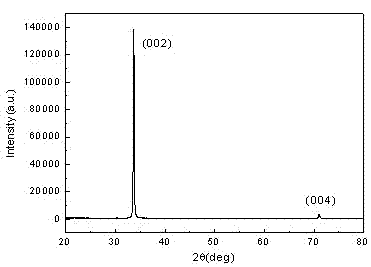
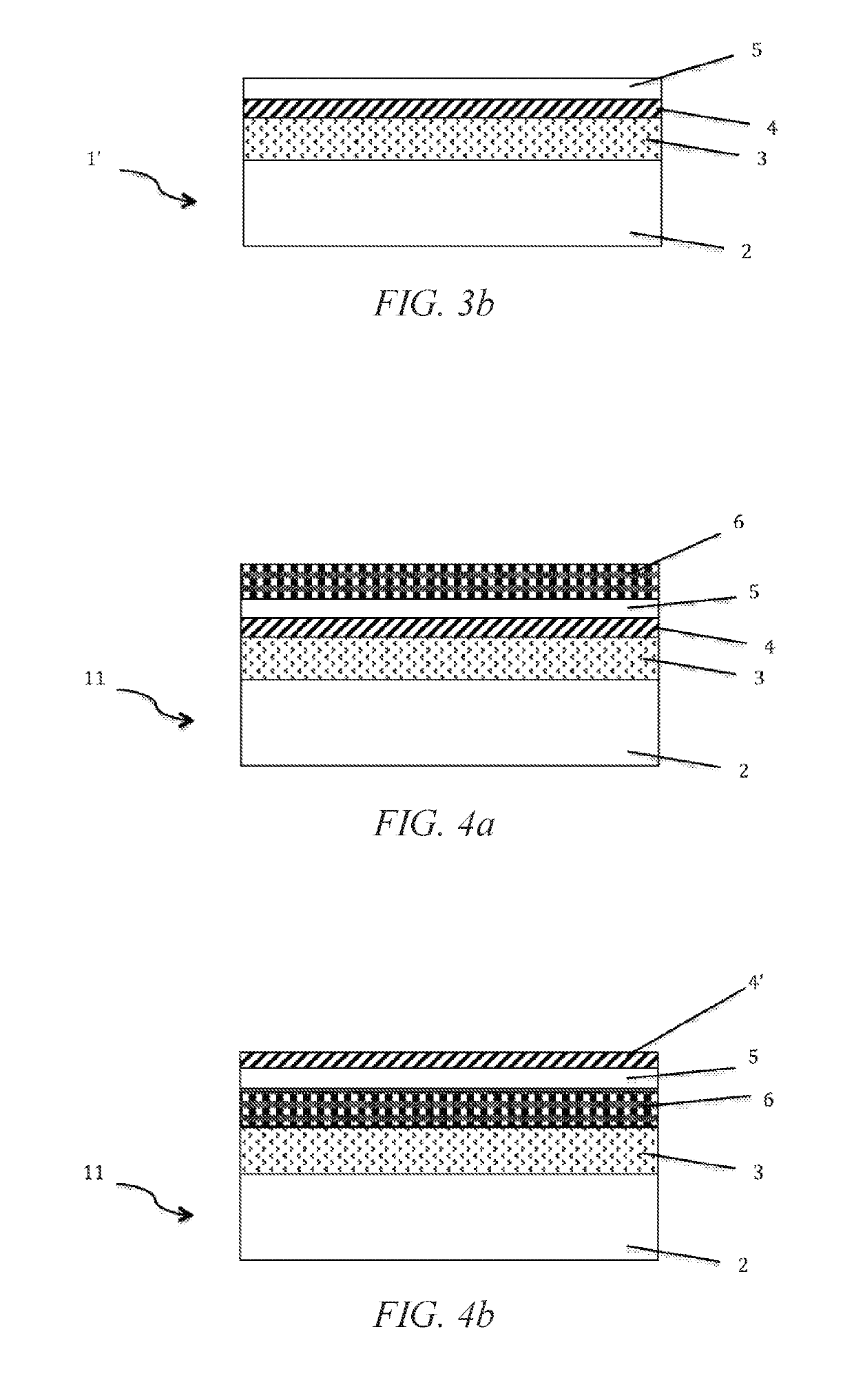
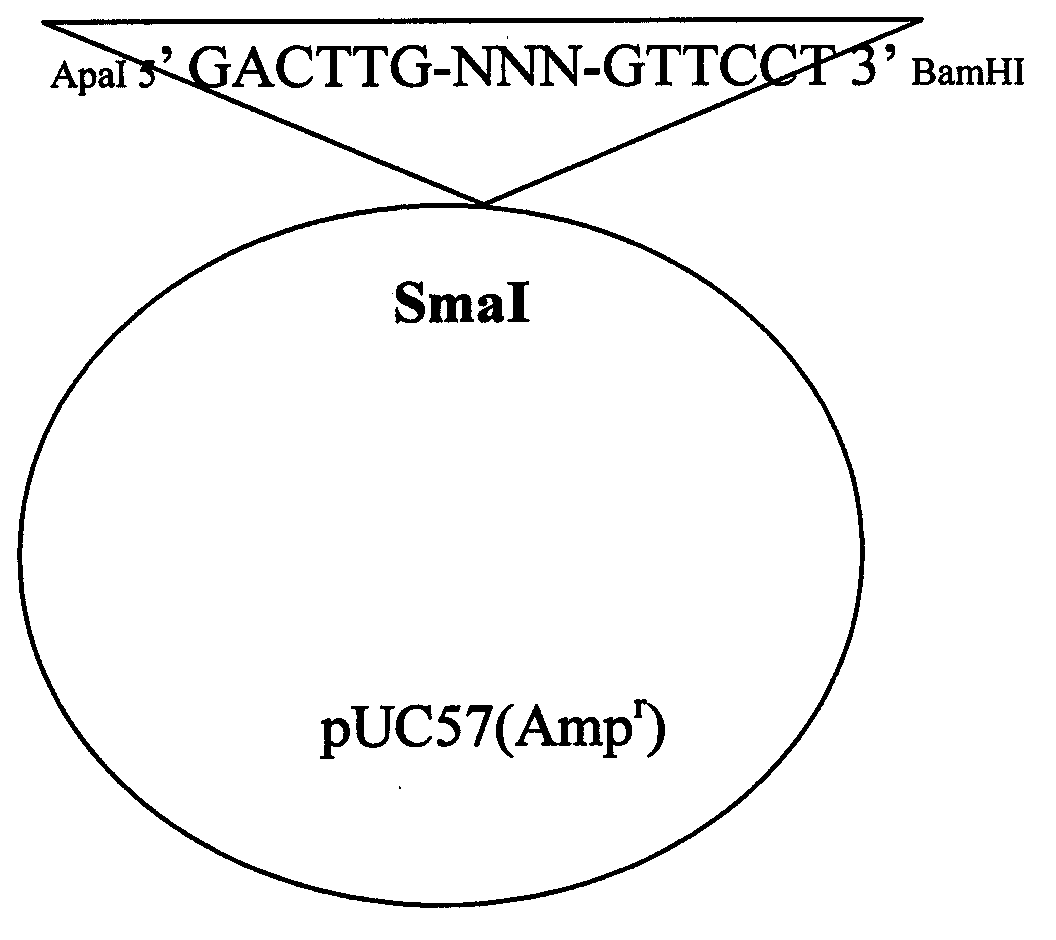
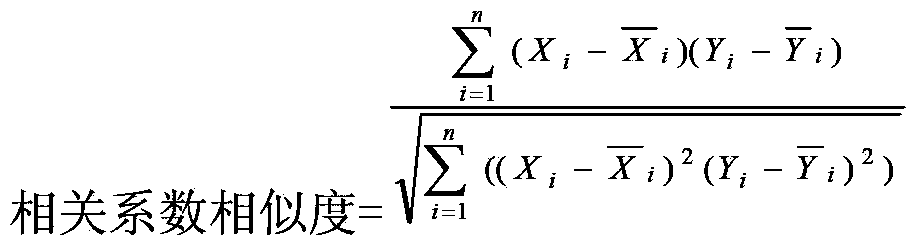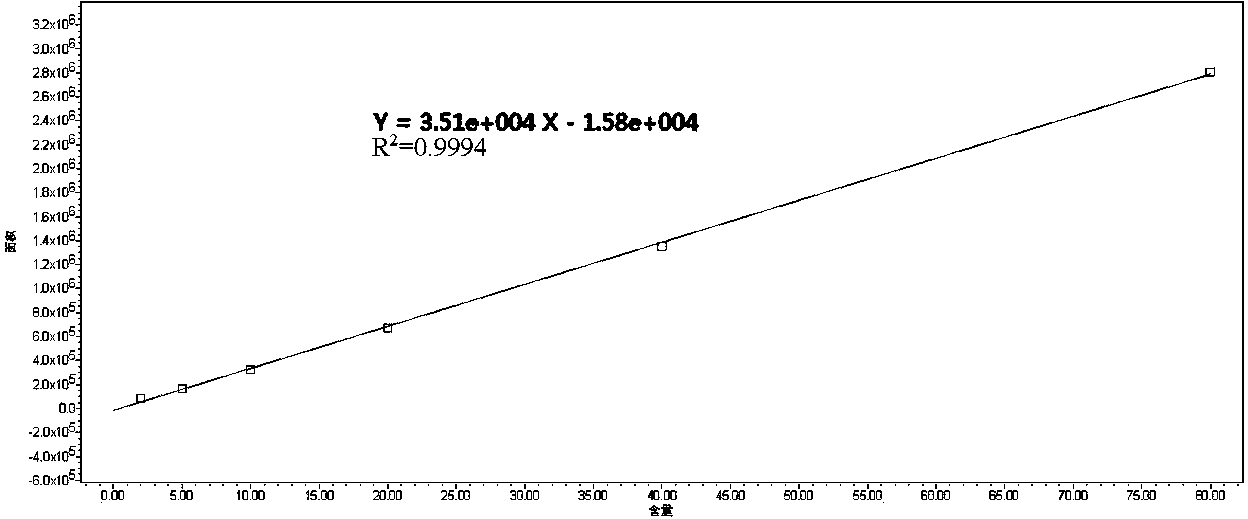Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43results about How to "Good stability and repeatability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
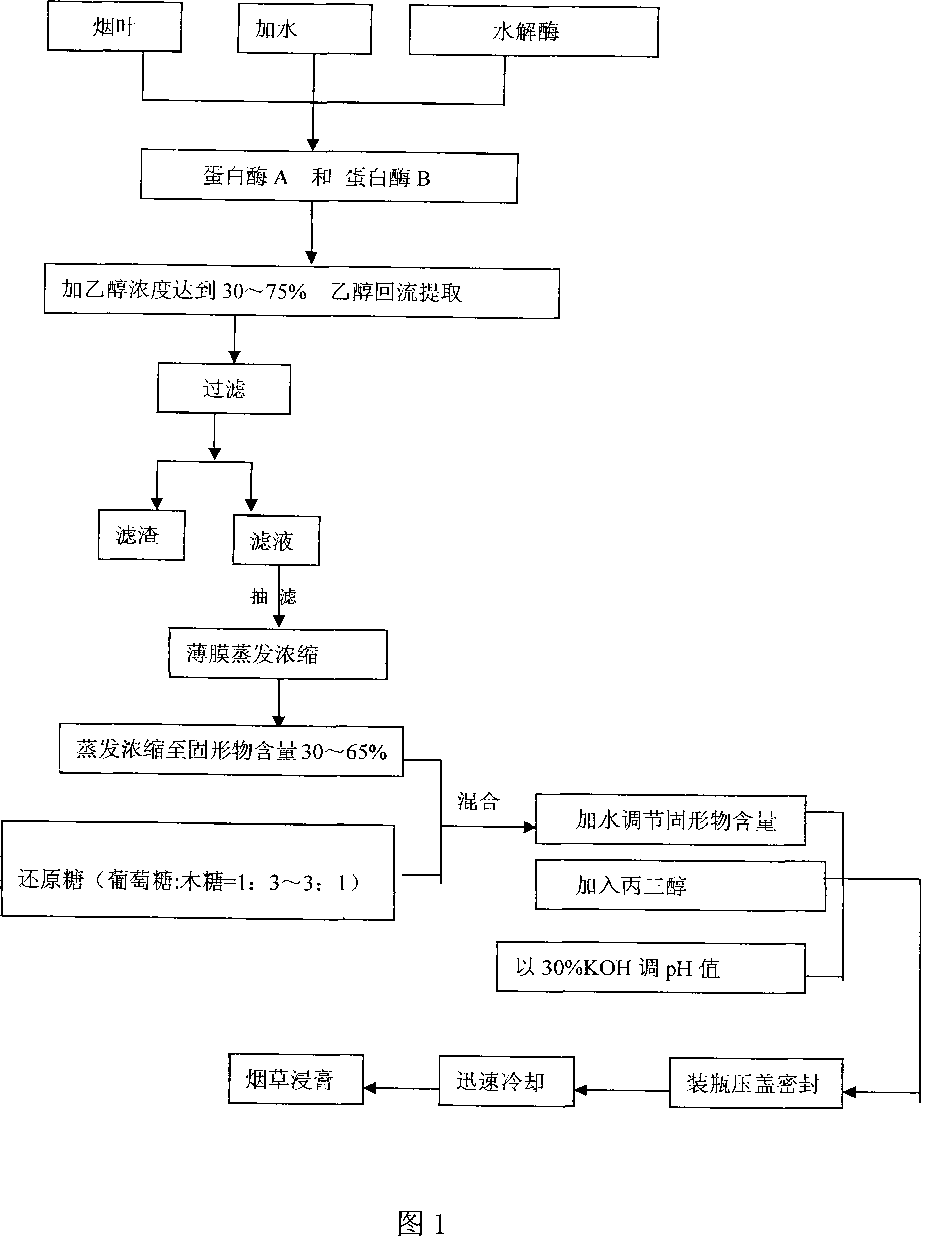
Preparation method for tobacco extracting
The invention discloses a preparing method for a tobacco-made extract. The invention includes the following steps: tobacco leaves are firstly blended with water and enzyme for enzymolysis; and then ethanol is applied for extraction, filtration and concentration; and lastly the tobacco-made extract is obtained through maillard