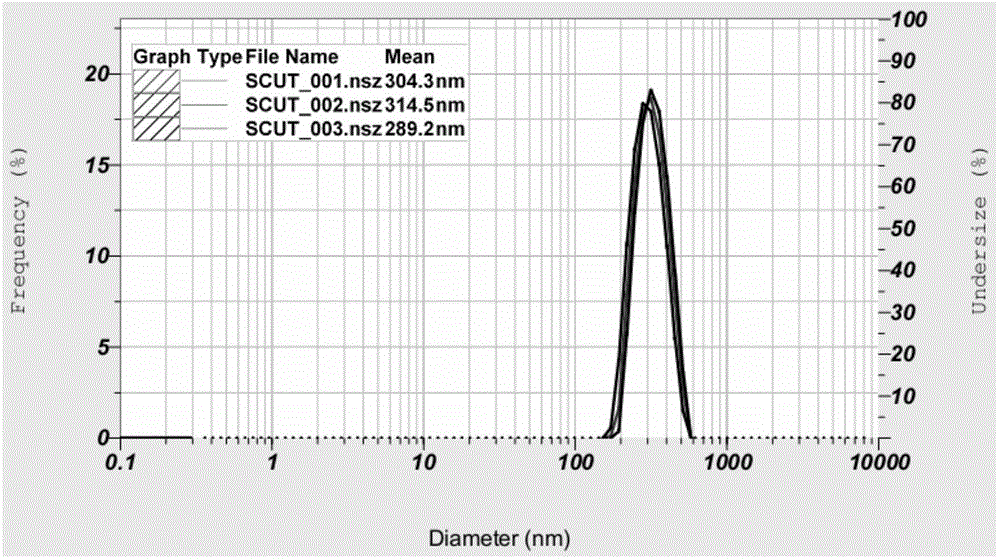
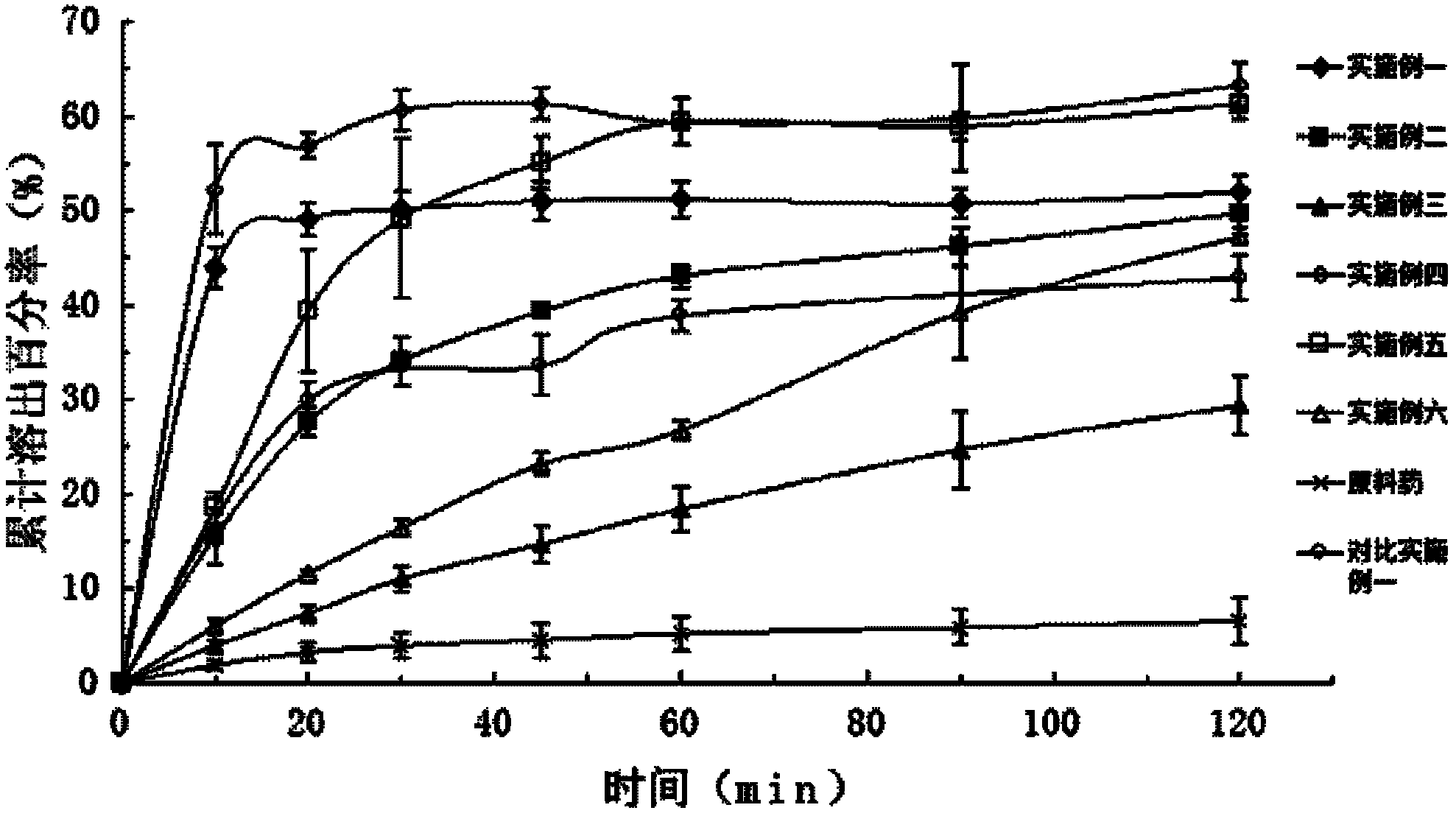
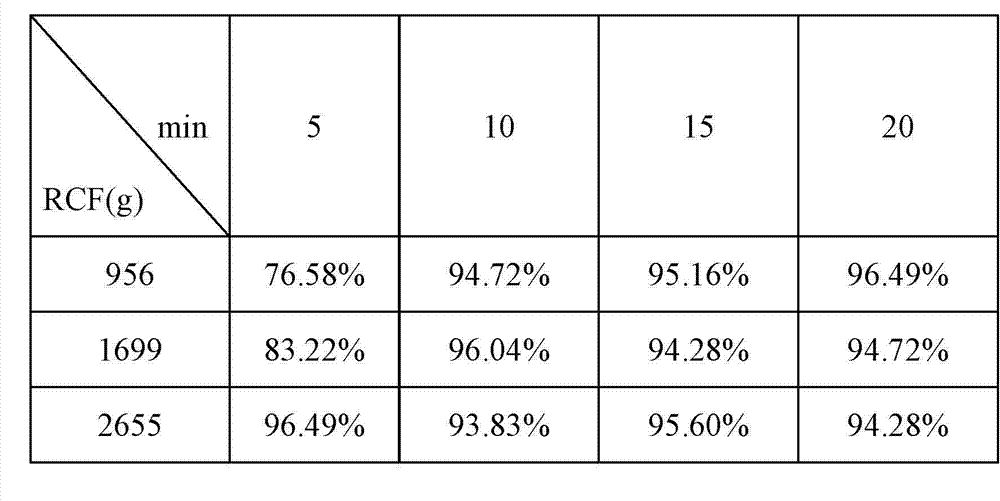
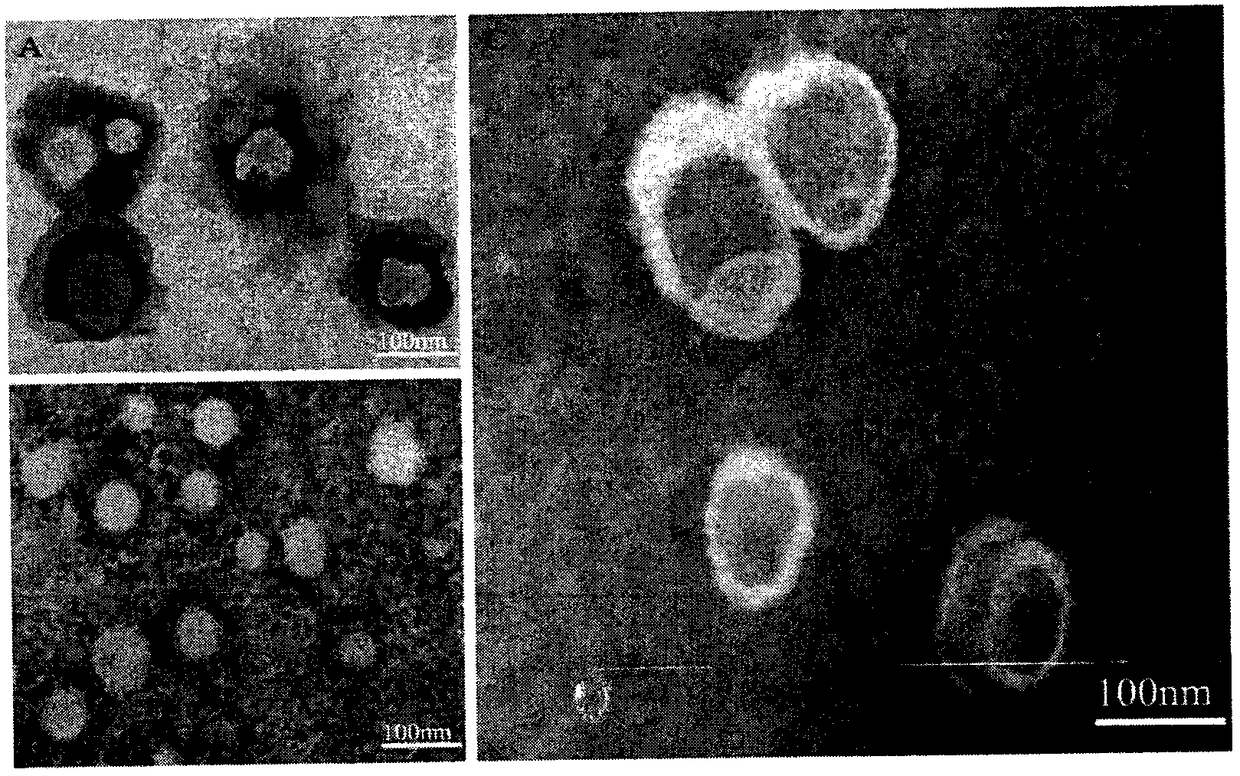
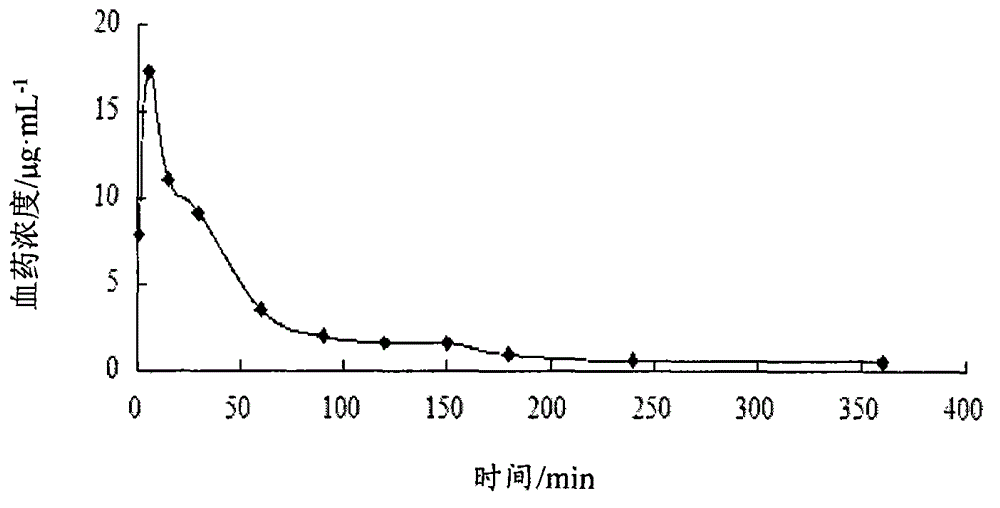
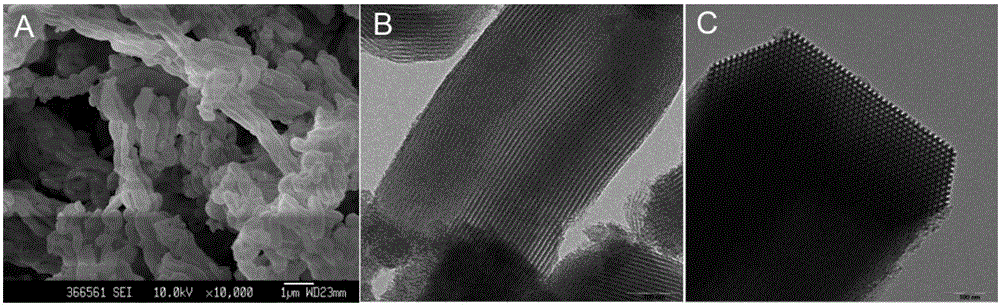
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
92results about How to "Improve bioavailability in vivo" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions comprising fenofibrate and rosuvastatin
InactiveUS20050096391A1Improve bioavailability in vivoSubstance may accumulateBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor rosuvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCrosuvastatin) of between about 150 and about 12,000. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and rosuvastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
Compositions comprising fenofibrate and pravastatin
InactiveUS20050096390A1Substance may accumulateImprove bioavailability in vivoBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor pravastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCpravastatin) of between about 90 and about 6300. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and pravastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
Preparation method of coating with bone induction and antibiosis functions on surface of medical metal
InactiveCN103203039AImprove bioavailability in vivoSmall toxicityCoatingsProsthesisAntibiosisNanoparticle coating
The invention discloses a method for preparing a nanoparticle coating with the bone induction and antibiosis functions on the surface of a medical metal. According to the method, nanoparticles with different electric properties as used as a carrier, the nanoparticles are formed by mutual crosslinking of high-molecular polymers, and growth factors and antibiotics are respectively loaded in the process of preparing the nanoparticles with dissimilar electric properties, wherein factors and factor-friendly polyanions are sufficiently mixed to react first and are then immobilized into the nanoparticles through ionic crosslinking; and amino-containing antibiotics are grafted to a polyaldehyde anionic polymer through chemical crosslinking first and are then loaded into the nanoparticles through ionic crosslinking. The two types of nanoparticles are alternately assembled on the surface of the medical metal through electrostatic adsorption, covalent crosslinking is also formed among the particles, and the stability of the coating is enhanced. The coating prepared by using the method has the function of controlling adsorption and release of the growth factors and the antibiotics under the normal physiological environment, the activity of the growth factors are keep well and a strong bone induction function is achieved; and in addition, the antibiotics can be slowly released for a long time, and a good antibiosis effect is achieved.
