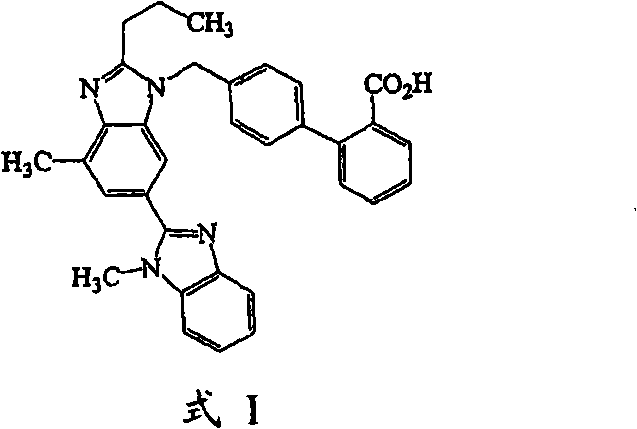
Compound tablet containing telmisartan and hydrochlorothiazide
A technology for hydrochlorothiazide and telmisartan, applied in the field of medicine, can solve the problems of incompleteness, slow dissolution of hydrochlorothiazide, hinder the release of hydrochlorothiazide, etc., and achieve the effects of stable quality, improved efficiency and good dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
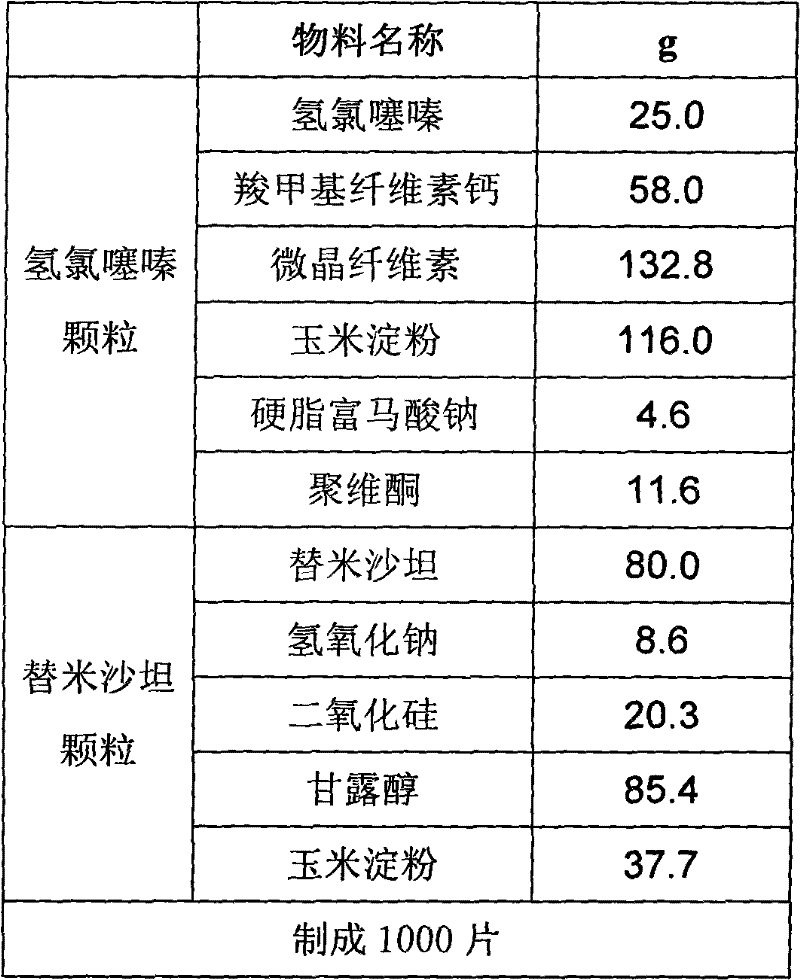
[0043] Preparation of hydrochlorothiazide granules:
[0044] Mix 25.0g of hydrochlorothiazide, 58.0g of carmellose calcium, 132.8g of microcrystalline cellulose, 116.0g of cornstarch, 11.6g of povidone, and 4.6g of sodium stearyl fumarate, and use 300g of purified water as a wetting agent. One-step granulation in a fluidized bed, controlling the material temperature at 22-25°C, drying to obtain hydrochlorothiazide dry granules, and passing the dry granules through a φ1.2mm rotary sieve for granulation to obtain hydrochlorothiazide granules.
[0045] Preparation of Telmisartan Granules:
[0046] Dissolve 80g of telmisartan and 8.6g of sodium hydroxide in 200g of water to obtain a clear solution, add 20.3g of silicon dioxide to disperse evenly, place 85.4g of mannitol and 37.7g of cornstarch in a fluidized bed, and spray into the above solution , to prepare telmisartan granules, pass through a φ1.2mm rotary sieve after drying, and sieve the granules to obtain telmis...
Embodiment 2
[0050]
[0051] Preparation of hydrochlorothiazide granules:
[0052] Mix 25.0g hydrochlorothiazide, 58g carboxymethylcellulose calcium, 132.8g microcrystalline cellulose, 116.0g cornstarch, 11.6g povidone and 4.6g sodium stearyl fumarate, and use 300g purified water as a wetting agent , carry out one-step granulation in a fluidized bed, control the temperature of the material at 22-25°C, dry the granules after granulation, and use a Φ1.2mm rotary sieve to size the dry granules to obtain hydrochlorothiazide granules.
[0053] Preparation of Telmisartan Granules:
[0054] Dissolve 80g telmisartan and 8.6g sodium hydroxide in 200g water to obtain a clear solution, add 20.3g silicon dioxide and disperse evenly; place 66.8g mannitol, 37.7g cornstarch and 7.0g carboxymethylcellulose calcium In the fluidized bed, spray the above-mentioned solution, control the temperature of the material at 25-30 ° C, and make telmisartan granules; 11.6g povidone is made into an aqueous solution...
Embodiment 3
[0058]
[0059] Preparation of hydrochlorothiazide granules:
[0060] Mix 25.0g hydrochlorothiazide, 58g crospovidone, 132.8g microcrystalline cellulose, 116.0g corn starch, 11.6g povidone and 4.6g sodium stearyl fumarate, and use 300g purified water as a wetting agent, Carry out one-step granulation in a fluidized bed, control the temperature of the material at 22-25°C, dry the granules after the granulation is completed, and use a Φ1.2mm rotary sieve to size the dry granules to obtain hydrochlorothiazide granules.
[0061] Preparation of Telmisartan Granules:
[0062] Dissolve 80g telmisartan and 8.6g sodium hydroxide in 200g water to obtain a clear solution, add 20.3g silicon dioxide and disperse evenly; place 66.8g mannitol, 37.7g cornstarch and 7.0g carboxymethylcellulose calcium In the fluidized bed, spray the above-mentioned solution, control the temperature of the material at 25-30 ° C, and make telmisartan granules; 11.6g povidone is made into an aqueous solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com