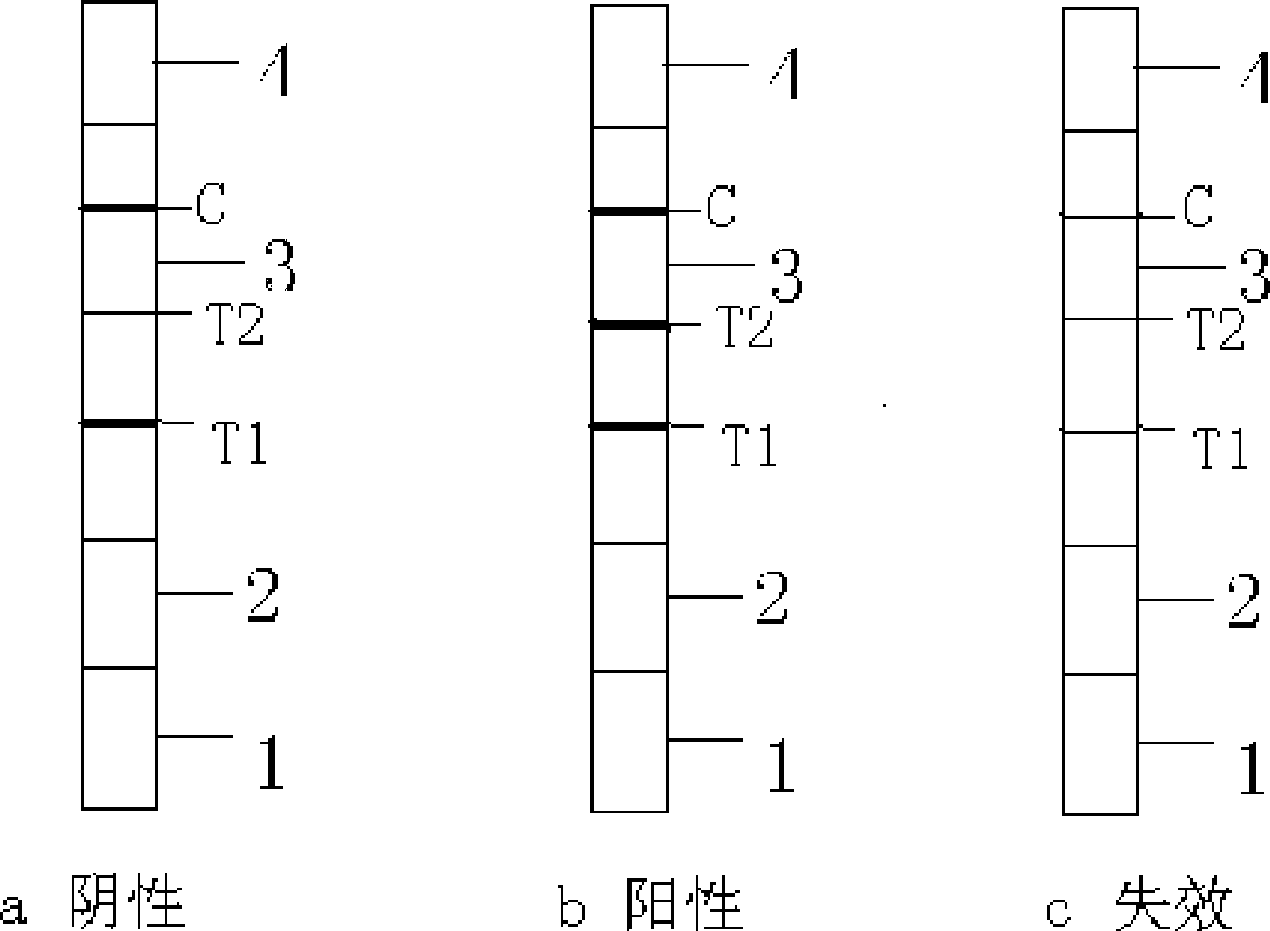
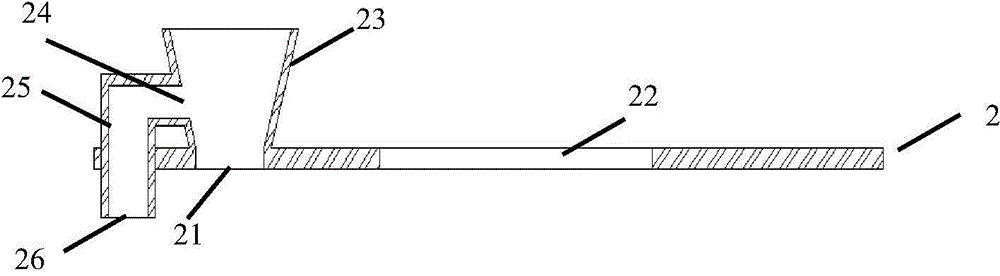
The invention discloses a test strip for semi-quantitative detection of microalbuminuria, belonging to medical test consumables. The test strip is characterized in that the test strip for semi-quantitative detection of microalbuminuria is a colloidal gold test strip. An anti-human albumin antibody and rabbit IgG with acolloidal gold label is enveloped on a gold label fiber pad; anti-rabbit IgG antibody is enveloped on a quality control line C, and human albumin is enveloped on a detection line T. The test strip is simple, sensitive and accurate, can be used for semi-quantitative detection of microalbuminuria in human urine and suitable for detection of early renal injury patients.
Owner:蓝十字生物药业(北京)有限公司
Urine analysis and test card for urine microalbumin/urine creatinine
ActiveCN103575913ASolution rangeAddress operational complexityDisease diagnosisBiological testingAlbuminUrine creatinine test
The invention relates to a urine analysis and test card for urine microalbumin / urine creatinine. The urine analysis and test card for the urine microalbumin / urine creatinine comprises a sample pad, wherein a loading hole is formed in the sample pad. The urine analysis and test card for the urine microalbumin / urine creatinine is characterized in that a urine microalbumin test card and a urine creatinine test card are arranged on the sample pad respectively; one end of the urine microalbumin test card and one end of the urine creatinine test card are connected with the loading hole respectively; an albumin detection line and a first quality control line are arranged on the urine microalbumin test card; the albumin detection line is positioned at the end near to the loading hole; a urine creatinine detection line and a second quality control line are arranged on the urine creatinine test card; the urine creatinine detection line is positioned at the end near to the loading hole; a first detection hole is formed in the back side of the urine microalbumin test card and at the position corresponding to the albumin detection line; a second detection hole is formed in the back side of the urine creatinine test card and at the position corresponding to the urine creatinine detection line. The urine analysis and test card for the urine microalbumin / urine creatinine can solve the problem of interference caused by the situation that urine protein is singly used as a detection index; a preparation method is simple, and easy to operate.
Owner:无锡博慧斯生物医药科技有限公司
Kit for measuring microalbuminuria by adopting immune competition turbidimetry
InactiveCN107741493AMeet the screeningTreatment Level MonitoringMaterial analysisNormal peopleMicrosphere
The invention provides a kit for measuring microalbuminuria by adopting immune competition turbidimetry. The kit comprises an anti-human albumin antibody, human albumin marked leatexbeads and a buffersolution. The anti-human albumin antibody and the human albumin marked leatexbeads are separately placed. The particle size of the leatexbeads is 80-267 nm. When the kit is used for measuring microalbuminuria in human urine, the maximum linear range is 5 mg / L-1500 mg / L. The kit can meet screening of clinical normal people, and can also avoid result underestimation or even false negative result arising from a hook effect caused by antigen excess.
Owner:HUNAN HY BIOPOCT TECH CO LTD
Colloid selenium test paper for semi-quantitative determination of urinary trace albumin
The invention provides a test paper for semi-quantitatively detecting colloid selenium in microdose albumin in urine. The test paper can be applied in the fields of early diagnosis of diabetic nephropathy, hypertensive nephropathy and preeclampsia and injury of kidney caused by various poisonous substances. A plastic bottom plate of the test paper is orderly glued with a sampling liquid absorption part, a colloid selenium labeling part, a detection reaction part and a water absorption part, wherein the colloid selenium labeling part is a single-cloning antibody 1 which is coated by a glass fiber membrane, or acetic acid fiber membrane, or nylon membrane and labeled by the colloid selenium; the detection reaction part is a nitrocellulose membrane which is provided with two detection bands and quality control bands, T1 is a single-cloning antibody 2 which is precisely quantitative, and T2 is the single-cloning antibody 2 with a certain concentration; and the quality control bands are coated by antiplague IgG. The invention provides a method for semi-quantitatively detecting the microdose albumin in a urine sample conveniently, visually and quickly.