reaction. The invention utilizes the tobacco dust generated from the tobacco production, takes into full consideration the feasibility and operability of industrial production with respect to the preparing process, and furthermore, conducts a series of exploration on factors relating to the enzymolysis, extraction and maillard reaction forms the final preparing process and optimal technical parameters, and thereby the product quality reaches the requirements for adding fragrance to the tobacco-made extract with outstanding steadiness and repeatability.
Owner:CHINA TOBACCO GUANGDONG IND
High heat-resisting ceramic cooking cook ware suitable for electromagnetic induction furnace
InactiveCN101077274ADeliciousImprove heat utilizationCooking-vessel materialsLithiumThermal stability
The present invention discloses one kind of high temperature resistant ceramic cooker suitable for use in electromagnetic induction furnace. The high temperature resistant ceramic cooker is produced with the main materials, including lithium-containing mineral material, quartz, kaolin, etc, and through grinding, mixing, forming, drying, glazing and high temperature baking to obtain the ceramic body; coating far infrared radiation layer, electromagnetically inducing eddy flow heating layer and far infrared reflecting layer to the bottom of the ceramic body; and roasting. It has high heat stability, no cracking even in no-water cooking state, high heat utilization rate and long service life. It is especially suitable for cooking in electromagnetic induction furnace.
