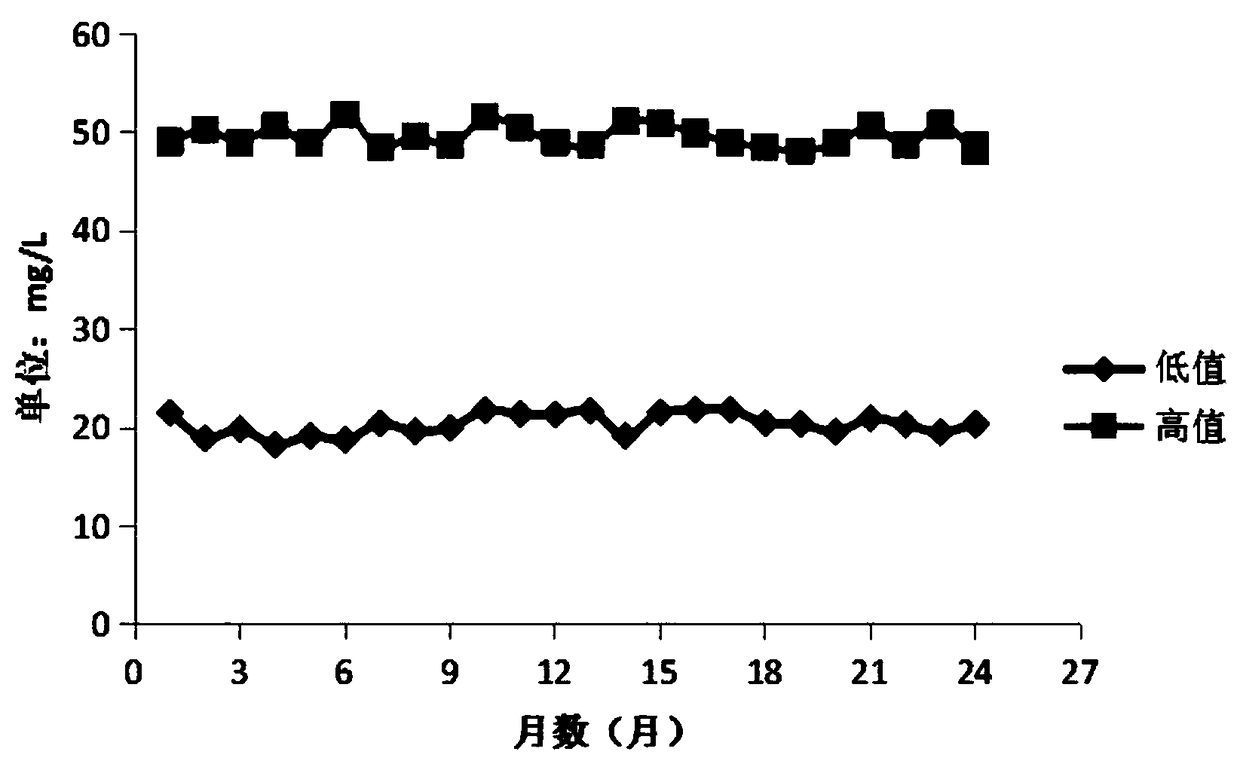
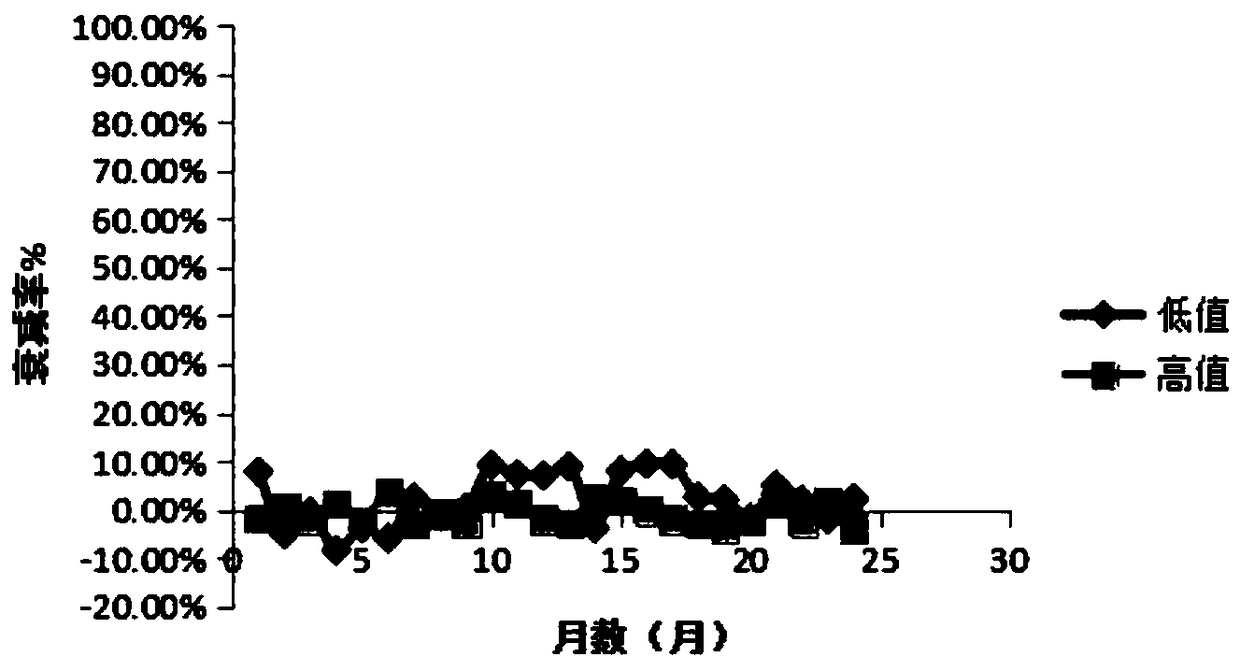
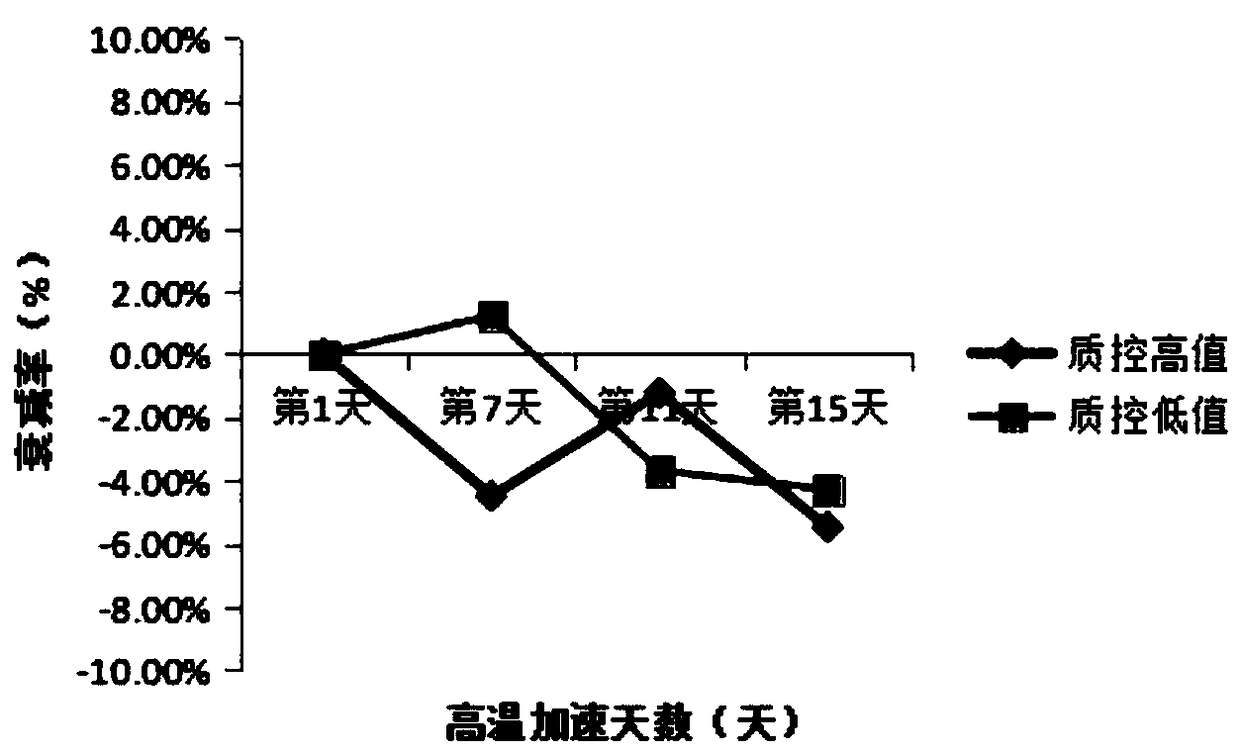
Stable urine microalbumin quality control material and preparation method thereof
A technology for urine microalbumin and quality control products, which is applied in the preparation of test samples, measuring devices, sampling, etc., can solve the problems of urine microalbumin control products attenuation and long-term storage, and achieve increased permeability, Avoid protein attenuation and apply to a wide range of pH
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The preparation method of the above-mentioned stable urine microalbumin protein control product comprises the following steps: respectively take 1L of purified water, add buffer salts of the stated concentration, protective agent A, protective agent B and surfactant in sequence, and stir in turn until completely Dissolve; filter once with a 0.22μm filter; adjust the pH value to 7.2-7.4 with concentrated HCl or 40mmol / LNaOH, the pH value is the value measured at 20±0.5°C, and then add albumin according to the concentration calculation , to obtain the urine microalbumin high-value quality control product and urine microalbumin low-value quality control product.