Owner:LANZHOU UNIVERSITY
Microalbuminuria detection kit and preparation thereof
The invention provides a microalbuminuria (mAlb) detection kit, which is based on a latex enhanced turbidimetric immunoassay method, is a liquid double reagent, namely reagent R1 and reagent R2, wherein the reagent R1 is a reactant and the reagent R2 is a solution containing albumin immuno latex particles, and the detection kit is characterized by crosslinking by applying a chemical process, and covalently crosslinking a goat anti-human albumin antibody (also called goat anti-human mAlb antibody) with carboxylated polystyrene latex through water-soluble carbodiimide (EDC) and H-hydroxy succinimide (NHS), so as to form an mAlb antibody latex reagent. The reagent has the advantages of high sensitivity, strong specificity and simple preparation, and is worthy of further promotion.
Owner:王贤俊
Kit for determining microalbuminuria and preparation method thereof
InactiveCN106053368AHigh analytical sensitivityRapid responseColor/spectral properties measurementsBiological testingSuspending AgentsSurface-active agents
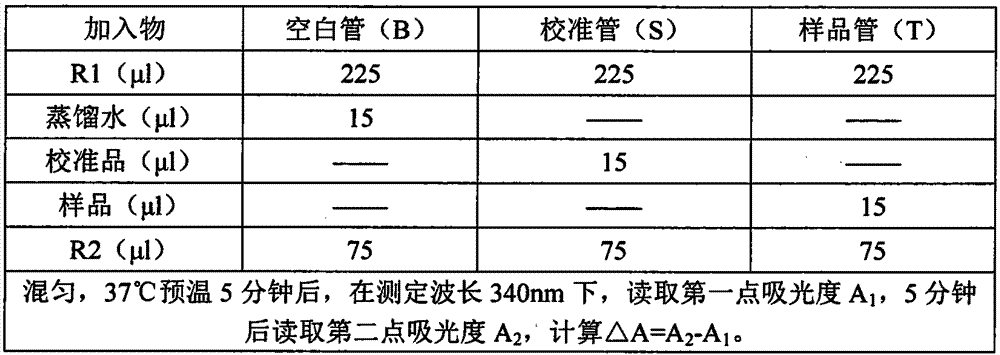
The invention discloses a kit for determining microalbuminuria and a preparation method thereof. The kit comprises dual-liquid components of a reagent R1 and a reagent R2 which are independent from each other. The reagent R1 is prepared from a buffer solution, an accelerator, inorganic salt ions and a corrosion remover; the reagent R2 is prepared from a buffer solution, a suspending agent, a surface active agent, inorganic salt ions, a corrosion remover and anti-albumin antibodies, and a solvent of the reagent R2 is purified water. The preparation method comprises the steps that the reagents are prepared according to the component content; a to-be-tested sample is mixed with the reagent R1 and the reagent R2 to be subjected to a sufficient reaction; a fully automatic biochemical analyzer is used for determining an absorptivity difference obtained after the reaction; according to an absorptivity change value, the concentration of microalbuminuria in the sample is worked out. The kit has the advantages of being convenient to operate, high in accuracy and the like.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Urine microalbumin detection method and kit
InactiveCN103091316AEasy to detectStrong specificityMethine/polymethine dyesMaterial analysis by observing effect on chemical indicatorTest sampleBuffer solution
The invention relates to a urine microalbumin detection method and a kit. The urine microalbumin detection method comprises the following steps of: (1) preparing a plurality of standard solutions witg different urine microalbumin concentrations from a metal-ion-contained buffer solution and microalbumin, wherein the pH of the buffer solution is 5.0-8.2; (2) adding a same amount of cyanine dyes and the metal-ion-contained buffer solution in the plurality of standard solutions to obtain a plurality of standard colorimetric solutions; (3) adding the cyanine dyes and the metal-ion-contained buffer solution in a to-be-tested liquid sample, so as to enable the concentrations and the pH values of the cyanine dyes in the to-be-tested sample to be same as those of the standard colorimetric solutions, so as to obtain a test solution; and (4) comparing the color of the test solution with that of the standard colorimetric solutions obtained in the step (2), finding out one standard colorimetric solution of which the color is the same or close to that of the test solution from the plurality of standard colorimetric solutions, so as to obtain the result that the urine microalbumin concentration of the standard solution corresponding to the standard colorimetric solution is the same or close to that of the to-be-tested liquid.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Microdose urine protein testing chip and its tester
InactiveCN1854738AQuick checkAid in early diagnosisMaterial analysis by observing effect on chemical indicatorBiological testingCelluloseUrine protein test
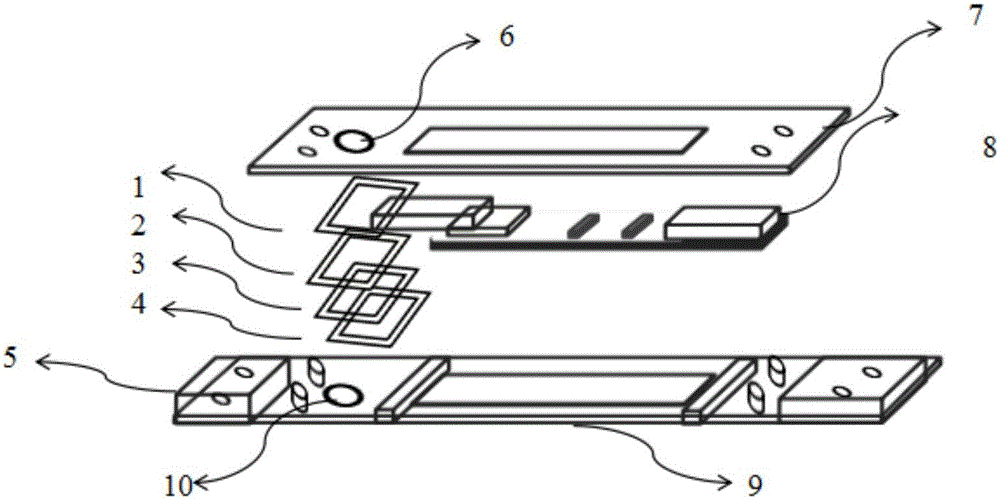
A test device of trace amount albumin in urine consists of a shell with cover, a test sheet and a window on shell. The said test sheet is prepared as setting flow guide membrane on substrate, setting glass cellulose membrane enveloped by colloidal gold labeled antibody at colloidal gold labeled antibody portion, setting cellulose membrane enveloped with quality control band and detection band on color developing portion as quality control band being enveloped with substance aimed to colloidal gold label and detection band being used to combine with colloidal gold labeled antibody and setting water absorption paper at water absorption portion.
Owner:BEIJING YICHENG BIOELECTRONICS TECHNOLOGY COMPANY
Application of medicinal composition for treating diabetes mellitus
The invention provides application of a Tibetan medicinal composition in preparing a medicament for treating diabetes mellitus. The Tibetan medicinal composition consists of the following raw material drugs in parts by weight: 60 to 600 parts of gymnadenia conopsea, 30 to 300 parts of radix pleurospermi tibetanici, 40 to 400 parts of sealwort, 30 to 300 parts of radix mirabilis himalaicae, 40 to 400 parts of asparagus fern, 10 to 100 parts of cordyceps sinensis, 30 to 300 parts of cynomorium songaricum, 10 to 100 parts of caltrop, 4 to 45 parts of przewalskia tangutica, and 50 to 500 parts of myrobalan. Tests prove that the medicinal composition can remarkably improve the physical sign of a patient, and has remarkable improvement to fasting plasma-glucose (FPG), 2 hour postprandial blood glucose (PBG), urinary albumin excretion rate (UAE), microalbuminuria (MAV) and glycosylated hemoglobin (HbAlc).
Owner:SHANDONG JINHE DRUG RES DEV
Kit for detecting content of urine microalbumin in serum, and preparation method thereof
The invention discloses a kit for detecting the content of urine microalbumin in serum. The kit comprises an anti-reagent A, an anti-reagent B, a magnetic particle reagent, a calibrator, a quality control product and a luminescent substrate solution, wherein the anti-reagent A is an alkaline phosphatase-labeled urine microalbumin derivative; the anti-reagent B is a FITC-labeled urine microalbuminpolyclonal antibody; and the magnetic microparticle reagent is carboxyl magnetic particles coated with a FITC antibody. The invention discloses a preparation method for the kit. The kit combines chemiluminescence with immunomagnetic particles, provides a near-homogeneous reaction system and adopts a one-step reaction mode, so detection performances (such as sensitivity, precision, a detection range, etc.) are greatly improved, and reaction time is greatly shortened (wherein less than 35 min is needed from the beginning of sample feeding to acquisition of detection results, and the reaction time is obviously less than the reaction time of kits of a same kind); high coupling efficiency and firm bonding are realized; and the preparation method is stable in process, and can greatly reduce product cost while improving product performance.