Owner:JINGDEZHEN CERAMIC INSTITUTE
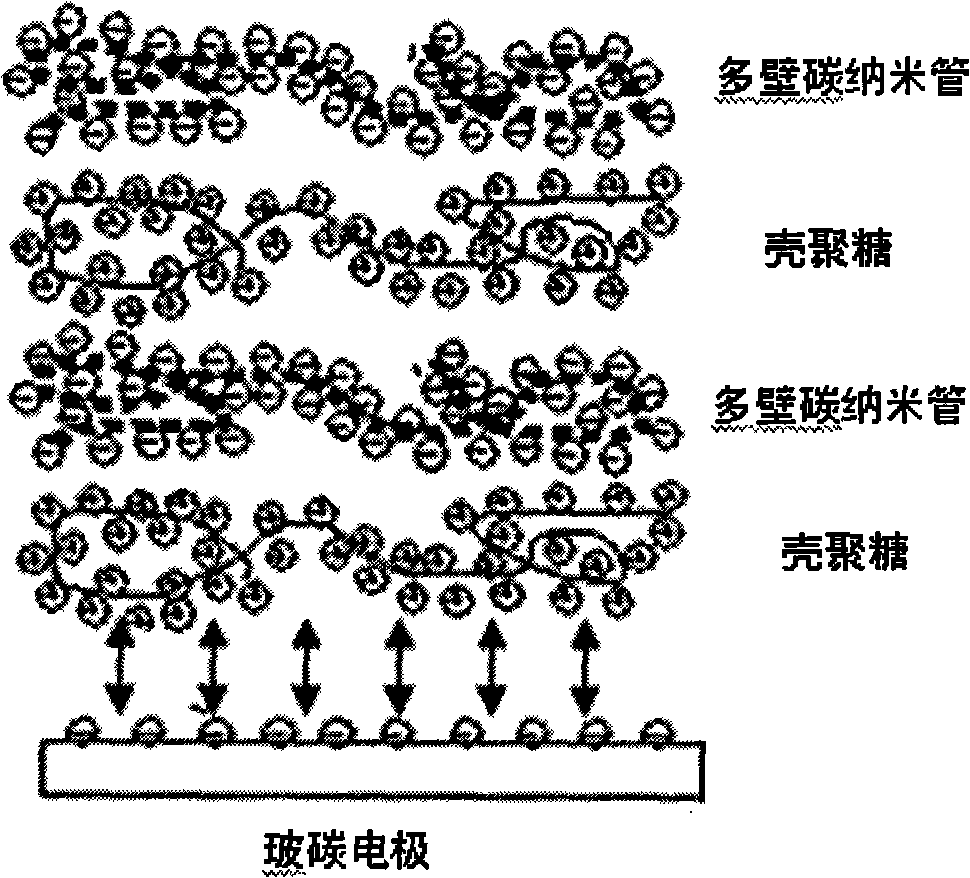
Electrochemical biosensor based on chitosan-immobilized acetylcholinesterase and application thereof
InactiveCN101526493ANot easy to fall offGood stability and repeatabilityBiocideMaterial analysis by electric/magnetic meansEnzyme electrodeCarbamate pesticides
The invention relates to an electrochemical biosensor and application thereof, in particular to an electrochemical biosensor based on chitosan-immobilized acetylcholinesterase and application thereof. The acetylcholinesterase is immobilized with chitosan by way of layer-by-layer self-assembly, but not immobilized by simple cross-linking. The biosensor detects organic phosphorus and carbamate pesticides at a higher sensitivity, and a prepared enzyme electrode is not easy to fall off has better repeatability, better stability and longer service life.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
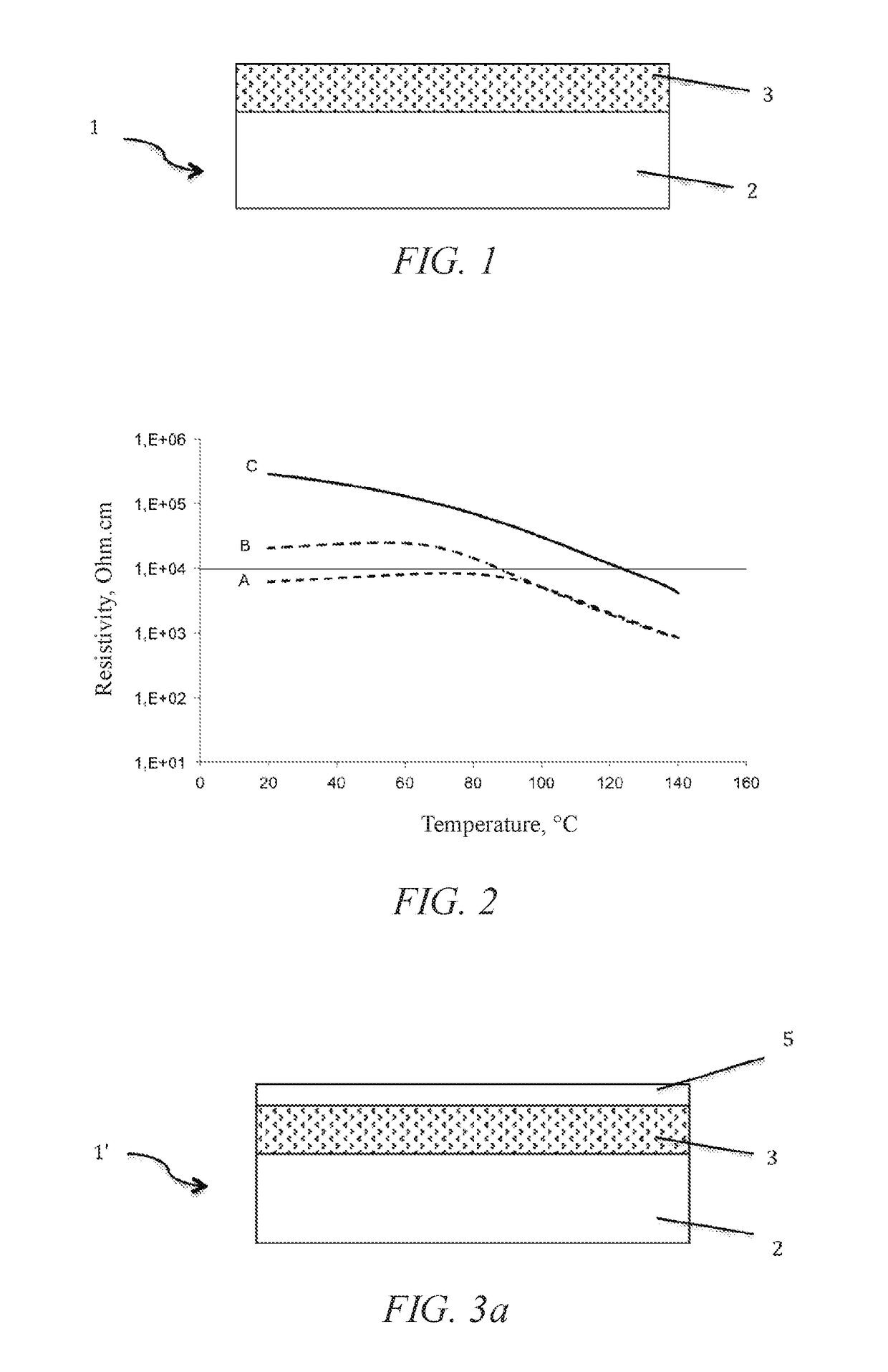
Structure for radiofrequency applications
ActiveUS20170331501A1Improve stabilityGood repeatabilityElectrode carriers/collectorsSemiconductor/solid-state device manufacturingTrappingElectric resistivity
A structure for radiofrequency applications includes: a semiconducting supporting substrate, and a trapping layer arranged on the supporting substrate. The trapping layer includes a higher defect density than a predetermined defect density. The predetermined defect density is the defect density beyond which the electric resistivity of the trapping layer is no lower 10,000 ohm·cm over a temperature range extending from −20° C. to 120° C.
