Fenofibrate solid dispersion body, and preparation method and application thereof
A technology of solid dispersion and fenofibrate, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas. It can solve the problems of poor powder fluidity and achieve high dissolution rate, Improvement of wettability and improvement of in vitro dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of Mesoporous Silica SBA-15
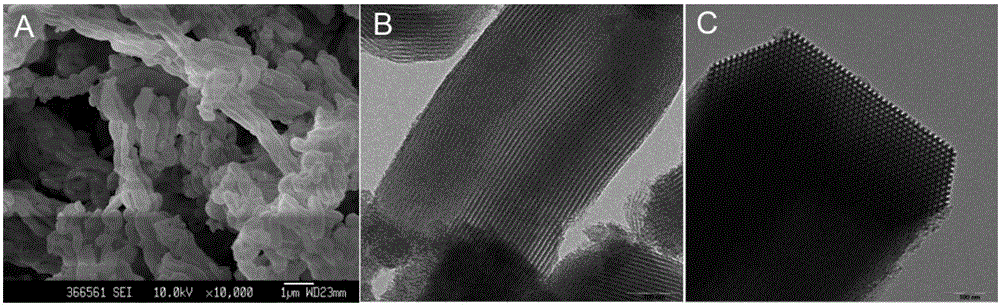
[0040]Dissolve 8g of triblock copolymer polyvinyl ether-polypropylene ether-polyethylene (P123) in 363mL of 2mol / L hydrochloric acid, and stir at 40°C until the solution forms a light blue transparent homogeneous system. Under continuous stirring, slowly add 17.53g of tetraethyl orthosilicate (TEOS) dropwise, continue stirring for 24h, and move the reactant to the reaction solution for crystallization at 100°C for 48h. Then the product is taken out and cooled, collected by centrifugation, washed with water until neutral, washed with ethanol, and collected by centrifugation. The product was vacuum-dried at 40° C., and then calcined in a muffle furnace at 550° C. for 6 hours to remove the template agent P123 to obtain the mesoporous silica SBA-15.
[0041] The appearance and pore structure of the synthesized mesoporous silica SBA-15 were characterized by scanning electron microscope and transmission electron microscope...
Embodiment 2
[0043] Example 2: Preparation and phase characterization of fenofibrate solid dispersion
[0044] This embodiment prepares the solid dispersion of fenofibrate according to the weight ratio of raw materials shown in the table below:
[0045] prescription number Fenofibrate SBA-15 PVP / VA64 1 2 copies 1 copy 7 copies 2 2 copies 2 copies 6 servings 3 2 copies 3 copies 5 copies 4 2 copies 4 parts 4 parts
[0046] The preparation of the fenofibrate solid dispersion of the present embodiment comprises the following steps:
[0047] 1. Loading of fenofibrate
[0048] Dissolve fenofibrate in absolute ethanol, add mesoporous silica SBA-15 prepared in Example 1, stir magnetically for 6 hours, and remove ethanol by rotary evaporation to obtain drug-loaded SBA-15. SBA-15 is designated FNB-SBA-15.
[0049] 2. Preparation of fenofibrate solid dispersion by hot melt extrusion
[0050] Grind and mix FNB-SBA-15 and PVP / VA64, add the...
Embodiment 3
[0055] Embodiment 3: Investigation on the powder properties of fenofibrate solid dispersion
[0056] The powder properties of the fenofibrate solid dispersion prepared in Example 2 were investigated, mainly including the density, angle of repose and contact angle of the powder. The objects of investigation are fenofibrate solid dispersions FSP1, FSP2, FSP3, FSP4 and FNB-SBA-15 prepared according to Example 2 (the mass ratio of fenofibrate: SBA-15 is 2:4) and fenofibrate Special raw materials. The test method is as follows:
[0057] 1. Tap density and bulk density
[0058] The sample is passed through a 80-mesh sieve, and accurately weighed (W powder ), carefully transfer to a graduated cylinder, and record the initial volume of the sample (V o ), mechanically vibrate the measuring cylinder containing the sample powder, tap until the volume no longer changes, record the powder volume at this time (V f ). with the formula W powder / V o and W powder / V f Calculate the b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com