Key intermediate of telmisartan, synthesis method thereof and method for synthesizing telmisartan by intermediate
A technology of telmisartan and a synthetic method, applied in directions such as organic chemistry, can solve problems such as unfavorable analysis judgment and central control, high price, unfavorable product separation and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
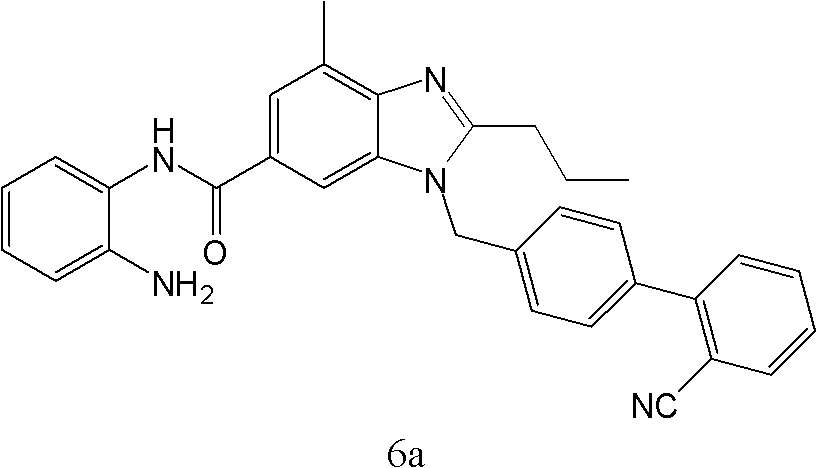
[0057] Example 1: N-(2-aminophenyl)-1-((2′-cyanobiphenyl-4-yl)methyl)-4-methyl-2-propyl-1H-benzimidazole- Preparation of 6-Carboxamide (Compound 6a).
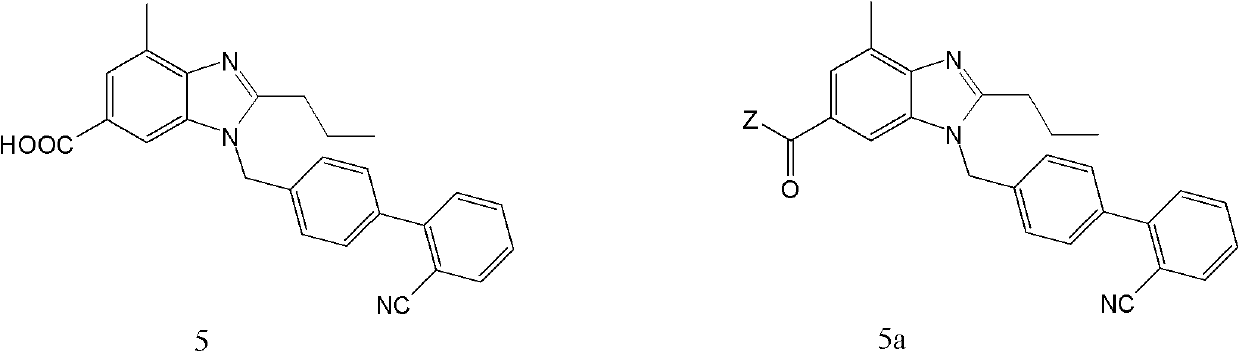
[0058] In a 1000ml four-necked flask, add 50g (0.122mol) 1-((2′-cyanobiphenyl-4-yl)methyl)-4-methyl-2-propyl-1H-benzimidazole-6- Carboxylic acid (5), 300ml of dichloroethane and 200ml of thionyl chloride were heated to 80°C, refluxed for 4 hours, evaporated to dryness under reduced pressure, and the residue was dissolved in 200ml of dichloromethane for the next amide reaction.
[0059] Add 26g (0.244mol) of o-phenylenediamine, 300ml of dichloromethane and 49g (0.488mol) of triethylamine into a 1000ml four-neck flask, control the temperature below 10°C and add the acid chloride solution in one step dropwise, after the addition is complete, stir for 3h , add 400ml of water, separate the liquids, add 1000ml of water and 100ml of hydrochloric acid to the organic phase for extraction, adjust the pH value of the aqueous phase to 9 w...