Owner:SOUTHWEST JIAOTONG UNIV
RhBMP-2-loaded chitosan microspheres, preparation method thereof and application thereof
InactiveCN102138905AImprove adsorption capacityConducive to the maintenance of activityPeptide/protein ingredientsSkeletal disorderChitosan microspheresHuman bone
The invention discloses recombinant human bone morphogenetic protein-2 (rhBMP-2)-loaded chitosan microspheres, a preparation method thereof and application thereof. A substrate of the rhBMP-2-loaded chitosan microspheres is chitosan microspheres, and a loaded medicament is rhBMP-2. Chitosan reacts with an ionic cross-linking agent, and a reaction product is processed by solution of sodium hydroxide to prepare the chitosan microspheres; and the rhBMP-2 is loaded by a passive loading mode, so that the rhBMP-2 is adsorbed on the chitosan microspheres, wherein a weight ratio of the rhBMP-2 to thechitosan is (1-30):100. The preparation method is simple and easy, has a mild preparation condition and is favorable for maintaining the bioactivity of the rhBMP-2. The rhBMP-2-loaded chitosan microspheres can control and release the rhBMP-2 for a long term, promote the formation of new bones, and can be used for preparing medicaments for treating bone coloboma, nonunion and delayed union.
Owner:EAST CHINA UNIV OF SCI & TECH
Compound turmeric lipid cubic liquid crystalline nano-particles and preparation method thereof
InactiveCN104622812AImprove stabilityImprove solubilityAntipyreticAnalgesicsLiquid crystallineSolubility
The invention provides compound turmeric lipid cubic liquid crystalline nano-particles and a preparation method thereof. The compound turmeric lipid cubic liquid crystalline nano-particles comprise the following components in parts by weight: 0.05-0.15g of phytantriol, 20-30mg of F127, 5-7mg of turmeric, 1-3mg of piperine and 15-25mL of water. Compared with a raw medicine group consisting of turmeric and piperine, the compound turmeric lipid cubic liquid crystalline nano-particles provided by the invention are significantly improved to show the properties that the in-vitro stability and solubility of turmeric can be improved on the one hand, the in-vivo bioavailability of turmeric can be significantly improved on the other hand, slow release and targeting effects can also be achieved, and the curative effects of turmeric on therapy in vivo can be enhanced so as to lay a foundation for turmeric to play the extensive pharmacological effects of resisting tumor, improving cardiovascular functions, resisting inflammation, resisting virus, protecting the liver and enhancing the immunity.