Owner:TAIZHOU ZECEN BIOTECH CO LTD
Urinary microalbumin (U-mALb)/urinary creatinine (U-Cr) integrated assay bigeminy strip and preparation method thereof
InactiveCN106353511AEasy to detectEasy to operateDisease diagnosisBiological testingCreatinine riseUrine volume
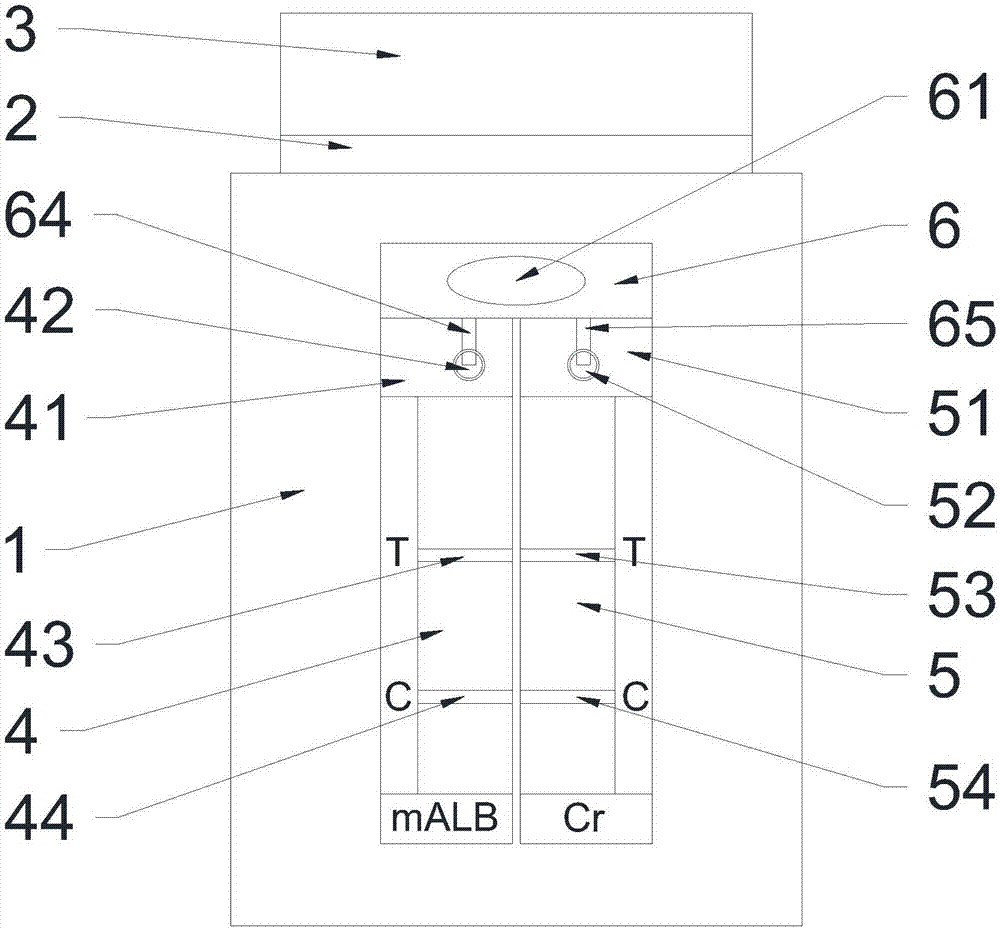
The invention relates to a urinary microalbumin (U-mALb) / urinary creatinine (U-Cr) integrated assay bigeminy strip, comprising a sample area and reaction areas. The U-mALb / U-Cr integrated assay bigeminy strip is characterized in that the sample area comprises a diffusion membrane and blood filtering membranes; the reaction areas comprise two detection windows, and the two detection windows can be used for detecting the ratios of the U-mALb and the U-Cr at the same time; the sample area for detecting the U-Cr by adopting a dry chemical method is arranged at the position where the reaction area is positioned, and the reaction area for detecting the U-mALb by using a colloidal gold dry type immune chromatography technology is arranged at a position which is 0.5cm away from the sample area. The different reaction areas contain different reagent pads, and the reagent pads respectively and independently react with corresponding components in urine. Synchronous detection can be realized only by dripping the random urine of a person to be tested into the sample area, so that the U-mALb / U-Cr integrated assay bigeminy strip is very suitable for medical institutions or individuals. By using the U-Cr for correction, the influence, caused by a sampling way and urine volume, on the excretory amount of urinary albumin can be eliminated, so that the U-mALb / U-Cr integrated assay bigeminy strip can provide more accurate evaluation for the albumin excretion rate and has a greater clinical significance for preventing diabetic nephropathy.
Owner:GETEIN BIOTECH
Urine microalbumin detection kit and preparation method thereof
The invention relates to a urine microalbumin detection kit and a preparation method thereof. The detection kit is prepared from a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from buffer solution (1), inorganic salt ions (1), accelerator (1), stabilizer (1) and preservative (1), and the reagent R2 is prepared from buffer solution (2), inorganic salt ions (2), preservative (2), stabilizer (2), suspending aid (1) and anti-human albumin antibody. The preparation method of the detection kit disclosed by the invention comprises the steps of preparing reagents according to constituent content; mixing a sample to be tested with the reagent R1 and the reagent R2 to enable the sample, the reagent R1 and the reagent R2 fully react; utilizing a full-automatic biochemical analyzer to measure an absorbance difference value after reaction; calculating out the concentration of urine microalbumin in the sample according to an absorbance change value. The detection kit disclosed by the invention has the advantages of low equipment cost, high sensibility, good accuracy and precision, easiness to store, good data repeatability and wide application to clinical biochemical analyzers.
Owner:吉林省富生医疗器械有限公司
Stable urine microalbumin quality control material and preparation method thereof
InactiveCN108918217AStable pHWide adaptabilityPreparing sample for investigationUltrasound attenuationQuality control
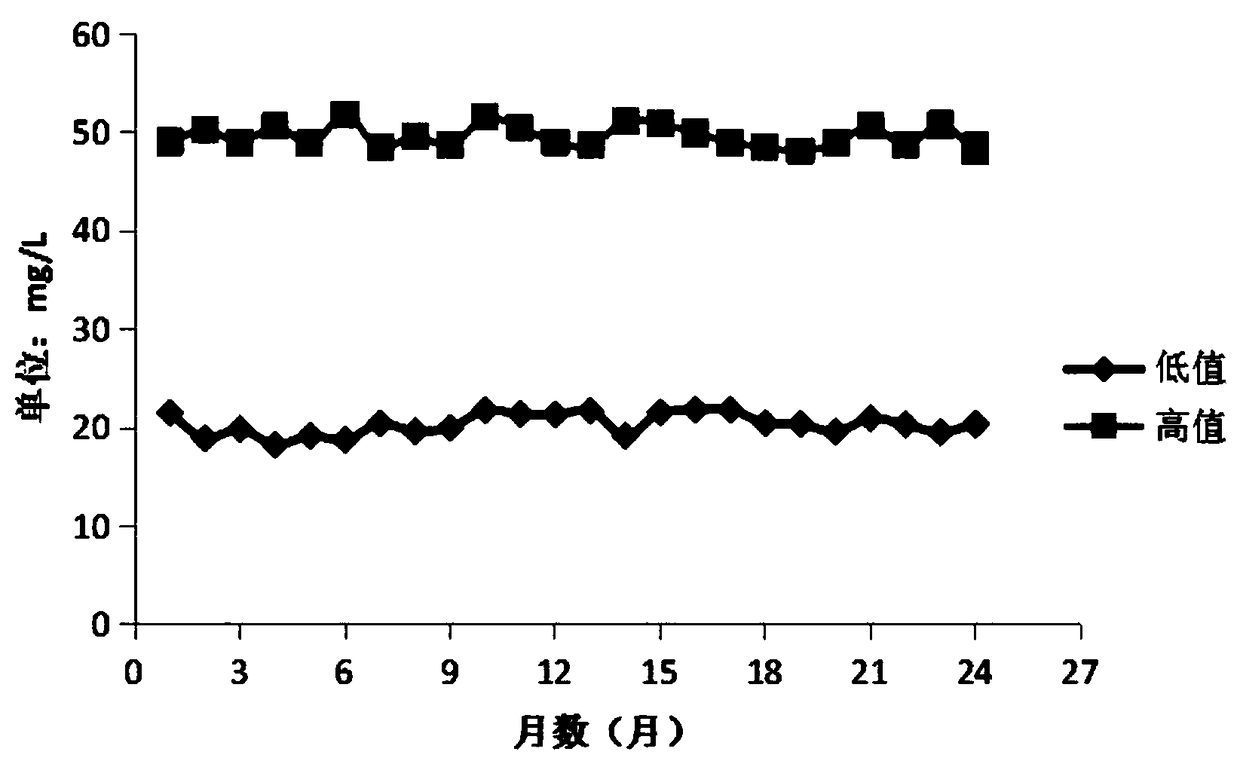
The invention discloses a stable urine microalbumin quality control material and a preparation method thereof. The stable urine microalbumin quality control material solves the technical problem thatthe existing urine microalbumin quality control material easily produces attenuation and cannot be preserved for a long time. The stable urine microalbumin quality control material belongs to the technical field of medical device biochemical in-vitro diagnosis. The stable urine microalbumin quality control material comprises 1-50mmol / L of a buffer with pH of 7.2-7.4, 5-15g / L of salt, 0.01-0.1% ofa protective agent A, 1-10% of a protective agent B, 1-10mL / L of a surfactant and 8.67-21.33mg / L or 45.2-62.8mg / L of albumin. The stable urine microalbumin quality control material has broad-spectrumantibacterial activity, has a wide application range, prevents microbial damage to quality control products, prevents protein attenuation, improves product stability and has an effect period of 24 months.
