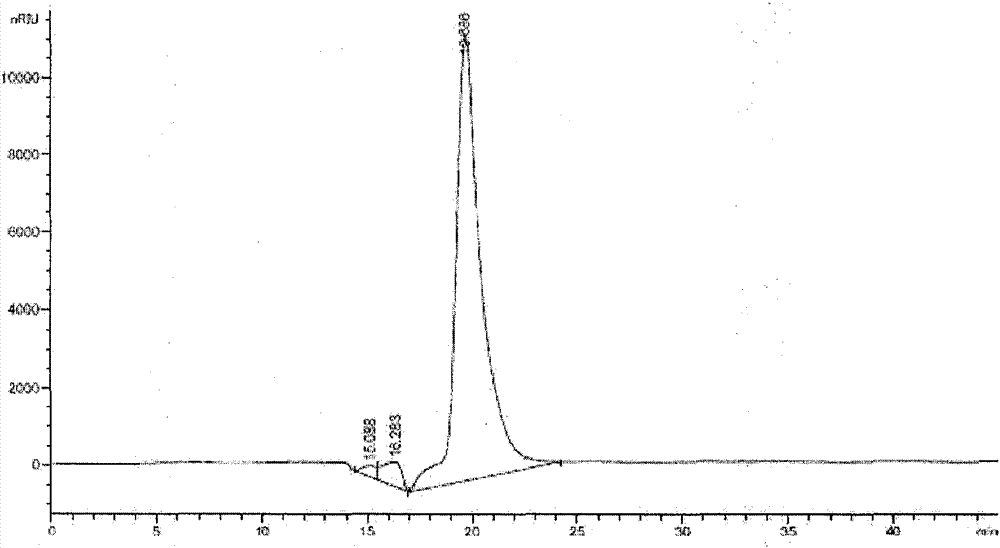
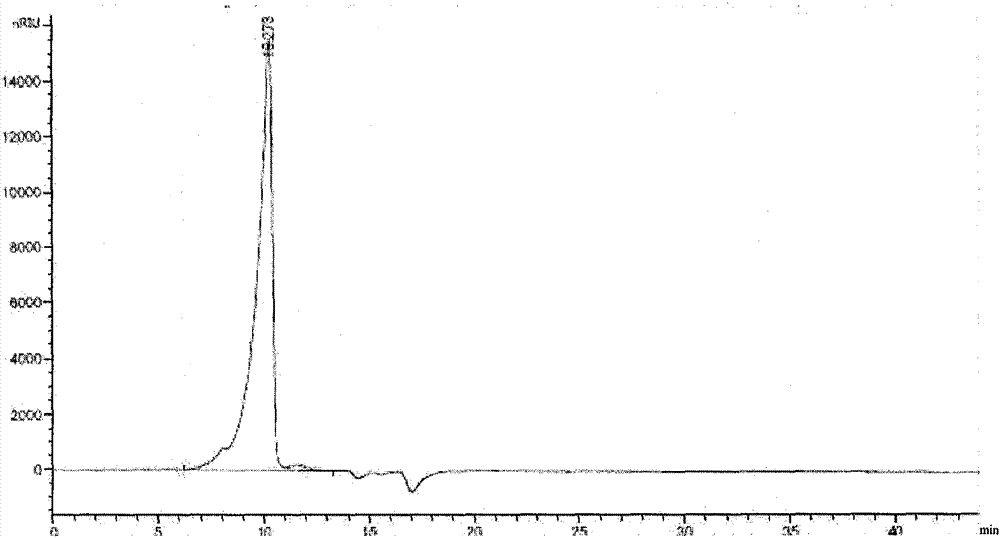
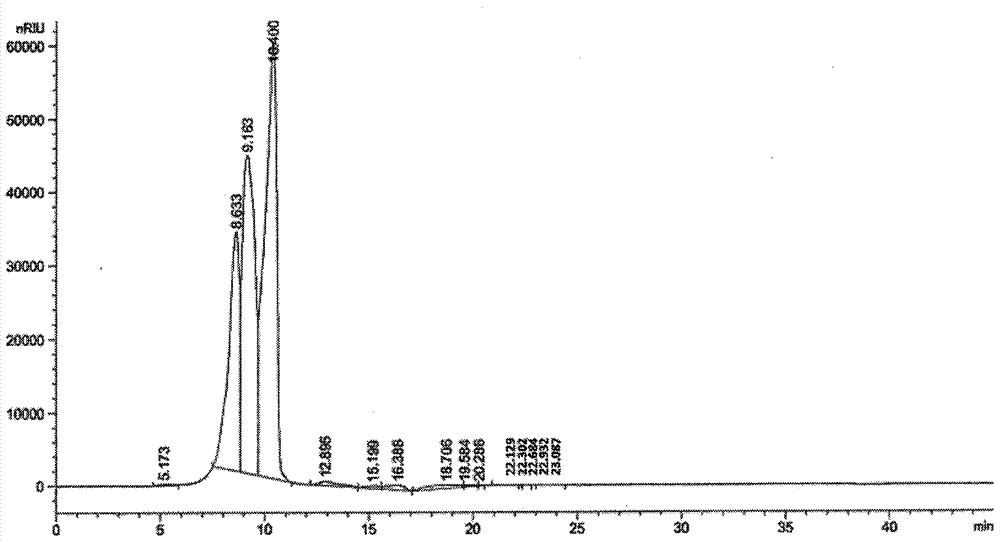
High-purity colloidal bismuth pectin compound and confirmation of structural formula, molecular formula and molecular weight thereof
A colloidal bismuth pectin, high-purity technology, applied in the directions of medical preparations containing active ingredients, inorganic active ingredients, organic active ingredients, etc., can solve the problems of low chemical purity of colloidal bismuth pectin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Take 165kg of dry citrus peel particles (particle size 2.5mm-3.5mm), add 1200L of purified water and heat (50-90°C) to soak for 30-60 minutes, centrifuge to remove decolorized water, and then wash with water for 3-4 times. The decolorized wet peel residue is added to dilute HCl aqueous solution (pH 2-2.5), kept at a temperature of 70-90°C for 90-120 minutes, and centrifuged to separate the glue. The glue is removed a small amount of solid suspended matter and solid impurities by a high-speed solid-liquid centrifugal separator to obtain refined glue, which is concentrated under reduced pressure (vacuum degree -0.7MPa, material temperature is about 60°C), so that the volume of the glue is reduced to thin glue 1 / 3rd-1 / 