[0050] The buffer is phosphate buffer, HEPES buffer, PIPES buffer, MOPS buffer, disodium hydrogen phosphate-citric acid buffer, TRIS buffer, TRIS-HCl buffer, barbiturate sodium-hydrochloric acid buffer One or more of the above-mentioned buffer solutions can effectively stabilize the pH value of the quality contro...
Embodiment 1
[0056] A stable urine microalbumin low-value quality control product consists of the following components:
[0057]
[0058] The preparation process is as follows: add 1L of purified water into a clean preparation container, then add the above-mentioned buffer solution, salt, protective agent A, protective agent B and surfactant in sequence, and stir until completely dissolved; use Filter once with a 0.22um filter membrane; adjust the pH value to 7.2-7.4 (20±0.5°C) with concentrated HCl or 40mmol / LNaOH, and add albumin according to the concentration calculation. Pack according to the specification of 1ml / bottle.
[0059] The details of the equipment used are high-density polyethylene barrels or bottles, 100,000-grade electronic balances, pH meters and matching calibration buffers, 1000mL graduated cylinders and special filters.
Embodiment 2
[0061] A stable urine microalbumin low-value quality control product consists of the following components:
[0062]
[0063]
[0064] The preparation process is as follows: add 1L of purified water into a clean preparation container, then add the above-mentioned buffer solution, salt, protective agent A, protective agent B and surfactant in sequence, and stir until completely dissolved; use Filter once with a 0.22um filter membrane; adjust the pH value to 7.2-7.4 (20±0.5°C) with concentrated HCl or 40mmol / LNaOH, and add albumin according to the concentration calculation. Pack according to the specification of 1ml / bottle.
[0065] The details of the equipment used are high-density polyethylene barrels or bottles, 100,000-grade electronic balances, pH meters and matching calibration buffers, 1000mL graduated cylinders and special filters.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com