Owner:DIRUI MEDICAL TECH CO LTD
Urine microalbumin/urine creatinine detection kit
ActiveCN108088839AEasy to operateImprove automationChemiluminescene/bioluminescenceCreatinine riseSarcosine
The invention discloses a urine microalbumin / urine creatinine detection kit. The kit comprises a urine creatinine detection reagent and a urine microalbumin detection reagent, wherein the urine creatinine detection reagent comprises a reagent R1 used for eliminating endogenous creatine and sarcosine, a reagent R2 used for converting creatinine into creatine and a luminescent substrate 1 used for detecting hydrogen peroxide; the urine microalbumin detection reagent comprises an immobilized rabbit-anti-human serum albumin polyclonal antibody, a reagent R3 used for binding urine microalbumin andthe immobilized rabbit-anti-human serum albumin polyclonal antibody, an alkaline phosphatase labelled reagent R4 used for binding with urine microalbumin and a luminescent substrate 2 used for detecting alkaline phosphatase. Urine microalbumin and urine creatinine are detected with a glow-type chemiluminiscence method; the detection method can detect two indexes on a chemiluminescence instrument and enables measurement to be more accurate, more convenient and rapider.
Owner:GUANGZHOU JINDE BIOTECH
Quantitative testing reagent for liquid microalbumin in urine and method
InactiveCN102680700AUndisturbedQuantitatively accurateBiological testingAntiendomysial antibodiesPhosphate
The invention discloses a quantitative testing reagent for liquid microalbumin in urine and a method. The quantitative testing reagent for the liquid microalbumin in urine consists of an R1 reagent and an R2 reagent, wherein the R1 reagent is obtained by dissolving a certain amount of sodium chloride, sodium hydrogen phosphate, potassium dihydrogen phosphate, sodium azide and polyethylene glycol in distilled water; and the R2 reagent is obtained by dissolving a certain amount of sodium azide in phosphate buffer solution and then adding a certain amount of anti-human albumin antibody into the mixture. The self-developed testing reagent for the liquid microalbumin in urine is combined with an automatic analyzer system to precisely determine the content of microalbumin in urine, so as to provide accurate information for a clinician and possibly test the urine sample quantitatively, automatically and fast.
Owner:JIAXING JIUQIJIU BIOLOGICAL TECH
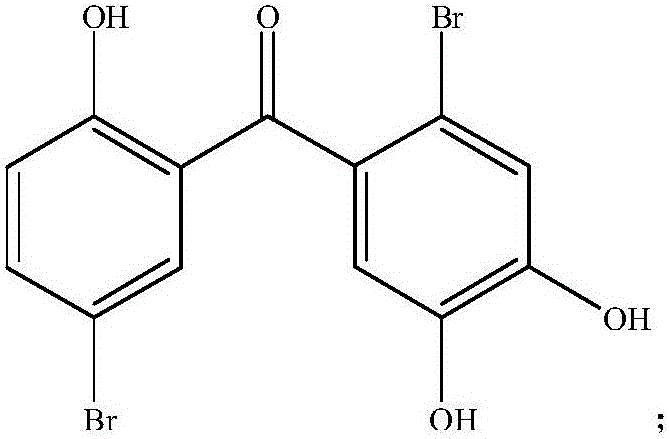
Application of two halophenol compounds in preparation of medicines of resisting type II diabetic nephropathy
ActiveCN105816446AImprove protectionImprove kidney functionMetabolism disorderKetone active ingredientsCaptoprilMorpholine
The invention discloses a medical new application of two halophenol compounds 4,5,2'-trihydroxy-2,5'-dibromobenzophenone and 4,5,2'-tri(4-morpholine methanoyl)-2,5'-dibromobenzophenone, in particular an application in preparation of medicines of resisting type II diabetic nephropathy. Meanwhile, the invention also discloses a medicine of resisting type II diabetic nephropathy employing the two halophenol compounds as active ingredients. By the two compounds, the renal function of the type II diabetic nephropathy can be effectively improved; the main treatment indexes, namely uric acid, urine protein, microalbuminuria, transforming growth factor TGF-beta1, blood sugar, low-density lipoprotein and the like are superior to those of an existing clinic first-line medicine captopril; and the important application prospect in treatment of the diabetic nephropathy is displayed.
Owner:SHANXI MEDICAL UNIV
Microalbuminuria and urine creatinine integrated test card
The invention relates to a microalbuminuria and urine creatinine integrated test card, which comprises a backboard. A microalbuminuria detecting part and a urine creatinine detecting part are arrangedon the backboard and share one sample pad. Reacting areas and detecting areas of the microalbuminuria detecting part and the urine creatinine detecting part are located at the two ends of the samplepad correspondingly. The microalbuminuria and urine creatinine integrated test card has the advantages of being simple in preparation method, convenient to operate, quick and accurate in detecting andthe like.
Owner:BEIJING YICHENG BIOELECTRONICS TECHNOLOGY COMPANY
Traditional Chinese medicinal composition for treating diabetes mellitus and preparation method thereof
InactiveCN104083640AFully functionalGood treatment effectMetabolism disorderPlant ingredientsSalvia miltiorrhizaPollen
The invention belongs to the technical field of traditional Chinese medicines, relates to a traditional Chinese medicinal composition and a preparation method thereof, and particularly relates to a traditional Chinese medicinal composition for treating diabetes mellitus and a preparation method thereof. Against existing technical defects, the traditional Chinese medicinal composition for treating or preventing diabetes mellitus comprises the following components in parts by weight: 10-20 parts radices trichosanthis, 5-20 parts of raw rehmmania root, 5-15 parts of astragalus membranaceus, 5-15 parts of kudzu vine root, 1-10 parts of dendrobium nobile, 9-15 parts of schisandra chinensis, 5-15 parts of salvia miltiorrhiza, 1.5-9 parts of radix ophiopogonis, 9-15 parts of poria and 5.5-15 parts of liquorice. The traditional Chinese medicinal composition can be used for remarkably improving symptoms of diabetes mellitus in the aspects of blood glucose, microalbuminuria and glycosylated hemoglobin of a diabetes rat.