Owner:崔景朝
Telmisartan solid dispersion and preparation method thereof
InactiveCN102178642AImprove solubilityImprove bioavailability in vivoOrganic active ingredientsPharmaceutical delivery mechanismMass ratioDissolution
The invention relates to the technical field of medicines, in particular to a telmisartan solid dispersion as a specific angiotensin II receptor antagonist (ATI type) and a preparation method thereof. The telmisartan solid dispersion comprises medicine telmisartan, a carrier and an alkali matter, and also comprises a surfactant, wherein the carrier is a hydrophilic polymeric carrier, and the mass ratio of the medicine telmisartan to the hydrophilic polymeric carrier to the alkali matter to the surfactant is 1:(1-9):(0.1-0.5):(0.1-0.5). The telmisartan solid dispersion has better dissolution rate during the use, and is convenient for gastrointestinal tract absorption, thereby improving bioavailability.
Owner:SUZHOU UNIV
Valsartan solid dispersion and preparation method thereof
InactiveCN102357078AImprove solubilityImprove bioavailability in vivoPowder deliveryPharmaceutical non-active ingredientsValsartanBioavailability
The invention relates to the technical field of medicine, particularly a valsartan solid dispersion and a preparation method thereof. The valsartan solid dispersion comprises a pharmaceutical valsartan, a carrier and an alkaline matter, and can also comprises a surfactant, wherein the carrier is a hydrophilic high-polymer carrier, and the mass ratio of pharmaceutical valsartan:hydrophilic high-polymer carrier:alkaline matter:surfactant is 1:(2-4):(0.1-0.3):(0.1-0.2). The valsartan solid dispersion has favorable leaching rate in use, and can be conveniently absorbed by the gastrointestinal tract, thereby improving the bioavailability.
Owner:SUZHOU UNIV
Silybin nanometer suspension and preparation method thereof
InactiveCN106580877ASmall particle sizeNarrow distributionOrganic active ingredientsMetabolism disorderMass ratioDissolution
The invention relates to the field of medicinal preparations, and provides a silybin nanometer suspension and a preparation method thereof. The silybin nanometer suspension is prepared from silybin, a stabilizer and water. The mass ratio of silybin to the stabilizer is 2:1-10:1. The mass ratio of the stabilizer in the suspension is 2-5%, and the mass ratio of the medicine is 10-20%. An improved medium grinding method is adopted, the obtained silybin nanometer suspension is small in grain size, narrow in distribution and good in stability, the medicine dissolution rate can be remarkably increased, and the medicine in-vivo bioavailability can be improved.
Owner:CHINA PHARM UNIV
Myricetin lipid microcapsule taking lecithin as carrier and preparation method thereof
InactiveCN102920681AImprove stabilityGood biological activityOrganic active ingredientsMetabolism disorderChemistryStearic acid
The invention provides a myricetin lipid microcapsule taking lecithin as a carrier and a preparation method thereof. The myricetin lipid microcapsule is prepared by the following preparation method: weighing myricetin, rape lecithin or soybean lecithin, and stearic acid monoglyceride in proportion, adding adequate anhydrous ethanol for dissolving, evaporating the obtained solution under the conditions of vacuum degree of 0.080-0.085 MPa and temperature of 50-70 EDG C to recover ethanol to be dry, adding adequate tween 40 aqueous solution with the concentration of 0.3-0.4g / L into a residual material, fully dissolving under the ultrasonic condition, and standing for 5-10 minutes after completely dissolving to prepare the myricetin lipid microcapsule. The preparation method utilizes the lecithin of the vegetable oil materials for preparing the myricetin into the lipid microcapsule, with the particle size controlled to be 100nm-300nm; and the stability of the prepared plant flavonoid lipid microcapsule is obviously improved compared with flavonoid powder, especially the in vivo bioavailability is increased. Animal studies show that the absorption rate of the lipid microcapsule is increased by more than 2 times compared with original powder so as to more prominently give play to the biological activities of the plant flavonoid.
Owner:ZHEJIANG UNIV OF TECH
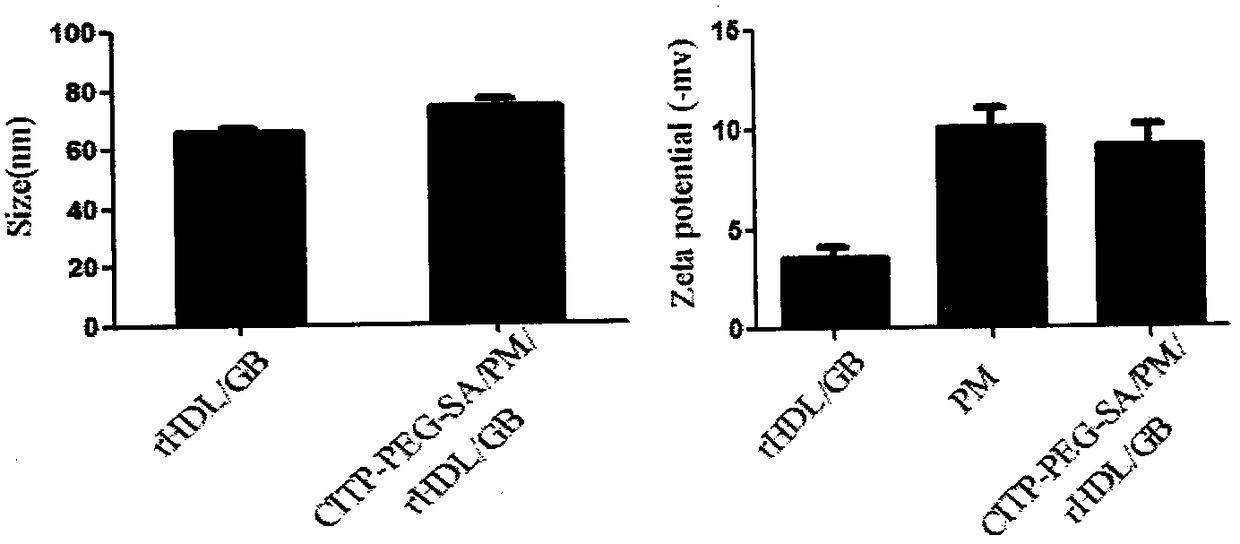
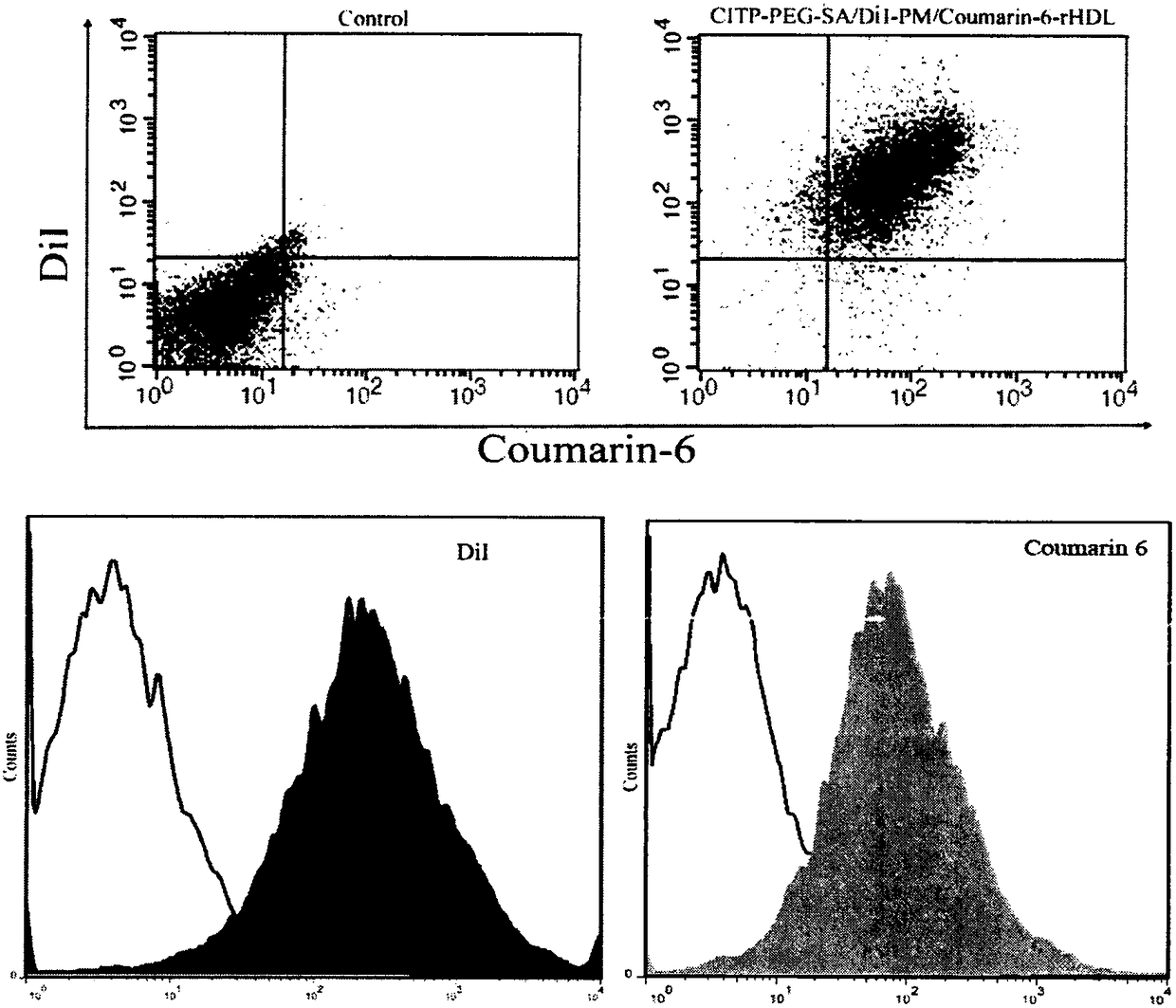
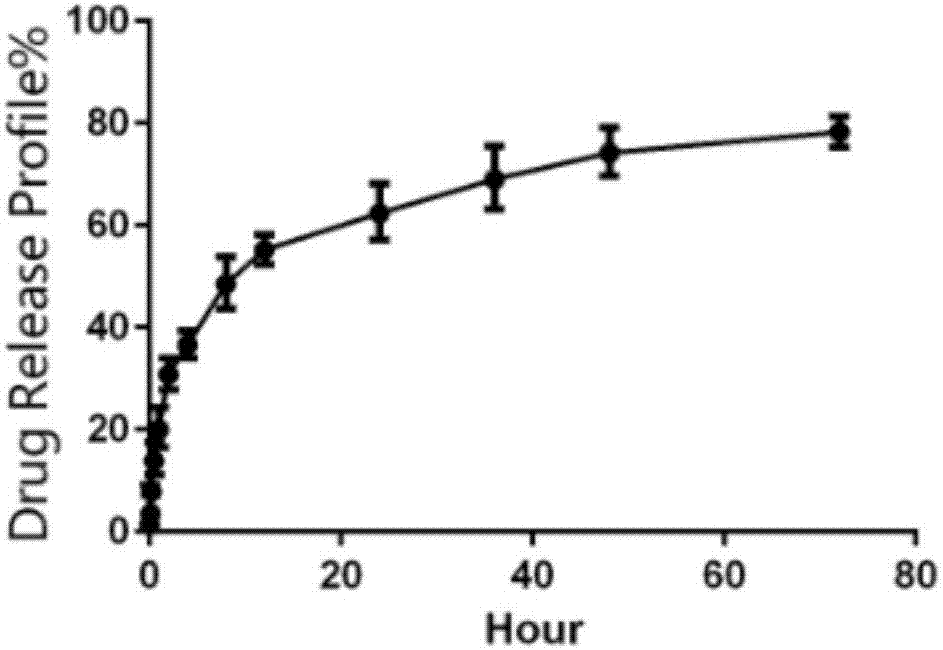
Preparation and applications of biologically camouflaged targeting nano drug delivering system for treating ischemic cerebral stroke
ActiveCN108815134AGood water solubilityInhibit aggregationOrganic active ingredientsAntipyreticBilobalidesTarget peptide