4th, get pectin thick mucilage. Add 11.11 kg of bismuth hydroxide to 100 kg of purified water, heat and stir to disperse into a colloidal solution, add 25 kg of potassium hydroxide (50%) aqueous solution, and fully stir to completely dissolve into a bismuth s...
Embodiment 2
[0061] Preparation of high-purity colloidal bismuth pectin bioadhesive time-lag tablets
[0062] (1) Preparation of bioadhesive composite excipients: take 333.33 g of high-purity colloidal bismuth pectin, another 18.52 g of croscarmellose sodium, 18.52 g of crospovidone, and 35 g of microcrystalline cellulose, add water Make it dissolve, spray dry to make compound auxiliary material.
[0063] (2) Tablet core preparation: mix high-purity bismuth pectin powder and compound excipients evenly, and directly dry (DC) compress the tablet, use this as the tablet core material, and measure the disintegration time limit in 0.1mol / L hydrochloric acid solution, Should be less than 10 minutes.
[0064] (3) Coating: Prepare a mixed solution of ethanol (70%) containing 3% of HPMC and 2% of PEG6000 to coat the isolation layer. A 95% ethanol solution containing EudragitE 100 10%, PVP 6%, and PEG6000 2% was prepared as a lagging layer coating solution for lagging layer coating. The drug prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com