Owner:QINGDAO CENT HOSPITAL
Urinary microalbumin detection apparatus
ActiveCN104535768ASimple and fast operationRapid responseBiological testingReagent stripNitrocellulose
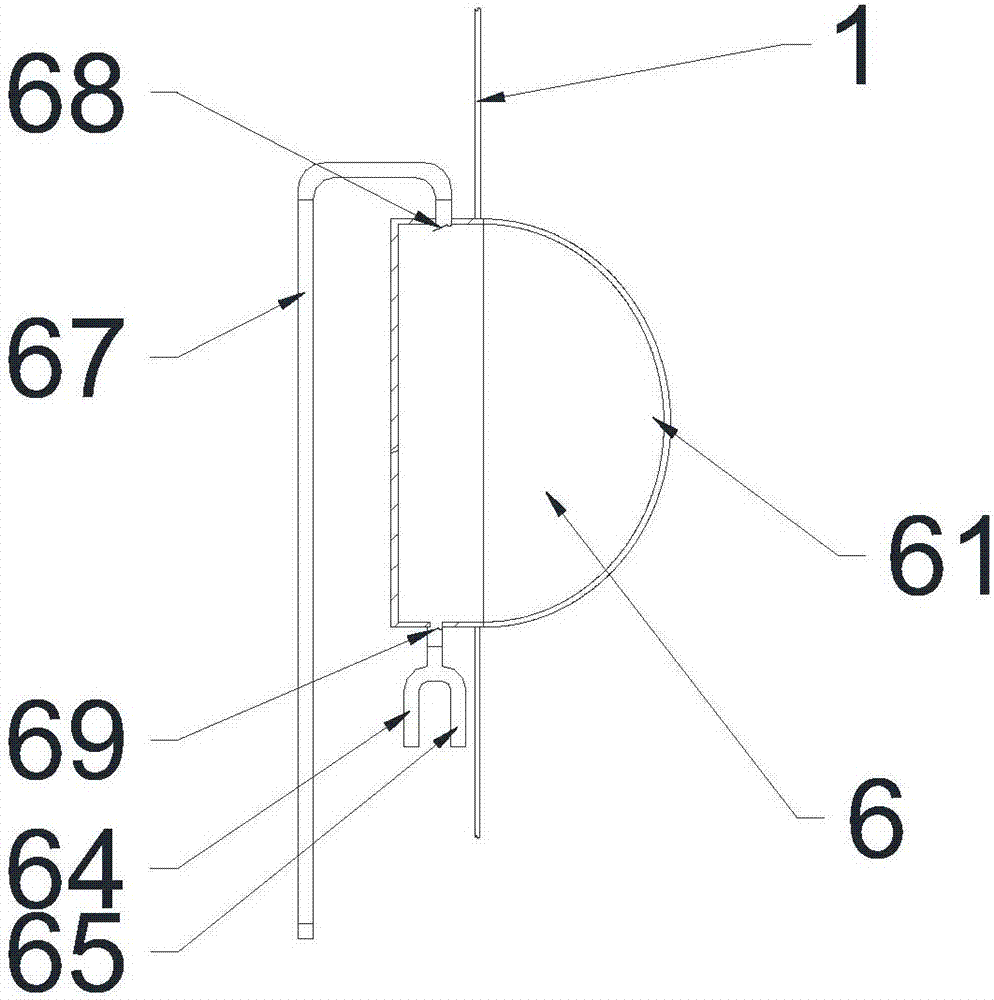
A disclosed urinary microalbumin detection apparatus comprises a box holder, a box cover and a reagent strip; the box cover is provided with a sample adding hole and an observation port; the reagent strip comprises a support base sheet and a chromatography structure disposed on the base sheet; the chromatography structure comprises sample filtering paper, an immune colloidal gold paper sheet, an immune nitrocellulose membrane and water adsorption paper which are in successive head-to-tail connection arrangement; and the apparatus is characterized in that the sample adding hole also is provided with an urine cup opened at the bottom, and the bottom opening of the urine cup has the shape and the size both same to those of the sample adding hole, the immune nitrocellulose membrane is provided with a quality control band and multiple detection lines, and the quality control band and the multiple detection lines are exposed in the observation port. The urinary microalbumin detection apparatus has the advantages of convenient operation, rapid reaction, high sensitivity, high specificity, economy and utility, uneasiness to contaminate hands, capability of quantificationally obtaining the concentration interval of urinary microalbumin, and the like, and is suitable for on-site detection and personal usage.
Owner:海宁经开产业园区开发建设有限公司
Detection method, system and kit of microalbuminuria
ActiveCN103115904AStrong specificityOperation is not affectedMethine/polymethine dyesFluorescence/phosphorescencePotassium ionsLength wave
The invention relates to a detection method, a system and a kit of microalbuminuria. The method for detecting the concentration of microalbuminuria in a solution comprises the following steps of: (1) preparing a plurality of solution samples with different concentrations of microalbuminuria, wherein each solution sample contains cyanine dyes with same concentration; (2) detecting fluorescence intensity values of an acquisition wavelength part; (3) obtaining a standard curve of the microalbuminuria concentration; (4) adding the cyanine dyes and a buffer solution containing potassium ions or sodium ions in a liquid sample to be detected, so that the concentration