The invention relates to preparation and applications of a biologically camouflaged targeting nano drug delivering system for treating ischemic cerebral stroke. The nano drug delivering system is compose of a drug, an inner core drug carrier, a biologically camouflaging shell, and a target finding material, wherein the drug is bilobalide B, the inner core drug carrier is recombinant high density lipoprotein, a drug is physically embedded into the drug carrier to form a drug loaded inner core; the biologically camouflaging shell is a blood platelet membrane, the drug loaded inner core is embedded in the blood platelet membrane in a co-extrusion mode to form a biomimetic drug loaded nano particle; the target finding material is a cerebral ischemia targeting peptide (CITP), and the CITP is used to modify the surface of the biomimetic drug loaded nano particle to form the biologically camouflaged targeting nano drug delivering system. Recombinant high density lipoprotein is used to wrap bilobalide B to form the drug loaded inner core, a blood platelet membrane and a blood platelet with a CITP modified surface are taken as the biomimetic shells to construct a nano particle for treatingischemic cerebral stroke, the circulation time of the nano particle in human body is prolonged, and the nano particle has a good targeting performance.
Owner:CHINA PHARM UNIV
Apixaban pellet and preparation method thereof
InactiveCN106913528ASimple methodEasy to operateOrganic active ingredientsGranular deliveryAdhesiveBioavailability
The invention relates to an apixaban pellet and a preparation method thereof. The apixaban pellet is composed of, from interior to exterior, a blank pellet core, a drug-containing layer and a coating layer, wherein the drug-containing layer is composed of micronized apixaban, a binder and a disintegrating agent. The apixaban pellet comprises, by weight, 60 to 84 parts of the blank pellet core, 2 to 7 parts of the micronized apixaban, 3 to 10 parts of the binder, 5 to 20 parts of the disintegrating agent and 2 to 8 parts of the coating layer. Thus, the apixaban pellet obtained in the invention has a rough and regular shape and uniform particle size; and compared with commercially available tablets, the apixaban pellet provided by the invention has better stability, faster dissolving-out rate and higher bioavailability.
Owner:WATERSTONE PHARMA WUHAN
Spherical micelle for packaging ginsenoside Rg3 and preparation method and application thereof
InactiveCN107320448AGood water solubilityIncrease intakeOrganic active ingredientsPharmaceutical non-active ingredientsOrganic solventVacuum drying
The invention discloses a preparation method of a spherical micelle for packaging ginsenoside Rg3. The method includes the steps of weighing and fetching ginsenoside Rg3 and carrier materials to be dissolved in appropriate quantity of organic solvents, removing the organic solvent after the mixtures of ginsenoside Rg3 and the carrier materials are completely dissolved, and placing the mixtures in a vacuum drying box over a night; adding appropriate amount of deionized water into the mixtures for hydration, and conducting ultrasonic treatment when the mixtures are completely dissolved; using a filtering membrane to filter the hydration liquid after the ultrasonic treatment to obtain a transparent polymeric micelle solution, and then freezing and drying the solution to obtain the spherical micelle. The invention also discloses a spherical micelle for packaging the ginsenoside Rg3 and an application of the spherical micelle for packaging the ginsenoside Rg3.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Ginkgolide composition and application thereof
InactiveCN103263411AOvercoming Quality Control FlawsEasy to separateOrganic active ingredientsCardiovascular disorderVascular diseaseMedicine
The present invention discloses a Ginkgolide composition and a method for the preparation thereof. The composition includes two active components, Ginkgolide A and Ginkgolide B, and the weight ratio of Ginkgolide A to Ginkgolide B is 1: (2-9). A strong synergistic effect is found between Ginkgolide A and Ginkgolide B, which has a remarkable application effect on the treatment of cardio-cerebral vascular disease. The invention realizes simultaneous determination for the component content of the Ginkgolides preparation. Experiments show that the Ginkgolide A and Ginkgolide B have the advantages of good separating degree with better linear relation, reproducibility, precision, stability, and recovery rate. The dosage form of the preparations provided by the present invention is beneficial for improving the bioavailability in vivo and drug efficacy.
