New process for preparing telmisartan
A technology of telmisartan and a new process, applied in the field of organic chemistry, can solve problems such as inability to use industrialized production, and achieve the effects of good product quality, shortened reaction time, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
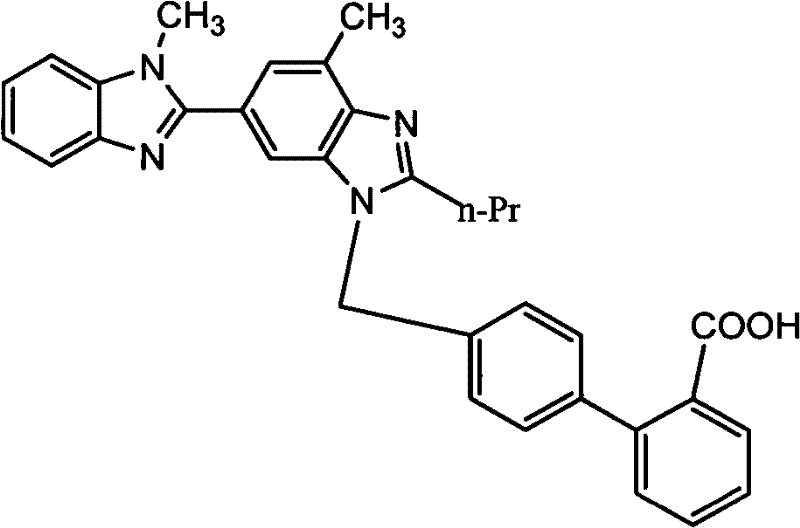
[0024] Example 1: 4'-[(1,4'-dimethyl-2'-propyl[2,6'-di-1H-benzimidazole]-1'-yl)methyl]-[1, 1'-biphenyl]-2-carboxylic acid (Compound III, Telmisartan)
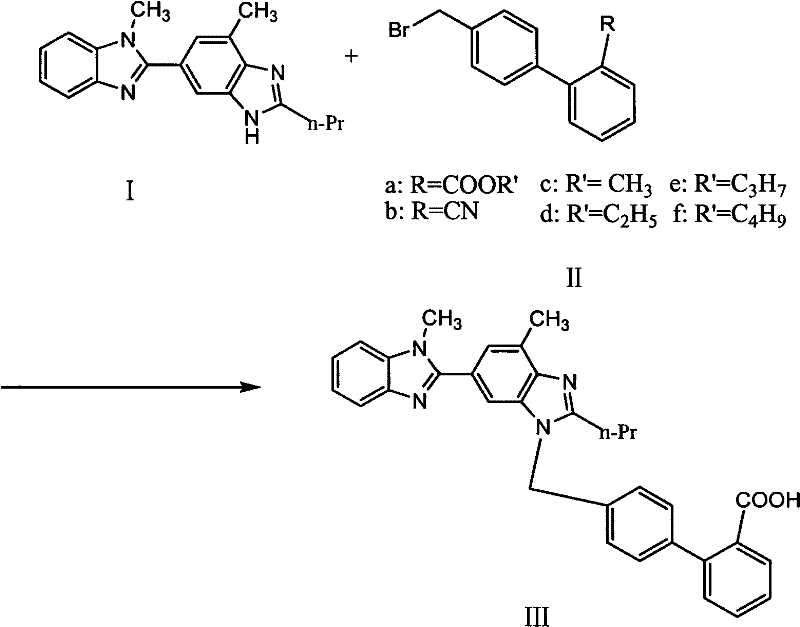
[0025] Put 50g compound I, 200ml dimethylsulfoxide (DMSO) into a 500ml reaction bottle, stir and dissolve at room temperature, add 55g, 4'-bromomethylbiphenyl-2-carbonitrile and 40gNaOH, 40ml dimethylsulfoxide (DMSO) React at about 25-30°C for 2 hours, raise the temperature to 160°C and react for 8 hours, after the reaction is completed, cool naturally, add glacial acetic acid to crystallize, filter to obtain 301g of crude telmisartan (wet weight)
[0026] Put 301g of crude telmisartan into a 1000ml reaction bottle, add 500ml of ethanol and 30ml of ammonia water to heat and dissolve, add 3g of activated carbon for decolorization for 30 minutes, filter, cool to room temperature, add glacial acetic acid to crystallize, filter, and dry to obtain 72.5g of telmisartan. Misartan fine product, the yield is 85.6%, the HPLC purity is 9...
Embodiment 2
[0027] Example 2: 4'-[(1,4'-dimethyl-2'-propyl[2,6'-di-1H-benzimidazole]-1'-yl)methyl]-[1, 1'-biphenyl]-2-carboxylic acid (Compound III, Telmisartan)
[0028] Put 50g of compound I and 200ml of dimethylformamide (DMF) into a 500ml reaction flask, stir at room temperature for 10 minutes, add 52g of 4'-bromomethylbiphenyl-2-carbonitrile and 40g of Na 2 CO 3 , 40ml of dimethylformamide (DMF), 25 ~ 30 ℃ for about 2 hours, heated up to 140 ℃ for 8 hours, after the reaction was completed, cooled naturally, added hydrochloric acid to crystallize, filtered to get 319g telmisartan crude product (wet Heavy).
[0029] Put 319g of crude telmisartan into a 1000ml reaction bottle, add 500ml of ethanol and 35ml of ammonia water to heat and dissolve, add 5g of activated carbon for decolorization for 30 minutes, filter, cool to room temperature, add glacial acetic acid to crystallize, filter, and dry to obtain 70.14g The refined product of telmisartan has a yield of 82.9%, an HPLC purity of...
Embodiment 3
[0030] Example 3: 4'-[(1,4'-dimethyl-2'-propyl[2,6'-di-1H-benzimidazol]-1'-yl)methyl]-[1, 1'-biphenyl]-2-carboxylic acid (Compound III, Telmisartan)
[0031] Drop into 50kg compound I, 300 liters of ethylene glycol in 1000 liters reactor, stir at room temperature for 10 minutes, add 55kg4'-bromomethylbiphenyl-2-carboxylate methyl ester and 40kg potassium hydroxide, 50 liters of ethyl alcohol Diol, 25~30 DEG C left and right reaction 2 hours, be warming up to 50 DEG C and react 3 hours, after completion of reaction, natural cooling, add glacial acetic acid crystallization, filter, obtain 328kg telmisartan crude product (wet weight).
[0032] Put 328 kg of crude telmisartan into a 1000 liter reactor, add 500 liters of ethanol and 50 liters of ammonia water, heat to dissolve, add 5 kg of activated carbon for decolorization for 30 minutes, cool to room temperature, add glacial acetic acid to crystallize, filter and dry , to obtain 79.2kg of fine telmisartan, yield 93.6%, HPLC pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com