and the pH value of the cyanine dyes in the liquid solution to be detected is consistent with those of the solution sample in the step (1), so as to obtain a test solution and record a dilution ratio of the liquid sample to be detected; (5) detecting the fluorescence intensity values of the acquisition wavelength part; and (6) finding the microalbuminuria concentration value of the corresponding test solution in the standard curve, and calculating the microalbuminuria concentration of the sample to be detected, wherein the acquisition wavelength is in the range of 550 to 650 nanometers.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Micro-albumin creatinine ratio (ARC) detection device
ActiveCN107132352AConvenient interceptionEasy loadingMaterial analysis by observing effect on chemical indicatorCreatinine riseEngineering
The invention relates to a micro-albumin creatinine ratio (ARC) detection device which comprises a detection device body, an ice bag and a heat insulating bag. The detection device body comprises a sample bottle, an albumin detection device and a creatinine detection device, the albumin detection device and the creatinine detection device are parallelly arranged on the outer wall of the sample bottle, and a sample feeding device is arranged at the upper end of the albumin detection device and the creatinine detection device. By the aid of a sampling device, urine samples are conveniently intercepted, the sample bottle is conveniently filled with urine, the micro-albumin creatinine ratio of the urine can be simply monitored, daily inspection is conveniently forecasted in advance, the samples are conveniently reserved in the sample bottle and quantitatively detected in a hospital, and the urine cannot be polluted or go bad.
Owner:烟台星齐医学检验有限公司
Application of verbascoside in treating and preventing type II diabetic nephropathy
InactiveCN108451961AIncrease the areaIncrease the average optical density valueOrganic active ingredientsMetabolism disorderTG - TriglycerideProtein C
The invention relates to new application and a new action mechanism of verbascoside, in particular to application of verbascoside in the drugs for treating and preventing type II diabetic nephropathy,and the research on the protection function of the verbascoside for the type II diabetic nephropathy and the action mechanism thereof. Results show that the verbascoside can alleviate