Embodiment 2
[0062] Example 2: N-(2-aminophenyl)-1-((2′-cyanobiphenyl-4-yl)methyl)-4-methyl-2-propyl-1H-benzimidazole- Preparation of 6-Carboxamide (Compound 6a).
[0063] In a 1000ml four-necked flask, add 50g (0.122mol) 1-((2′-cyanobiphenyl-4-yl)methyl)-4-methyl-2-propyl-1H-benzimidazole-6- Carboxylic acid (5), 15g (0.139mol) of triethylamine, 300ml of tetrahydrofuran, stirring and dissolving, under the protection of nitrogen, lower the temperature to below 5°C, add 13g of methyl chloroformate dropwise, and keep the temperature below 10°C for 3 hours. Then, 26 g (0.241 mol) of o-phenylenediamine was added to the flask, 22 g (0.204 mol) of triethylamine was added dropwise under ice-cooling, and the mixture was reacted at room temperature for 4 h under nitrogen protection. Aftertreatment according to the method of Example 1, the product N-(2-aminophenyl)-1-((2'-cyanobiphenyl-4-yl)methyl)-4-methyl-2-propyl- 1H-benzimidazole-6-carboxamide 55.1g, yield 90.5%, detection parameters are as fol...
Embodiment 3
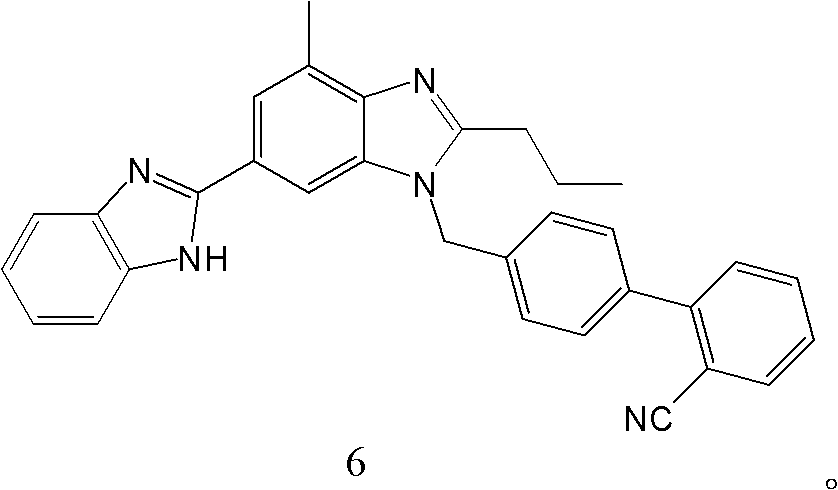
[0066] Example 3: 4'-[(4'-methyl-2'-propyl[2,6'-di-1H-benzimidazol]-1'-yl)methyl]-1,1'-link Preparation of Benzene-2-carbonitrile (Compound 6).
[0067] In a 1000ml three-necked flask, add 50g (0.1mol) N-(2-aminophenyl)-1-((2′-cyanobiphenyl-4-yl)methyl)-4-methyl-2-propane Base-1H-benzimidazole-6-carboxylic acid amide and 300ml (4.5mol) of glacial acetic acid, heated up to 120°C, refluxed for 4h, cooled to room temperature, concentrated to dryness under reduced pressure, added 500ml of water to the residue to dilute, Adjust the pH value to 9 with ammonia water, precipitate a white solid, filter and dry to obtain the product 4'-[(4'-methyl-2'-propyl[2,6'-di-1H-benzimidazole]-1 '-yl)methyl]-1,1'-biphenyl-2-carbonitrile 47.2g, yield 98%, detection parameters are as follows:
[0068] HNMR1 (CDCl 3 ): 1.01(t, 3H), 1.80(m, 2H), 2.14(s, 3H), 2.84(t, 2H), 5.22(s, 2H), 6.99~7.93(m, 14H);
[0069] MS (M+1): 482.33.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com