Owner:S O I TEC SILICON ON INSULATOR THECHNOLOGIES
Enzyme-linked immunologic detection method of bisphenol A
InactiveCN101413945ALow costGood stability and repeatabilityMaterial analysis by observing effect on chemical indicatorPolyclonal antibodiesChemistry
An enzyme-linked immunoassay method of bisphenol A belongs to the technical field of immunodetection. The enzyme-linked immunosorbent assay method utilizes the immunization of a synthetic bisphenol A immunogen to obtain a polyclonal antibody, takes the bisphenol A as a standard product, takes a conjugate of a hapten of diphenolic acid and OVA as a coating antigen and establishes the indirect competitive enzyme-linked immunosorbent assay method of the bisphenol A. The enzyme-linked immunosorbent assay method establishes the indirect competitive ELISA method of the bisphenol A and provides a rapid and high-efficient detection method for detecting the residual bisphenol A, as the method adopts the polyclonal antibody, the cost is lower, and the stability and the repeatability are good. The sensitivity is 0.1ng / ml, and the linear range is 0-100ng / ml. The high specificity and the affinity of the immune reaction lead the ELISA to have very high selectivity and sensitivity.
Owner:JIANGNAN UNIV
Automatic welding machine
InactiveCN102615458AGuaranteed stabilityGood stability and repeatabilityWelding/cutting auxillary devicesAuxillary welding devicesEngineeringPortal frame
The invention discloses an automatic welding machine, which comprises a seat and a worktable arranged on the seat. X-axis guide tracks are arranged at two ends of the worktable, a portal frame capable of moving front and back is arranged on the X-axis guide tracks, a Y-axis guide track is arranged on the portal frame, a Y-axis seat capable of moving left and right is disposed on the Y-axis guide track, a Z-axis guide track is arranged on the Y-axis seat and provided with a Z-axis seat capable of vertically moving, a U-axis seat is disposed on the Z-axis seat, a welding gun seat capable of rotating horizontally is disposed on the U-axis seat, and a welding gun angle adjusting mechanism capable of swinging on a perpendicular plane is disposed on the welding gun seat and provided with a welding gun. During operation, after definite setting of the angle adjusting mechanism is finished, an operator only needs to place a workpiece on a positioning fixture, then the welding machine can automatically finish a welding process, the workpiece is taken down after welding is finished, and the operator only needs to carry out feeding and discharging operations. Besides, as production factors are stable and repeatability is good in a welding process, quality stability can be guaranteed while work efficiency is improved.
Owner:东莞市双力数控设备有限公司
ELISA detecting method for dihexyl phthalate
InactiveCN101419232ALow costGood stability and repeatabilityMaterial analysisPolyclonal antibodiesEnzyme linked immunoassay
The invention relates to an enzyme-linked immunoassay method for dihexyl phthalate, and belongs to the technical field of immunodetection. The invention utilizes immunity of synthesized dihexyl phthalate immunogen to obtain a polyclonal antibody, and takes the dihexyl phthalate as standard and conjugate of dihexyl phthalate hapten and OVA as envelope antigen, to establish indirect competitive enzyme-linked immunoassay method for the dihexyl phthalate. The invention establishes the indirect competitive ELISA method for the dihexyl phthalate, and provides a quick and high-efficiency detecting method for detecting residual of the dihexyl phthalate. The method adopts the polyclonal antibody, so the cost is lower and the stability and repetitiveness are good; and high specificity and compatibility of an immunoreaction make the ELISA have extremely high selectivity and sensitivity, and the sensitivity is 0.01ng / mL and the linear range is between 0 and 100ng / mL.
Owner:JIANGNAN UNIV
Liquid crystal photon crystal fiber tunable narrowband filter and manufacturing method thereof
ActiveCN102012573AEnhanced couplingSimple structureNon-linear opticsUltraviolet lightsMicroscopic scale
The invention provides a liquid crystal photon crystal fiber tunable narrowband filter and a manufacturing method thereof. The method comprises the following steps of: directly plating a multilayer dielectric film on a photon crystal fiber end face to form a one-dimensional photon crystal, performing photosensitive self-assembly film treatment on the dielectric film, and then performing polarization treatment under ultraviolet light; and butting two photon crystal fibers treated likewise on the dielectric film sides, injecting liquid crystal at the butted position, and putting the liquid crystal into a variable magnetic field to realize wavelength tuning function. The invention overcomes the defects of difficult coupling of the filter and an optical communication system, complex structure and mechanical motion, and meanwhile solves the problem of difficult liquid crystal control in a microscopic system. The tunable narrowband filter is easy to couple and easy to match with an all optical network, and has the advantages of simple structure, large tuning range, low cost and the like.