liver injury caused by high glucose and lower the serum creatinine, urea nitrogen and urinary microalbuminuria levels, the blood fat level (total cholesterol and triglyceride) and the fasting blood-glucose and bloodinsulin level of spontaneous diabetes db / db mice; TGF (Transforming Growth Factor)-beta 1 and the signal transduction protein Smad3 and Smad4 and alpha-SMA protein expression thereof in a kidney tissue are obviously lowered; meanwhile, the verbascoside can alleviate the liver injury caused by high glucose; HK-2 proliferation and EMT (pithelial-Mesenchymal Transition) formation can be inhibited. In conclusion, the verbascoside has an obvious protection function on the type II diabetic nephropathy, the action mechanism of the verbascoside is that the kidney protection function of the verbascoside is implemented through oxidative stress reaction regulation and control, TGF-beta / smad signaling channel inhibition and kidney fibrosis alleviation.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
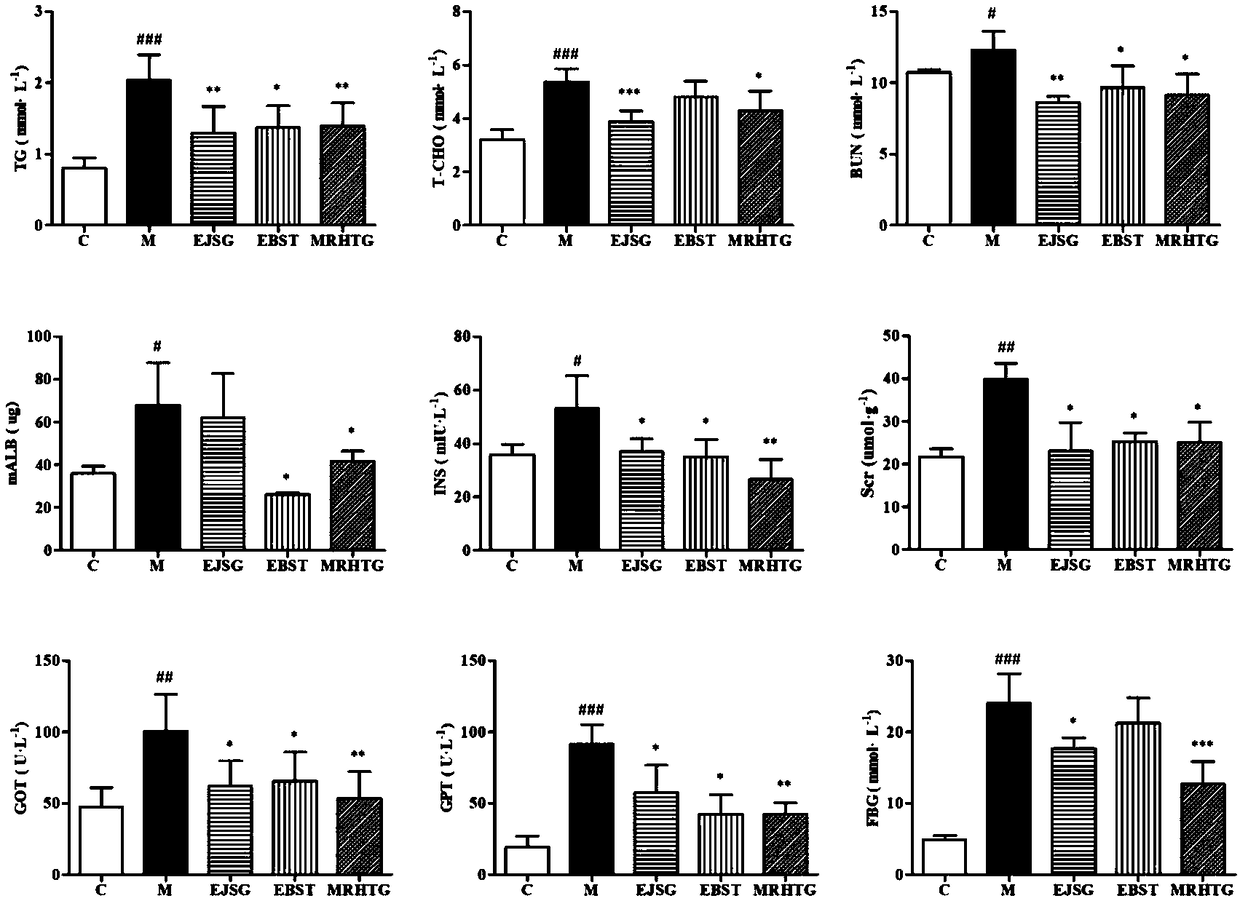
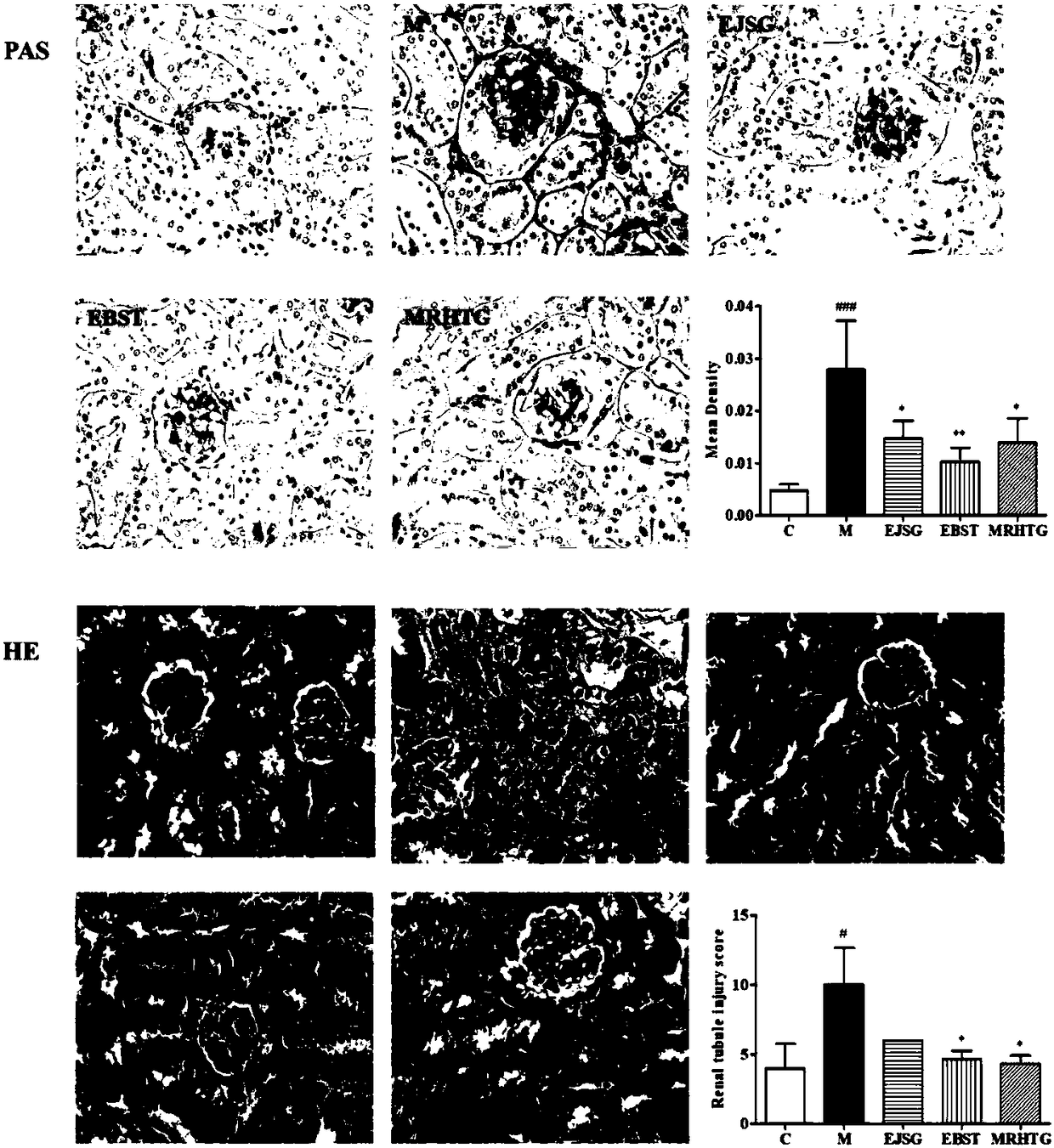
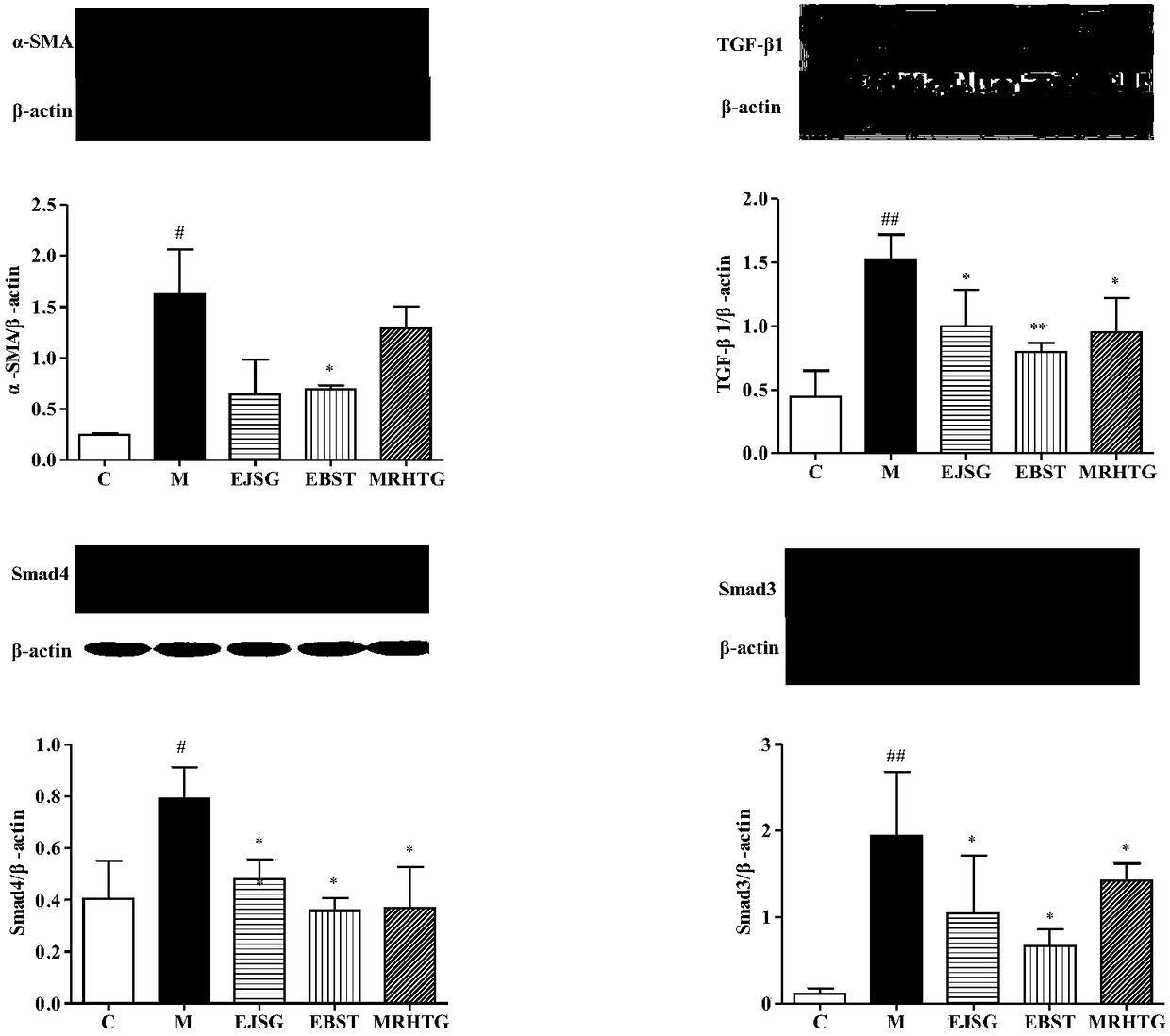
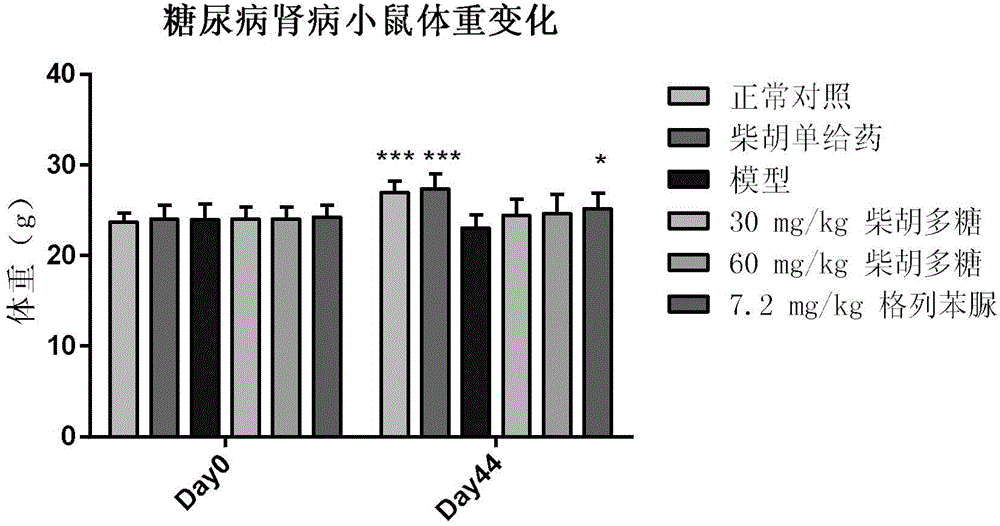
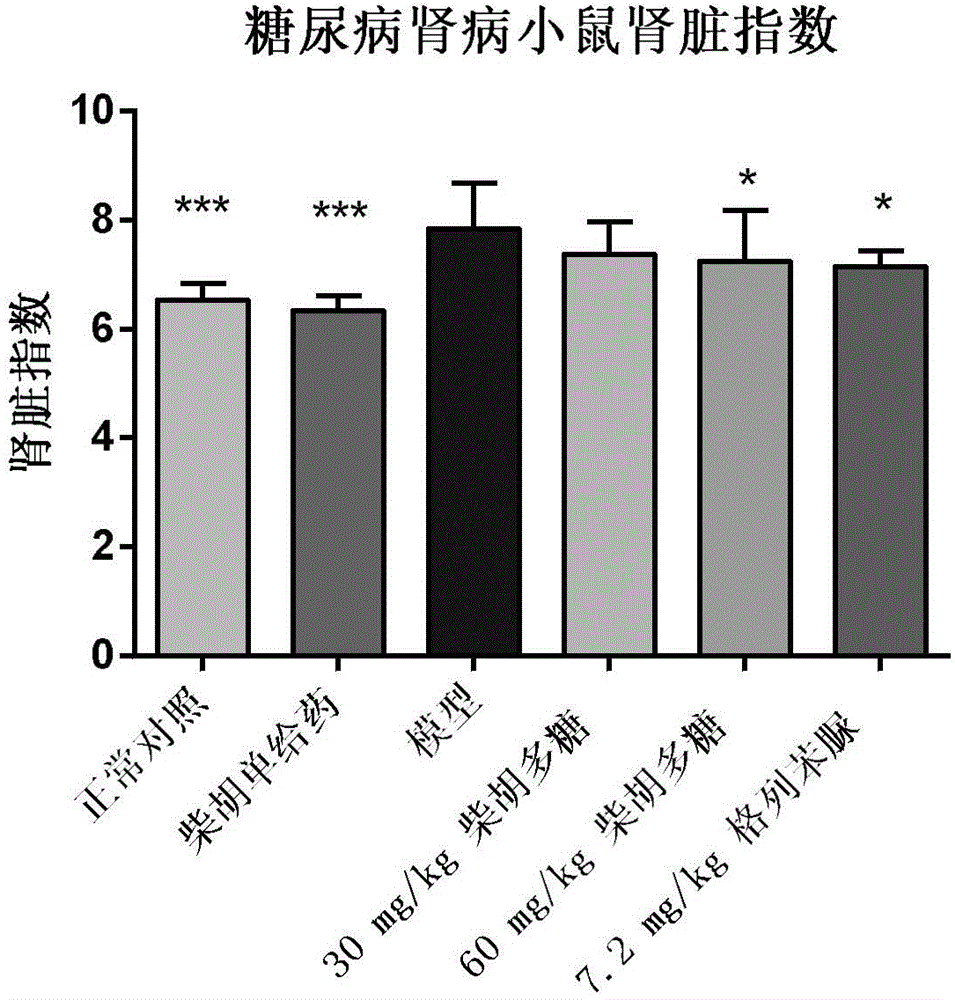
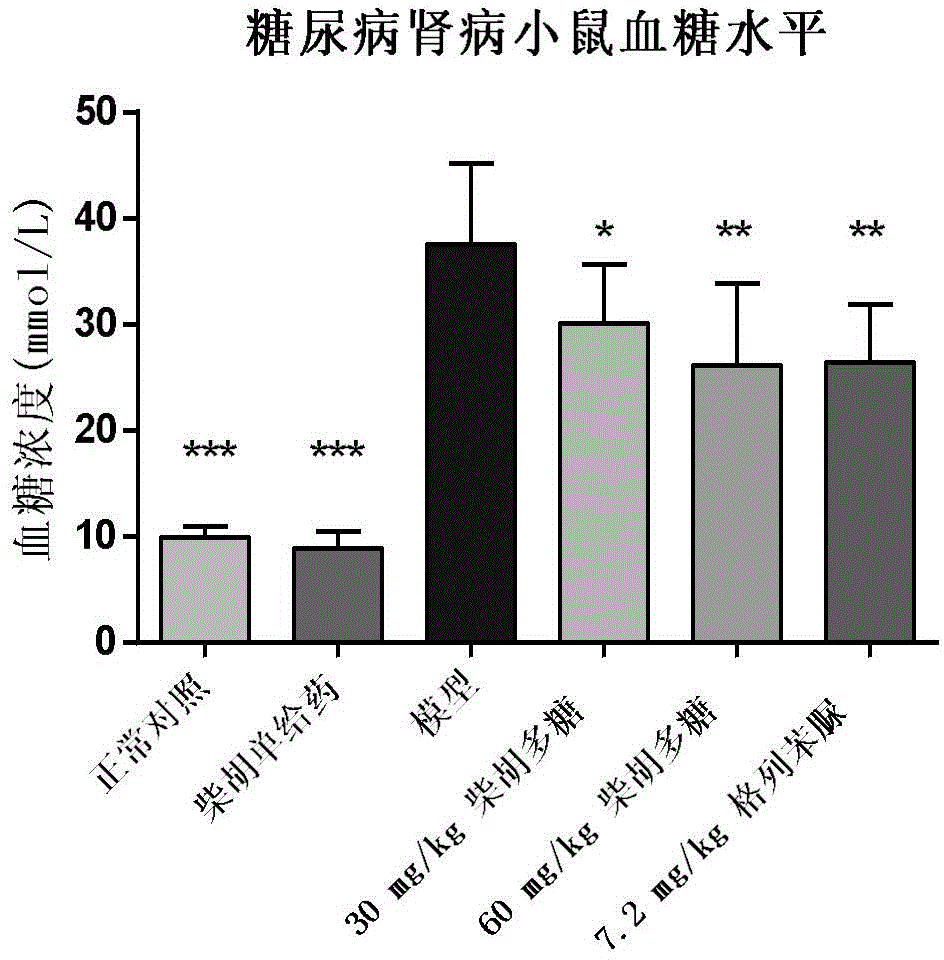
Application of bupleurum polysaccharide in preparation of drugs used for preventing and treating diabetic nephropathy
The invention specifically relates to application of bupleurum polysaccharide in preparation of drugs used for preventing and treating diabetic nephropathy, which belongs to the field of traditional Chinese medicine. According to results of integral experiments and in-vitro experiments, bupleurum polysaccharide extract has obvious prevention and treatment effect on diabetic nephropathy. Bupleurum polysaccharide can improve the body weight of diseased animals, substantially reduce the level of blood sugar and urine sugar, obviously promote secretion of insulin, decrease the level of serum creatinine, reduce secretion level of microalbuminuria and the renal index and enhance renal pathological symptoms, thereby exerting blood sugar-reducing and kidney-protecting effect. Bupleurum polysaccharide can reverse tubular cell transdifferentiation causec by TGF-beta. The bupleurum polysaccharide can be used for preparation of drugs used for preventing and treating diabetes and renal complications caused by diabetes.