Owner:JIANGSU KANION PHARMA CO LTD +1
Atorvastatin calcium submicroemulsion and preparation method thereof
InactiveCN107157929AExpand the form of medicationImprove in vivo efficacyMetabolism disorderPharmaceutical non-active ingredientsHigh concentrationSolubility
The present invention provides an atorvastatin calcium submicroemulsion and a preparation method thereof. The atorvastatin calcium submicroemulsion comprises atorvastatin calcium (phospholipid complex form), an oil phase solvent, an emulsifier, a stabilizer, a potential modifier, glycerol and water, wherein the atorvastatin calcium phospholipid complex is used as a precursor drug so as to increase the solubility of atorvastatin calcium in the oil phase, and a molar ratio of atorvastatin calcium to the phospholipid is 1:1. According to the present invention, the drug-containing phospholipid complex is adopted as the precursor drug of atorvastatin calcium, such that the solubility and the stability of the drug in the oil phase are improved, the drug loading is increased, and the use of the high-concentration organic solvent can be avoided; and compared to the commercially available lipitor (atorvastatin calcium tablet), the atorvastatin calcium submicroemulsion of the present invention has the following characteristics that the absorption of the drug in the small intestine is improved, and the high blood drug level is provided and is maintained for a long time after the atorvastatin calcium submicroemulsion is orally taken so as to effectively improve the bioavailability of the drug.
Owner:GUANGDONG PHARMA UNIV
Nasal gel agent containing risperidone and preparation method of nasal gel agent
InactiveCN104398474AProlong the action timeImprove bioavailability in vivoOrganic active ingredientsNervous disorderHigh absorptionClinical efficacy
The invention discloses a nasal gel agent containing risperidone and a preparation method of the nasal gel agent, and belongs to the field of pharmaceutical preparations. The nasal gel agent comprises risperidone, a matrix material, pharmaceutically acceptable water and other pharmaceutically effective auxiliary materials such as an osmotic pressure regulator, an absorption enhancer, a preservative, a humectant and a pH regulator. The preparation method comprises the following steps: dissolving risperidone and the other pharmaceutically effective auxiliary materials in the water in sequence, adding into a blank gel matrix in sequence, and fully and uniformly grinding. The nasal gel agent is simple in preparation process and has the characteristics of remarkable brain-targeting characteristic, high absorption and action speed, high bioavailability, good clinical curative effect and high convenience and safety in use.
Owner:内蒙古医科大学附属医院
Fenofibrate solid dispersion body, and preparation method and application thereof
ActiveCN106727338AImprove bioavailability in vivoImprove liquidityOrganic active ingredientsPowder deliveryPolyethylene glycolMesoporous silica
The invention relates to a fenofibrate solid dispersion body, and a preparation method and application thereof. The fenofibrate solid dispersion body is mainly prepared from following raw materials: fenofibrate, mesoporous silica, and a macromolecule carrier material, wherein the content of fenofibrate (in mass) is 10-40%; the mass ratio of mesoporous silica to the macromolecule carrier material is 1:(0.2-10); the macromolecule carrier material is selected from at least one of copovidone, povidone, polyethylene glycol, and a polyethylene glycol / vinyl-epsilon-caprolactam / vinyl acetate copolymer. The fenofibrate solid dispersion body disclosed by the invention is good in in vitro dissolution effect, and high in vivo bioavailability; and by using the fenofibrate solid dispersion body, powder has high liquidity, and drugs have high physical stability.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Breviscapine composition nano-particle and preparation method thereof
InactiveCN103690489AImprove solubilityImprove in vitro dissolutionOrganic active ingredientsPowder deliveryPrillNanoparticle
The invention belongs to the field of pharmacy, and relates to a breviscapine composition nano-particle and a preparation method thereof. The provided novel breviscapine composition nano-particle is prepared by a solution containing the breviscapine, a polymer, a surfactant and a solvent with the adoption of an electrostatic spraying technology. The composition preparation device is simple, the parameters are controllable, the drug loading capacity is high, , the direct and continuous nano-particles with uniform particle size distribution can be obtained, the specific surface area is increased, and the dissolution in vitro and bioavailability of the breviscapine are improved remarkably.
Owner:NANJING DACHUANG BIOLOGICAL SCI & TECH CO LTD
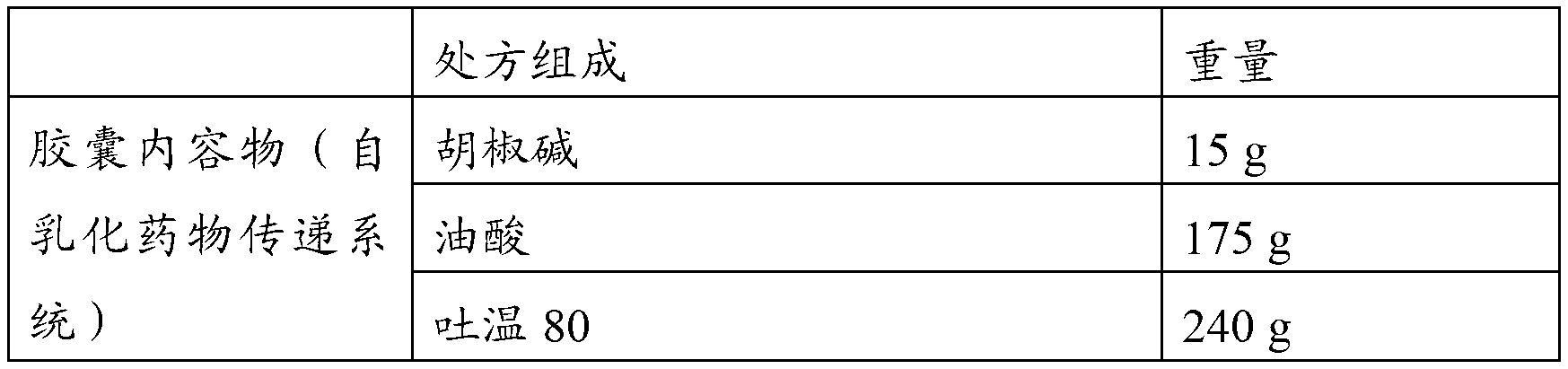
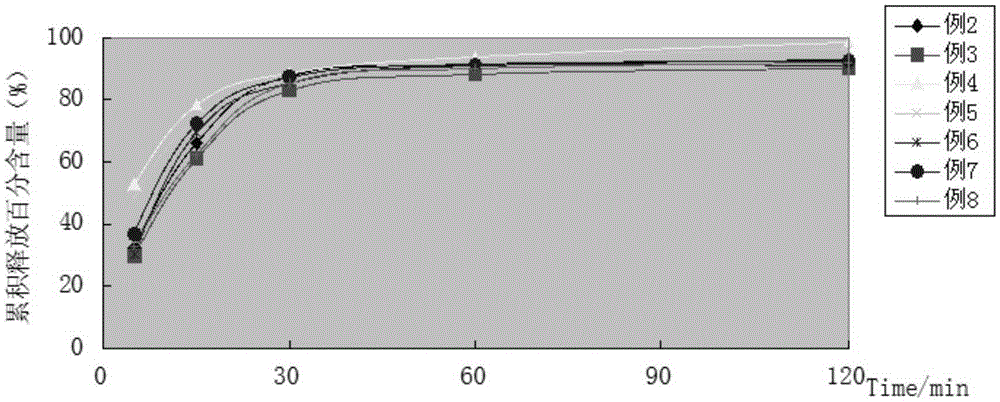
Piperine self-emulsifying soft capsule and preparation method thereof
ActiveCN103181913AImprove solubilityImprove in vitro solubilityOrganic active ingredientsNervous disorderPharmacologic actionMedicine
The invention discloses a piperine self-emulsifying soft capsule and a preparation method thereof. The preparation agent can improve the dissolution rate of piperine and bioavailability and further improves the curative effect. Piperine serving as the bioavailability reinforcer is adopted to prepare the self-emulsifying soft capsule, the bioavailability of other drugs can be improved in various ways, such as absorption, metabolism and the like, and great clinic significance is achieved on some medicines with excellent pharmacologic action, difficulty in absorption and easiness in metabolism. The piperine self-emulsifying soft capsule comprises piperine, an oil phase, a surfactant and a cosurfactant, of which the mass proportion is 1:(2.5-32.5):(15-35):(2.5-12.5). The preparation method comprises the following steps: dissolving a proper amount of piperine in the prescribed cosurfactant, uniformly mixing, then adding prescribed oil phase and surfactant, obtaining a transparent and homogeneous solution after complete dissolution, and pressing to obtain the soft capsule by adopting a conventional method.
