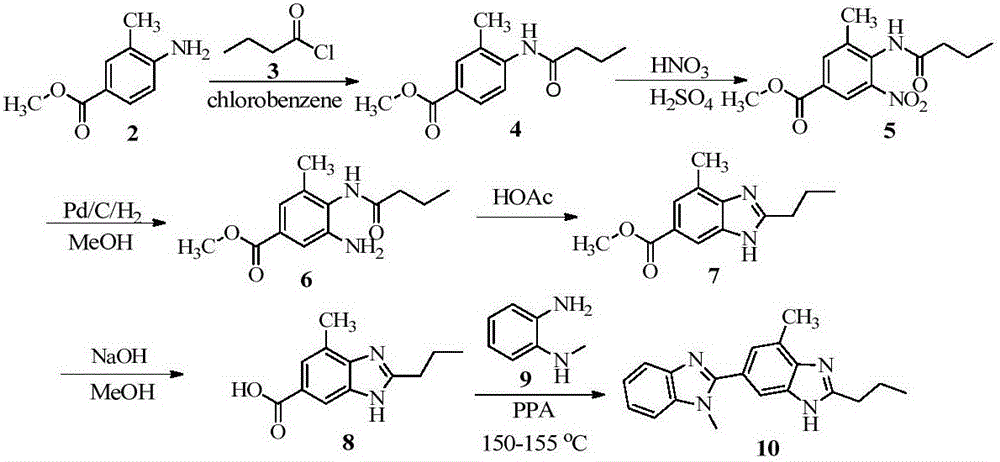
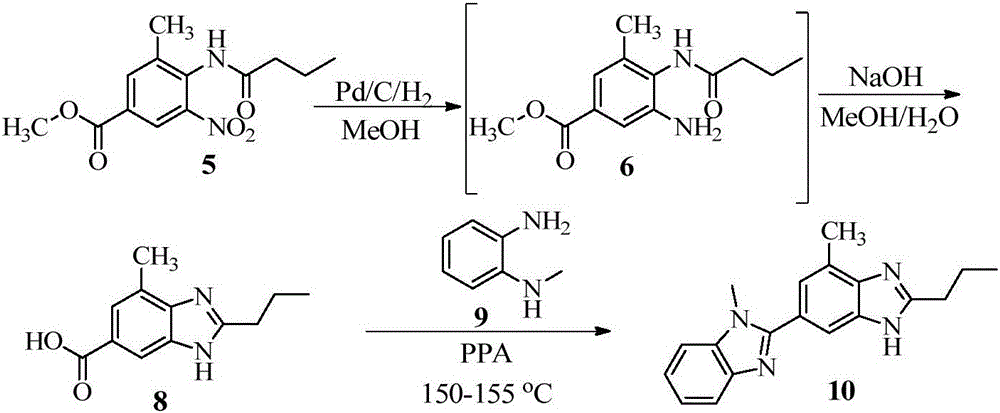
Benzimidazole compound and preparation method thereof
A compound and mixed solution technology, applied in the direction of organic chemistry, can solve the problems of high price and high cost of ytterbium perfluorooctane sulfonate, and the need for improvement of telmisartan, and achieve low cost, easy operation, product purity and yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 Synthesis of N-(2-methyl-4-(1-methyl-1H-benzo[d]imidazol-2-yl)phenyl)butamidine (compound shown in formula (I))
[0083]
[0084] Will P 2 o 5 (4.5g, 31.5mmol) and MsOH (45.0g, 468mmol) were added to a 500mL reaction flask equipped with a magnet and a thermometer, and the reaction mixture was heated to 150°C. After the reaction solution became clear, n-butyramide (22g, 252mmol,) and 2-methyl-4-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline (formula (II) indicated compound) (5 g, 21 mmol). After the reaction solution was stirred and reacted at 150°C for 21h, the reaction mixture was cooled to 60°C and 100mL of water was added. After the reaction solution was cooled to room temperature, 25mL of acetone was added to the reaction solution, and then the pH of the reaction solution was adjusted to Adjusted to 11-12, a large amount of solids were precipitated in the reaction solution, the reaction solution was stirred and reacted at room temperature for 5 hours, an...
Embodiment 2
[0086] Example 2 Synthesis of 1,7'-dimethyl-2'-propyl-1H,3'H-2,5'-benzo[d]imidazole (compound represented by formula (Ⅲ))
[0087]
[0088] At room temperature (17°C), N-(2-methyl-4-(1-methyl-1H-benzo[d]imidazol-2-yl)phenyl)butamidine (5.33g, 17.40mmol, formula ( The compound shown in I)) and NaOH (2.78g, 69.58mmol) were suspended in acetonitrile (200mL), and NaClO aqueous solution (65.83g, 2.42wt%, 21.40mmol) was added to the reaction mixture. The reaction mixture was heated to 30° C. and stirred at constant temperature for 2.5 h. The reaction mixture was lowered to room temperature, and water (154mL) was added dropwise to the reaction mixture. After the addition of water, the reaction mixture was stirred at room temperature for 2h, filtered with suction, and the filter cake was washed with water (3×20mL) and placed in a vacuum oven at 60°C. Drying for 24h gave 1,7'-dimethyl-2'-propyl-1H,3'H-2,5'-benzo[d]imidazole as a white solid (4.94g, yield: 93.30%, purity: 99.84 %) ...
Embodiment 3
[0090] Example 3 Synthesis of N-(2-methyl-4-(1-methyl-1H-benzo[d]imidazol-2-yl)phenyl)butamidine (compound shown in formula (I))
[0091] Will P 2 o 5 (0.9g, 6.3mmol) and MsOH (9.0g, 93.6mmol) were added to a 50mL reaction flask equipped with a magnet and a thermometer, and the reaction mixture was heated to 150°C. After the reaction solution becomes clear, add n-butyramide (according to the feeding ratio in the following table 1) and 2-methyl-4-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline (formula Compound shown in (II)) (1.0 g, 4.2 mmol). After the reaction solution was stirred and reacted at 150° C. for 21 h, the reaction solution was sampled and sent for testing. The HPLC results are shown in Table 1.
[0092] It can be seen that when the molar ratio of the compound represented by the formula (II) to n-butyramide is 1:12, the content of the compound represented by the formula (I) in the reaction solution is the most, which further shows that the compound represented by the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com