Owner:FUDAN UNIV
Resonance scattering spectrometry for detecting trace of albumin in human urine
InactiveCN101320044AThe instrument is simpleSimple and fast operationScattering properties measurementsRaman scatteringHorizontal axisSpectroscopy methods
The utility model discloses a resonance scattering spectroscopy method for quickly and sensitively detecting microalbumin in human urine. According to the method provided by the utility model, the detection system of Malb the concentration of which is known is firstly prepared to work out the resonance scattering spectroscopy I 610nm of Malb; and then Mabl can be taken as a blank and the blank value (I 610) b can be measured, and Delta I can be worked out according to the equation: Delta I is equal to I 610nm subtracting (I 610) b; a working curve can be drawn by taking the concentration of added Malb solution as a horizontal axis, and taking Delta I as a vertical axis; and then, the system to be detected of a detecting sample can be prepared, and the Delta I of a sample to be detected can be worked out; and the Malb concentration of the sample can be also worked out according to the working curve. The method provided by the utility model has the advantages that instruments included in the method are simple and can be operated easily; film regent is stable and suitable for storage; colloidal gold can be prepared easily; catalysis performance is excellent and quick; and the sensitivity is high.
Owner:GUANGXI NORMAL UNIV
A kit for early diagnosis of diabetic nephropathy
ActiveCN107091927BImprove the detection rateEasy to operateMaterial analysisCreatinine riseHaptoglobin
Owner:ANHUI HUIBANG BIOLOGICAL ENG
Novel application of Elabela polypeptide and medicine thereof
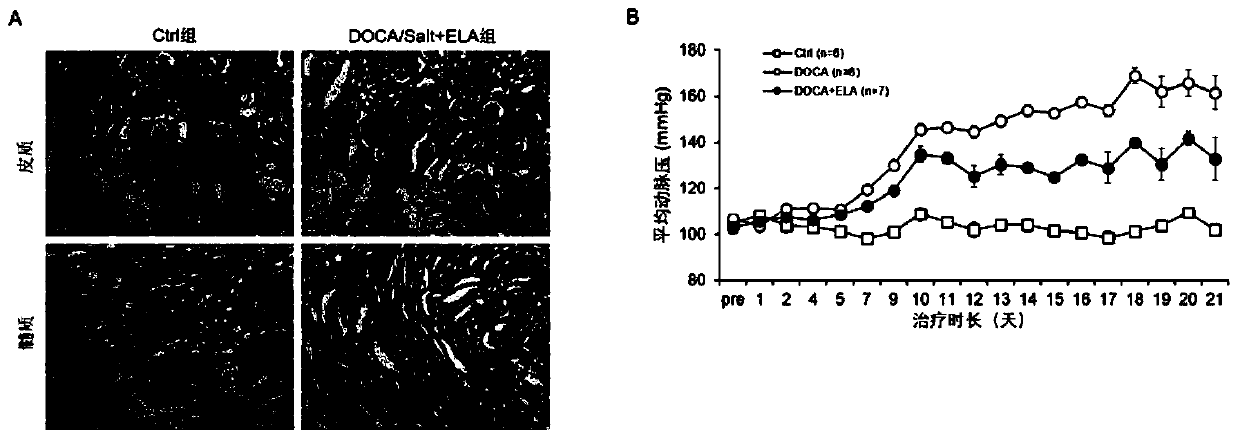
PendingCN110960671ARelieve arterial pressureReduce pathological damagePeptide/protein ingredientsUrinary disorderInflammatory factorsDisease
The invention discloses an application of Elabela polypeptide in preparation of a medicine or functional food for preventing and / or treating diseases related to chronic kidney injury and the medicinethereof. The diseases related to chronic kidney injury comprise (i) chronic renal insufficiency caused by hypertension; or ii) chronic renal insufficiency with hypertension. The Elabela polypeptide isstable in blood pressure reduction, can effectively relieve glomerular pathological injury of SD rats induced by deoxycorticosterone, reduce discharge of urine microalbumin, relieve development of renal fibrosis and reduce expression of inflammatory factors in kidney tissues, and has a remarkable effect on treatment of chronic kidney injury. Meanwhile, the Elabela polypeptide is an endogenous hormone synthesized by a human body and is expressed in kidney tissue, so that the toxic and side effects are smaller than those of the existing medicine for treating chronic kidney injury.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Test strip for combined and quantitative detection on urine microalbumin and cystatin C
InactiveCN108918876ALower requirementSimple and fast operationDisease diagnosisBiological testingHuman albuminPoint-of-care testing
The invention discloses a test strip for combined quantitative detection of urine microalbumin and cystatin C, comprising a bottom plate, a sample pad, a binding pad, a nitrocellulose membrane and anabsorbent pad, wherein the bonding pad is fixedly provided with an anti-human-albumin monoclonal antibody-fluorescent microsphere complex, a cystatin C paired monoclonal antibody-fluorescent microsphere complex and a goat anti-rabbit IgG-fluorescent microsphere complex; the anti-human albumin monoclonal antibody-fluorescent microsphere complex is formed by an anti-human albumin monoclonal antibodyand fluorescent microspheres coupled by an EDC and NHS two-step method, the cystatin C monoclonal antibody-fluorescent microsphere complex is obtained by a cystatin C monoclonal antibody and fluorescent microspheres coupled by the EDC and NHS two-step method, and the goat anti-rabbit IgG-fluorescent microsphere complex is formed by sheep anti-rabbit IgG and fluorescent microspheres coupled by anEDC one-step method. The invention provides the test strip for combined quantitative detection of the urine microalbumin and cystatin C, which has the advantages of simple and convenient operation, rapid detection, high sensitivity, low cost, and suitability for point of care testing.
Owner:河南省生物工程技术研究中心
Chinese medicinal compound with function of treating diabetes and preparation method and application thereof
ActiveCN101829271AHas a viscosityReduce generationAnthropod material medical ingredientsMetabolism disorderTG - TriglycerideShiny bugleweed
The invention discloses a Chinese medicinal compound with function of treating diabetes and a preparation method and application thereof. The Chinese medicinal compound consists of the following raw materials in part by weight: 8 to 20 parts of sealwort, 5 to 15 parts of roasted stiff silkworm, 5 to 15 parts of winged euonymus twig, 8 to 20 parts of Chinese wolfberry and 10 to 20 parts of hiraute shiny bugleweed herb. The Chinese medicinal compound provided by the invention is prepared and formulated by adopting the theory of syndrome differentiation and treatment according to the Chinese medicinal theory; experimental results show that the Chinese medicinal compound provided by the invention has multi-link and multi-target combined action, and can correct glucose metabolism turbulence, reduce blood sugar, improve pancreatic B cell function, improve insulin resistance and enhance immunologic function; and the experimental results show that the Chinese medicinal compound provided by the invention can effectively reduce serum total cholesterol, triglyceride, serum free fatty acid and urine micro-albumin, has good effects of resisting lipid peroxidation, improving blood flowage and preventing or delaying atherosclerosis, and can be used for preventing and treating diabetes mellitus associated with hyperlipaemia and cardiovascular diseases.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Urine microalbumin detection kit employing immunity transmission turbidity method
InactiveCN109946295AImprove accuracyImprove stabilityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsLinear correlationTurbidity
The invention discloses a urine microalbumin detection kit employing an immunity transmission turbidity method, and belongs to the technical field of clinical in-vitro detection reagents. The kit comprises a reagent R1 and a reagent R2. EDTA-Na2 is added to the reagent R2, so the stability of the reagent is improved, the linear correlation is good, the accuracy of the reagent is also good, and thereagent can be further popularized and used in the market.
Owner:济南腾奔生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com