Owner:HARBIN MEDICAL UNIVERSITY
Ginkgolide B quick release pellet and preparation method thereof
ActiveCN105412022AGood stabilityImprove solubility and dissolution rateOrganic active ingredientsNervous disorderDrugOral medication
The invention relates to a ginkgolide B quick release pellet and a preparation method thereof, and belongs to the field of medicinal preparations. The ginkgolide B quick release pellet comprises a blank pellet core and a medicated layer, wherein the weight ratio of the blank pellet core to the medicated layer is 1:(1.1-10.0); the medicated layer is prepared from ginkgolide B, a crystal stabilizer and a curing stabilizer, wherein the weight ratio of the ginkgolide B to the curing stabilizer is 1:(1.0-2.5). The ginkgolide B quick release pellet disclosed by the invention is rapid in effect, good in absorption, safe and stable, the bioavailability in vivo can be remarkably improved, and the individual difference of oral administration is reduced. The drug loading capacity of the quick release pellet is improved, at the same time, the situations that the grain diameter is increased and precipitation is generated caused by long-term standing of medicament are avoided, and the quick release pellet has high clinical use value, and is simple in preparation technique and suitable for industrial production.
Owner:JIANGSU KANION PHARMA CO LTD
Pyridazinone derivative prodrugs or pharmaceutically acceptable salt thereof as well as pharmaceutical composition and application of prodrugs
ActiveCN108383798AGood solubility in the bodyImprove bioavailability in vivoOrganic active ingredientsOrganic chemistrySolubilityBioavailability
The invention belongs to the technical field of medicinal chemistry, in particular relates to pyridazinone derivative prodrugs or a pharmaceutically acceptable salt thereof, and relates to a pharmaceutical composition of the prodrugs and an application in preparing an antitumor medicine. The pyridazinone derivative prodrugs having a structural formula (I) shown in the description provided by the invention have good in-vivo solubility, after the prodrugs enter a body, pyridazinone medicine molecules are released by metabolism, so that in-vivo bioavailability of the pyridazinone medicine molecules is greatly improved, and the pyridazinone medicine molecules bind to an in-vivo PARP enzyme in an irreversible manner to play pharmacological effects, and the antitumor activity is remarkable.
Owner:SHANGHAI BIOBOND PHARMA
Glibenclamide nanocrystal preparation and preparation method thereof
InactiveCN105878194AImprove efficiencyHigh yieldPowder deliveryMetabolism disorderFreeze-dryingDissolution