Owner:苏州易奥秘光电科技有限公司
Diagnostic reagent kit for trichinosis by employing dot-immunogold filtration assay
InactiveCN101782577AHigh sensitivity and specificityGood stability and repeatabilityFermentationAnimals/human peptidesChemistryAntibody labeling
The invention discloses a diagnostic reagent kit for trichinosis by employing dot-immunogold filtration assay. The invention has the following advantages: gold labeled SPA is taken as an antibody labeling tag, isopropyl beta-D-thiogalactoside is used for inducing the pMAL-c2X-Ts21 / TB1 recombinant strain, expressing and purifying trichina Ts21 gene-encoded protein (Ts21 antigenic gene, ORF17.20, the access number at GenBank is U88239), after being purified on a nitrocellulose membrane (NCM), the Ts21 recombinant protein coats the detection point T, and human IgG coats the quality control point C, thus constructing Ts21 recombinant protein-dot-immunogold filtration assay (Ts21-DIGFA) and assembling the rapid diagnostic reagent kit for trichina with Ts21 recombinant protein by employing dot-immunogold filtration assay. The kit is simple to operate and has high sensitivity and specificity and good stability and repeatability; the detection results can be observed and judged within 1min immediately after adding samples, the diagnosis results are accurate and clear and misjudgment can not happen; and the kit is suitable for rapid diagnosis and serum epidemiological survey at the scene and is easy to popularize and apply extensively.
Owner:ZHENGZHOU UNIV
Attenuation measurement device for waveguide system
InactiveCN103647612ASmall Signal Reception CapabilityGood stability and repeatabilityTransmission monitoringPhysicsMeasurement device
The invention relates to an attenuation measurement device for a waveguide system. The attenuation measurement device comprises a radio-frequency signal output end, an attenuation signal input end, a radio-frequency signal source, a local oscillator signal source, a frequency divider, a frequency mixer, a semi-automatic inductive voltage divider and a phase-locked amplifier, the radio-frequency signal source generates a radio-frequency signal and a time-based signal, the radio-frequency signal is coupled to a radio-frequency signal input end, the local oscillator signal source takes the time-based radio-frequency signal as a reference signal and outputs a local oscillator signal, the radio-frequency signal and the local oscillator signal share a time base, the frequency divider is used for dividing the frequency for the time-based signal, the frequency mixer is used for mixing the frequency of the local oscillator signal and the frequency of the inputted attenuation signal, the semi-automatic inductive voltage divider is used for dividing the voltage of a frequency mixing signal outputted by the frequency mixer, and the phase-locked amplifier takes a frequency dividing signal outputted by the frequency divider as a reference signal and detects an output signal of the semi-automatic inductive voltage divider. The attenuation measurement device can measure a 50GHz-110GHz waveguide attenuation standard device, and has small signal receiving capability and fine stability and repeatability.
Owner:BEIJING INST OF RADIO METROLOGY & MEASUREMENT
Preparation method of ilicin A and total triterpenoids contained in Hainan holly leaf and application of ilicin A and total triterpenoids
InactiveCN102872168AGood stability and repeatabilityStable curative effectSteroidsCardiovascular disorderSolventChemistry
The invention relates to a method for extracting total triterpenoids and monomer ilicin A of enriched Hainan holly leaf. The method is characterized by comprising the following extraction steps that raw materials of Hainan holly leaf is subjected to reflux extraction by 50 percent to 95 percent of ethanol, liquid supernatant and a precipitation part are obtained after the extraction liquid is concentrated and centrifuged, the precipitation part is subjected to mixed suspension by 30 percent to 40 percent of ethanol, then, petroleum ether is used for extraction, the lower layer solution is subjected to pressure reduction, concentration and drying to obtain total triterpenoids; in addition, the ethanol extraction liquid is subjected to pressure reduction, recovery and evaporation to dryness, water is used for dissolving, acid is added for hydrolysis, organic solvents are extracted, the recovery to dryness, the silicagel column coating and the sherwood oil-ethyl acetate gradient elution are carried out, eluant is collected, elution solvents are recovered through pressure reduction, and the monomer ilicin A is obtained. According to an extraction enriching method, the total triterpenoids content of the obtained product exceeds 50 percent, the purity of the monomer ilicin A is high, meanwhile, the repeatability and the stability of the method are good, the transfer rate of effective ingredients of the raw material is high, the cost is low, the operation is simple and convenient, and the method is suitable for industrial production. Meanwhile, the invention also relates to application of the total triterpenoids and the monomer ilicin A of Hainan holly leaf in the cardiovascular disease treating aspect, in particular to the atherosclerosis aspect, and the potential development value is realized.