The invention relates to a glibenclamide nanocrystal preparation and a preparation method and belongs to the technical field of pharmaceutical preparations. The glibenclamide nanocrystal preparation is prepared from, by weight, glibenclamide 35%-93%, a stabilizer 2%-7% and a freeze-drying protective agent 28-60%, wherein the ratio of the glibenclamide to the stabilizer is 30:1 to 15:1. The stabilizer is hydroxypropyl methyl cellulose, polyvinylpyrrolidone, poloxamer or a surface active agent. The glibenclamide nanocrystal preparation is controllable in particle size, good in stability and small in side and toxic effect and obviously improves the dissolution and bioavailability of the glibenclamide.
Owner:SHENYANG PHARMA UNIVERSITY
Nimodipine nanocrystallization method and dry suspension thereof
InactiveCN107837231ASmall particle sizeNarrow distributionOrganic active ingredientsNervous disorderBiochemical engineeringNimodipine
The invention relates to the field of pharmaceutical preparations and provides a nimodipine nanocrystallization method and a dry suspension thereof. The method comprises the following steps: nano-crystallizing nimodipine by adopting a medium grinding method; preparing a nano suspension in the presence of a stabilizer and water; then adding a suspending agent and a spray drying protective agent orother auxiliary materials to prepare nano suspension spray drying powder by further spraying and drying; and then adding other auxiliary materials and uniformly mixing to obtain the nimodipine dry suspension. The dry suspension is good in redispersibility, good in stability and convenient to take; nimodipine which is redispersed has small grain size and narrow distribution, and the potential of nimodipine meets the demand. The dissolution rate of the drug is improved obviously, and the bioavailability in vivo of the drug can be improved. Meanwhile, the dry suspension formula and preparation process are simple, and the preparation is safe and effective, has relatively low cost and is easy for industrial production.
Owner:CHINA PHARM UNIV
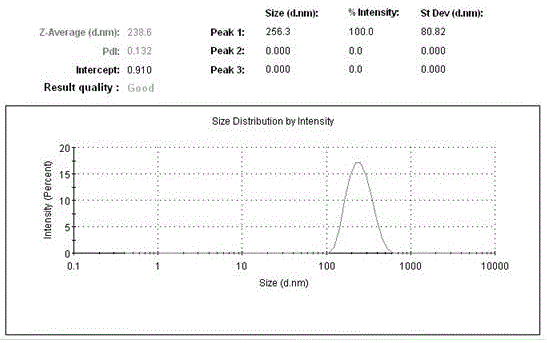
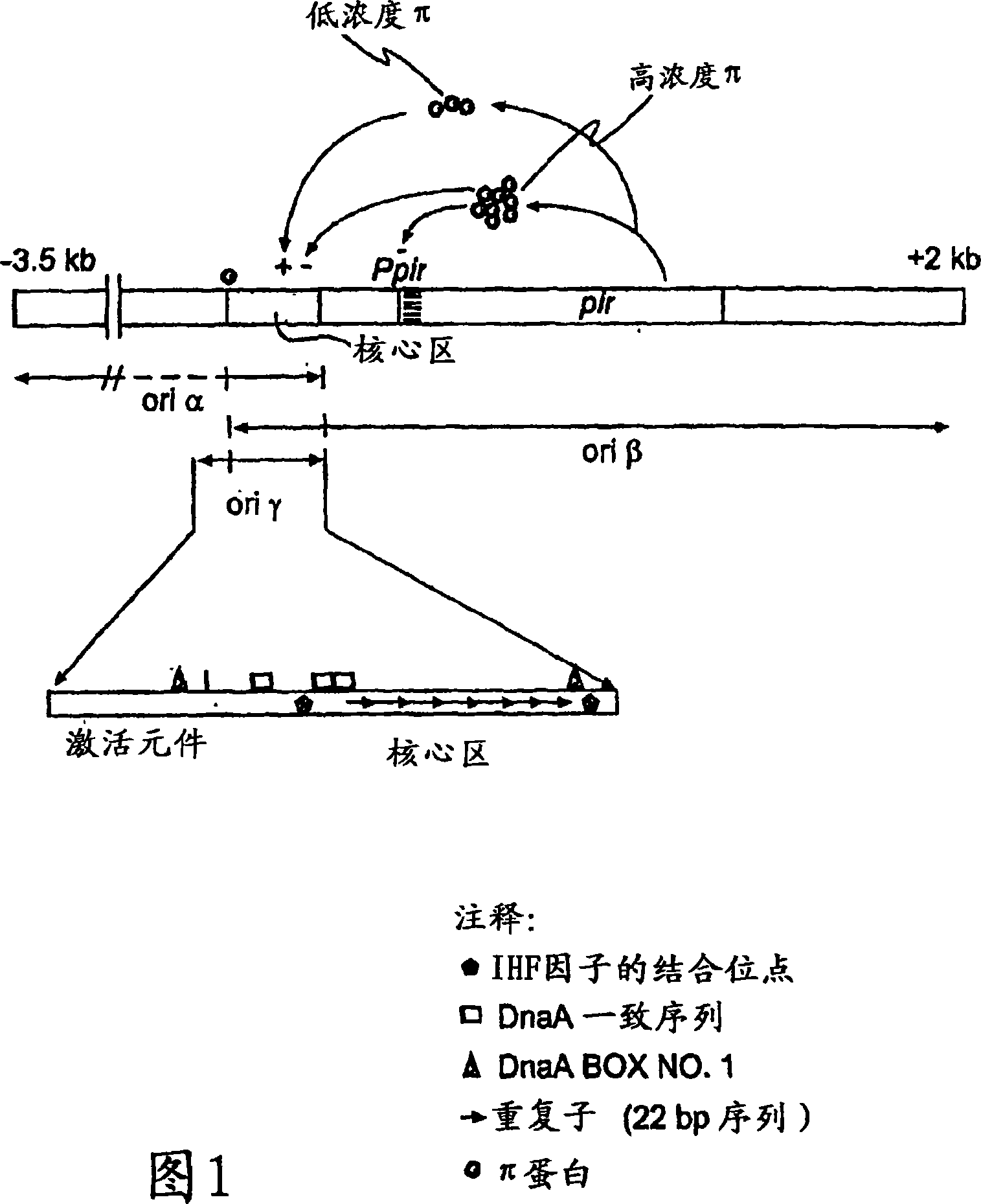
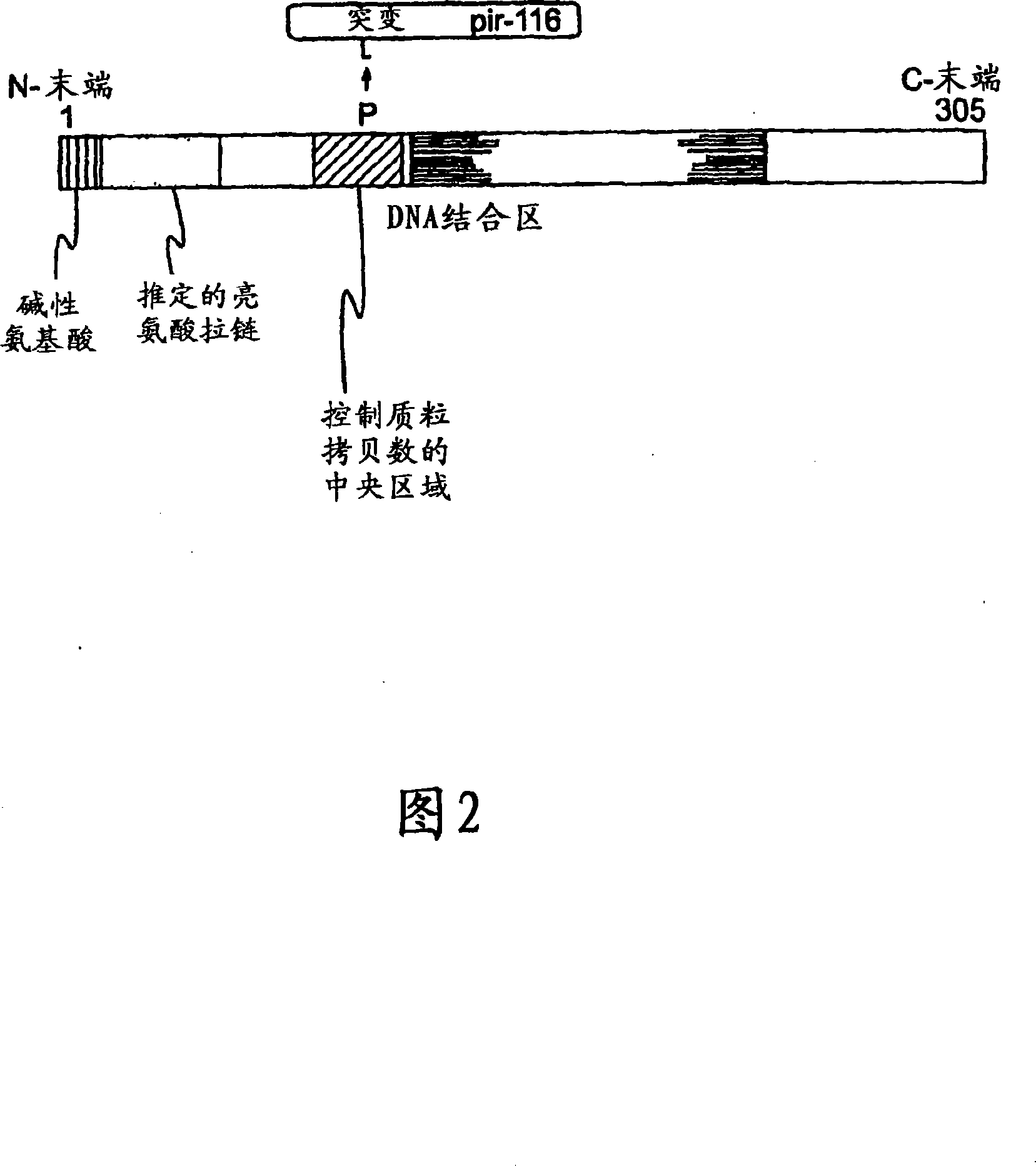
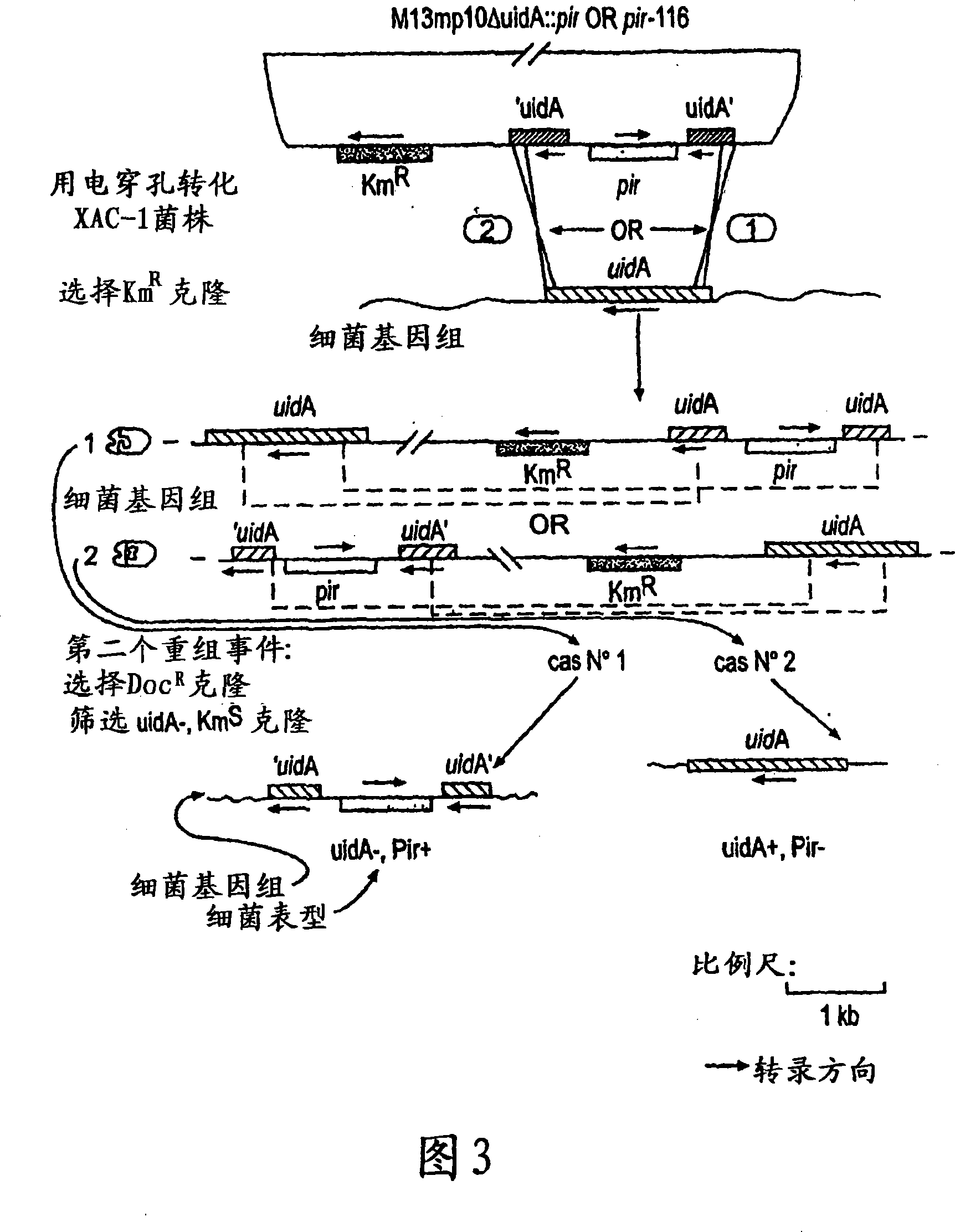
Circular DNA molecule having a conditional origin of replication, process for their preparation and their use in gene therapy
InactiveCN101182534AShorten the lengthImprove bioavailability in vivoNervous disorderBacteriaHeterologousOrigin of replication
A prokaryotic recombinant host cell comprising a heterologous replication initiation protein that activates a conditional origin of replication and an extrachromosomal DNA molecule comprising a heterologous therapeutic gene and a conditional origin of replication whose functionality in the prokaryotic recombinant host cell requires a replication initiating protein which is foreign to the host cell is described. The host cell may comprise a pir gene having at least one mutation, which may occur in the pir gene copy number control region, the pir gene leucine zipper-like motif, or the pir gene DNA binding region.