Owner:CHINA PHARM UNIV
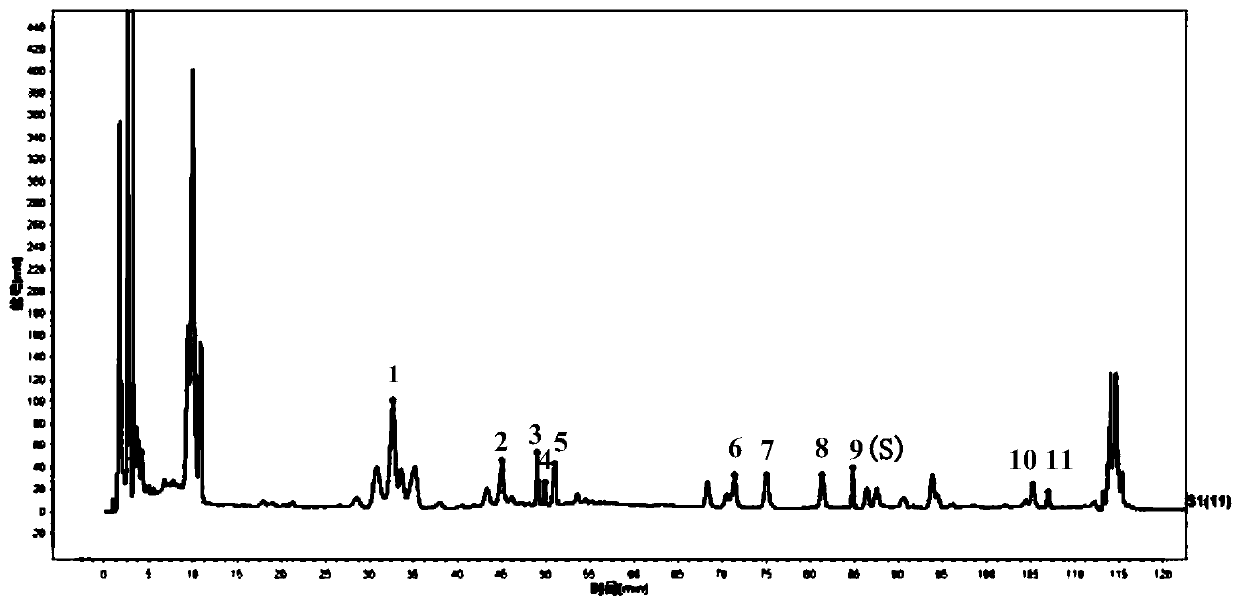
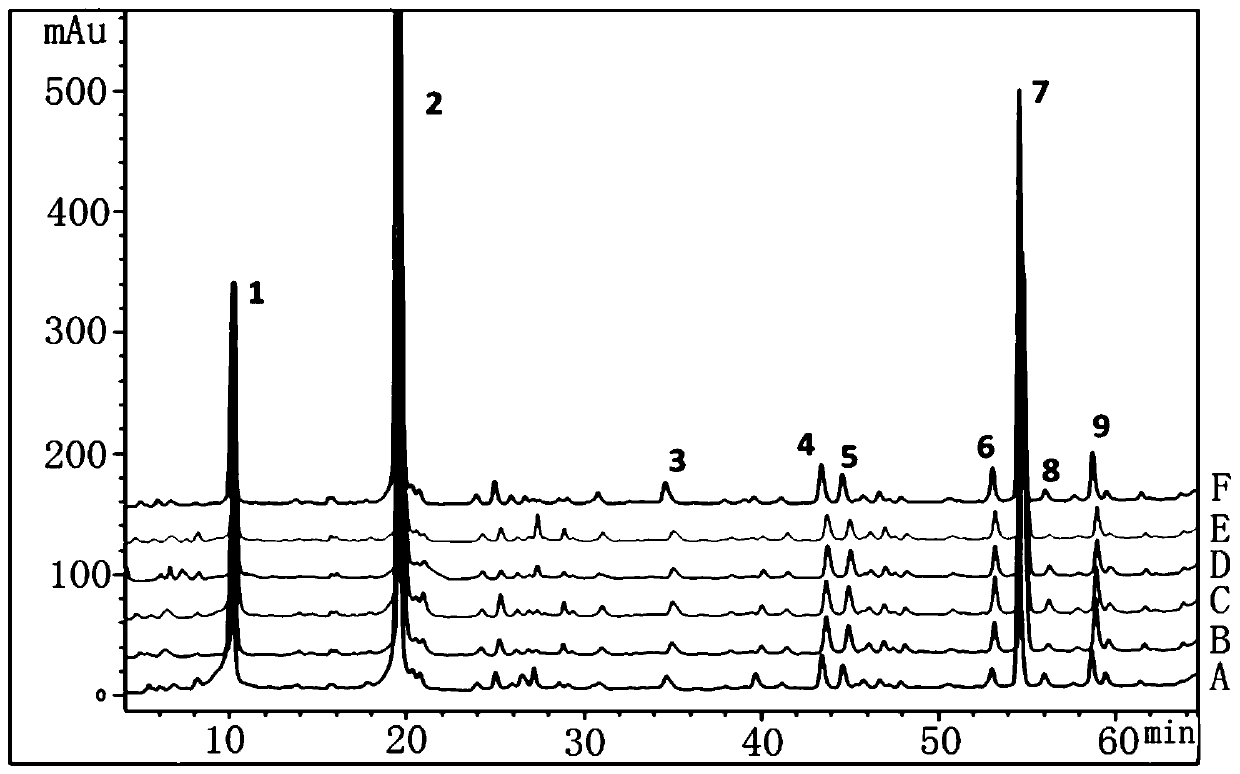
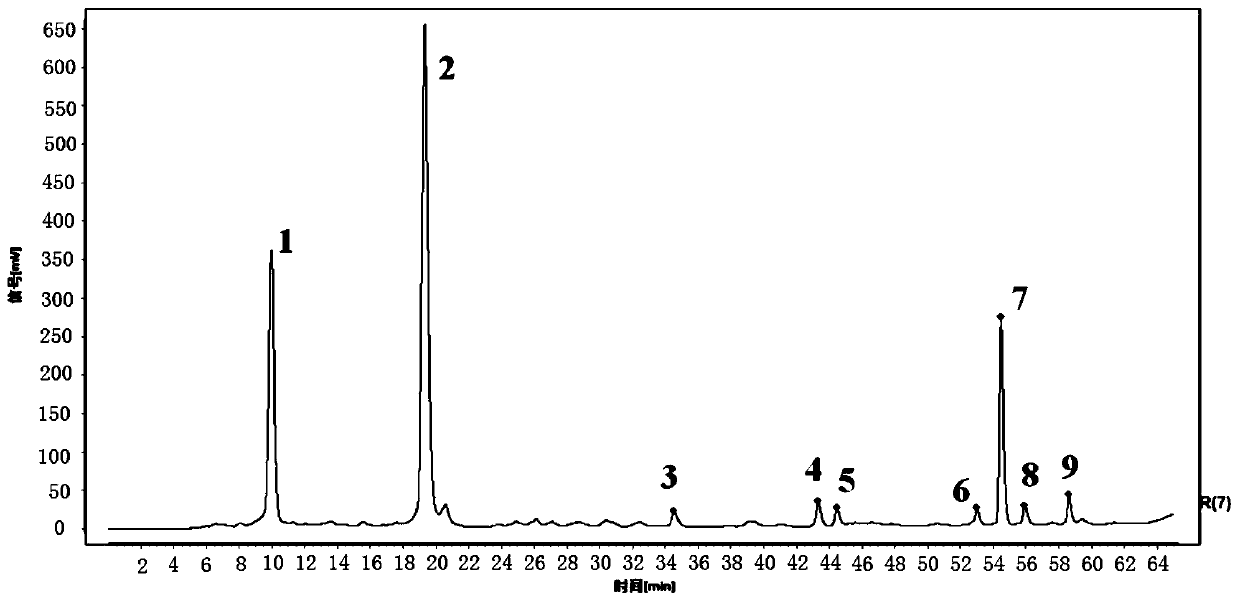
Method for detecting HPLC specific chromatogram of origin-preserving decoction
ActiveCN110907580AHigh accuracy and precisionGood stability and repeatabilityComponent separationTest solutionMedicinal chemistry
The invention discloses a method for detecting an HPLC specific chromatogram of an origin-preserving decoction. The method is characterized by comprising the following steps of (1) preparing a test solution, namely taking an origin-preserving decoction preparation to be detected, and preparing the test solution; (2) preparing a mixed reference substance solution, namely taking ginsenosides Rg1, Reand Rb1, and preparing the ginsenosides Rg1, Re and Rb1 into the mixed reference substance solution; and (3) formulating a specific chromatogram, namely respectively sucking the test solution and thereference substance solution, injecting the solutions into a high performance liquid chromatograph, and obtaining the specific chromatogram of the origin-preserving decoction according to a common peak in the chromatograms of more than N batches of tested products. The detection method provided by the invention can be used for effectively characterizing the variety and quantity of the chemical components in the origin-preserving decoction, and an effective detection method is provided for the comprehensive and effective quality control of the origin-preserving decoction.
Owner:XIAMEN TRADITIONAL CHINESE MEDICINE +1
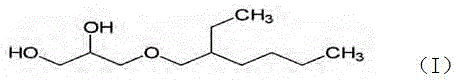
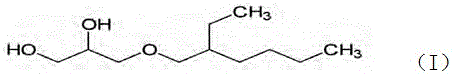
Method for preparing high-purity ethylhexylglycerin
ActiveCN104817436AThe reaction process is simpleSmall steric hindranceEther preparationEthyl phosphateEther