Owner:AVENTIS PHARMA INC
Pramipexole dihydrochloride sustained-release tablet composition and preparation method thereof
ActiveCN105456216AFacilitated releaseRelease stabilityOrganic active ingredientsNervous disorderSustained Release TabletMethyl cellulose
The invention provides a pramipexole dihydrochloride sustained-release tablet composition and a preparation method thereof. The composition comprises the following components by weight: 0.1%-1% of pramipexole dihydrochloride, 10%-40% of hydroxypropyl methyl cellulose, 25%-50% of polyacrylic acid resin, 20-60% of pregelatinized starch, 0.3%-1.5% of colloidal silicon dioxide and 0.5%-1% of magnesium stearate.
Owner:JIANGSU SHENLONG PHARMA +2
Composite nanometer particle obtained by coating curcumin eutectic crystal/piperine with polymers, and preparation of composite nanometer particle and application thereof to slow release pharmaceutical preparation
ActiveCN109432055AImprove physical and chemical propertiesImprove bioavailabilityAntibacterial agentsAntipyreticSupercritical anti solventAnti solvent
The invention belongs to the field of medicines, and particularly relates to a composite nanometer particle obtained by coating curcumin eutectic crystal / piperine with polymers. The composite nanometer particle comprises a core and a shell coating the core, wherein the core is a mixture of the curcumin eutectic crystal and the piperine; the shell is a hydrophilic polymer. The invention further discloses a preparation method and application of the composite nanometer particle. According to the preparation method provided by the invention, the composite nanometer particle with an in-situ coatingstructure is successfully prepared by creatively utilizing a curcumin eutectic crystal compound through a supercritical fluid anti-solvent technology (SAS). The invention provides the composite nanometer particle with the advantages of better dissolution performance, stability and bioavailability.
Owner:MEDONCARE PHARMA CO LTD
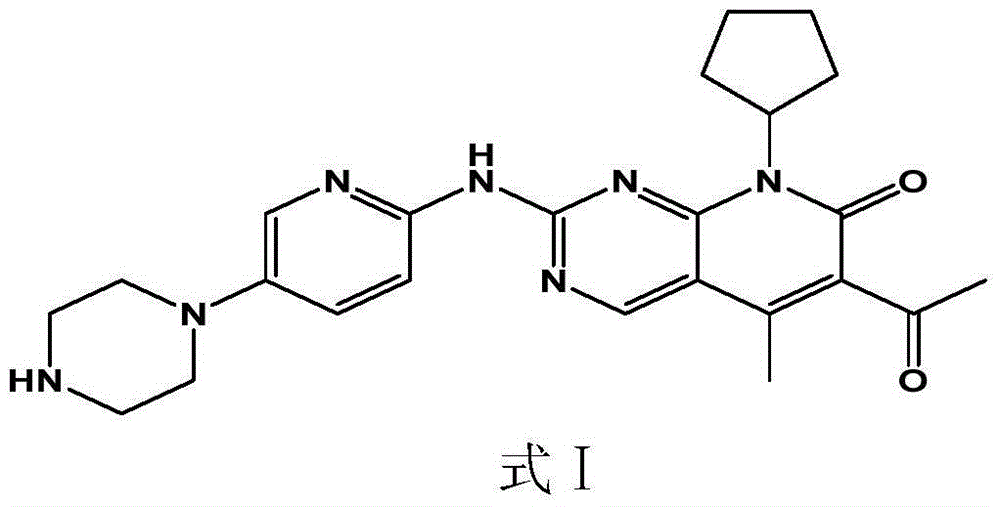
Pharmaceutical preparation containing cyclin inhibitor, and preparation method thereof
InactiveCN105616418AImprove dissolution behaviorImprove bioavailability in vivoOrganic active ingredientsCapsule deliverySulfonatePharmaceutical drug
The present invention discloses a pharmaceutical preparation containing a cyclin inhibitor, and a preparation method thereof, and particularly relates to a pharmaceutical preparation adopting 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazine-1-yl-pyridine-2-yl-amino)-8H-pyrido[2,3-d]pyrimidine-7-one represented by a formula I or a salt thereof as an active component, wherein the salt comprises a hydrochloride and an isethionate, and the dosage form of the pharmaceutical preparation comprises tablets and capsules, and has good stability and excellent dissolution behavior. The formula I is defined in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
Medicine composition containing magnesium isoglycyrrhizinate and preparation method
ActiveCN105616376ARealize joint usePromote dissolutionDigestive systemAntiviralsPatient complianceDissolution
The invention belongs to the field of medicine and particularly relates to an intelligent and novel medicine composition having anti-hepatitis B virus activity and a preparation method thereof.The medicine composition contains an enteric magnesium isoglycyrrhizinate sheet core and further contains other types of anti-hepatitis B virus medicine.To be specific, further improvement is made on the basis of the prior art, a dissolution layer is further added outside an enteric magnesium isoglycyrrhizinate coating layer, and unexpectedly, the magnesium isoglycyrrhizinate medicine composition high in dissolution rate and good in bioavailablity is obtained.Furthermore, the dissolution layer is sequentially wrapped by a medicine containing layer and a protection layer, so that a compound preparation is formed, the compound preparation is convenient to take, and patient compliance is greatly improved.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Anticoagulant immediate-release pharmaceutical preparation and preparation method thereof
ActiveCN108420798AMaximum drug absorptionHigh in vivo bioavailability and plasma concentrationOrganic active ingredientsPharmaceutical non-active ingredientsDrugPharmaceutical formulation
The invention relates to an anticoagulant immediate-release pharmaceutical preparation and a preparation method thereof, and belongs to the technical field of medicine, wherein the anticoagulant immediate-release pharmaceutical preparation contains a vicagrel compound or a pharmaceutically acceptable form thereof, wherein the preparation is in the form of tablets or capsules, the vicagrel or the pharmaceutically acceptable form has a suitable particle size, and the D90 is less than 50 [mu]m. According to the present invention, the in vitro dissolution test results of the pharmaceutical preparation formed by the drug-containing granules of the present invention show that the rapid release characteristic is provided, the considerable advantage is provided in pharmacokinetics in vivo, and thehigh area under the cure (AUC) and the rate (Cmax) are provided. The present invention further provides a preparation method of the anticoagulant immediate-release pharmaceutical preparation, whereinthe excellent-stability immediate-release preparation capsule or tablet can be obtained through the optional preparation step combination according to the drug-containing granule formula of the present invention.
Owner:JIANGSU VCARE PHARMATECH
Paeonol self-microemulsion preparation and preparation method thereof
InactiveCN109498573AImprove solubilityImprove bioavailability in vivoAntibacterial agentsAntimycoticsSolubilityEconomic benefits
The invention discloses a paeonol self-microemulsion preparation and a preparation method thereof. The paeonol self-microemulsion preparation is prepared from raw materials in percentage by weight asfollows: 0.1%-35% of paeonol, 5%-70% of oil phase, 5%-80% of an emulsifier and 5%-60% of a co-emulsifier, the liquid self-microemulsion preparation is prepared or a solid self-microemulsion preparation is further prepared from the liquid self-microemulsion preparation and an adsorbent. The paeonol self-microemulsion preparation and the preparation method thereof have the advantages as follows: theproblem of low paeonol solubility is solved, solubility of a drug is greatly increased, and in-vivo bioavailability of the drug is improved; by means of the solid self-microemulsion, softening and oil spilling of a traditional soft capsule can be improved, and stability of the preparation is improved; the required raw materials are easily available, the method is simple and feasible, special equipment is not needed, large-scale industrial production is facilitated, and the economic benefits are remarkable.
Owner:镇江市疾病预防控制中心 +1
Formulations of Phenyl Uracil Compounds
ActiveUS20140378490A1Improve bioavailabilityHigh dissolution rateOrganic active ingredientsBiocideCompound aActive agent
A pharmaceutical product comprising at least one phenyl uracil-based pharmaceutically active agent or an agent of related structural type and processes for obtaining such product.
Owner:ABBVIE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com