The invention provides a method for preparing high-purity ethylhexylglycerin. According to the method, an intermediate 4-alkoxymethyl-1,3-dioxoane is generated from 2-ethylhexylglycidyl ether and acetone under the action of boron trifluoride diethyl etherate, a terminator is added in good time before hydrolysis, and after liquid separation, an oil-phase substance is neutralized by use of sodium hydrogen carbonate and then washed, next, a stabilizer is added, and finally, the high-purity ethylhexylglycerin is obtained by use of short-path distillation. The method is suitable for cosmetic additives and suitable for large-scale industrial production, and has the advantages of simple process, small energy consumption, product yield of greater than 88%, purity of 99.3%, no color and no taste, and the like.
Owner:SHAANXI RES DESIGN INST OF PETROLEUM CHEM IND
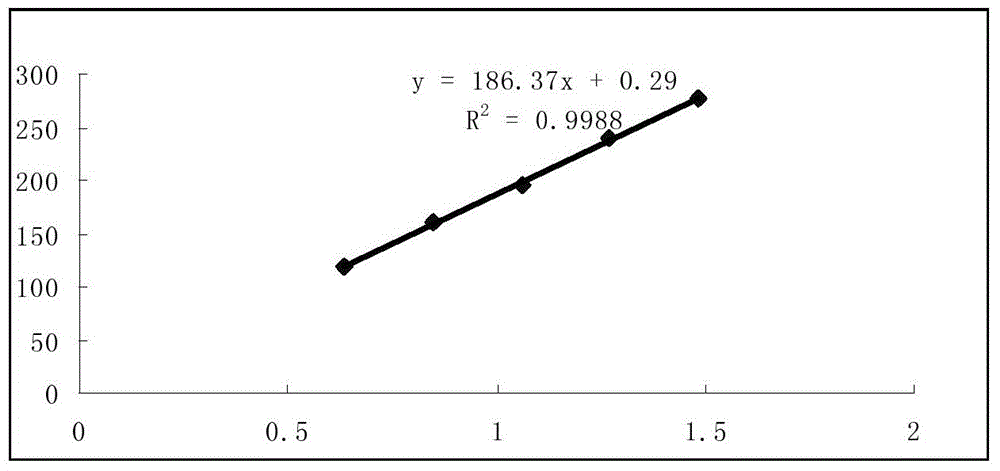
Method for detecting vitamin D content in vitamin D drop
ActiveCN105372337AGood stability and repeatabilityImprove analysis efficiencyComponent separationChromatography columnChemistry
The present invention discloses a method for detecting the vitamin D content in a vitamin D drop. According to the present invention, through a large number of optimizations, the optimal mobile phase composition, the optimal flow rate, the optimal detection wavelength, the optimal chromatography column and other analysis conditions are obtained, and the multiple experiment results show that the method has characteristics of good stability, good repeatability, high analysis efficiency and good separation, and can sensitively and accurately perform qualitative and quantitative detection on the vitamin D3, such that the quality of the vitamin D drop can be objectively, completely and accurately evaluated, and the important significance can be provided for the vitamin D drop quality control and the clinical treatment effect ensuring.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
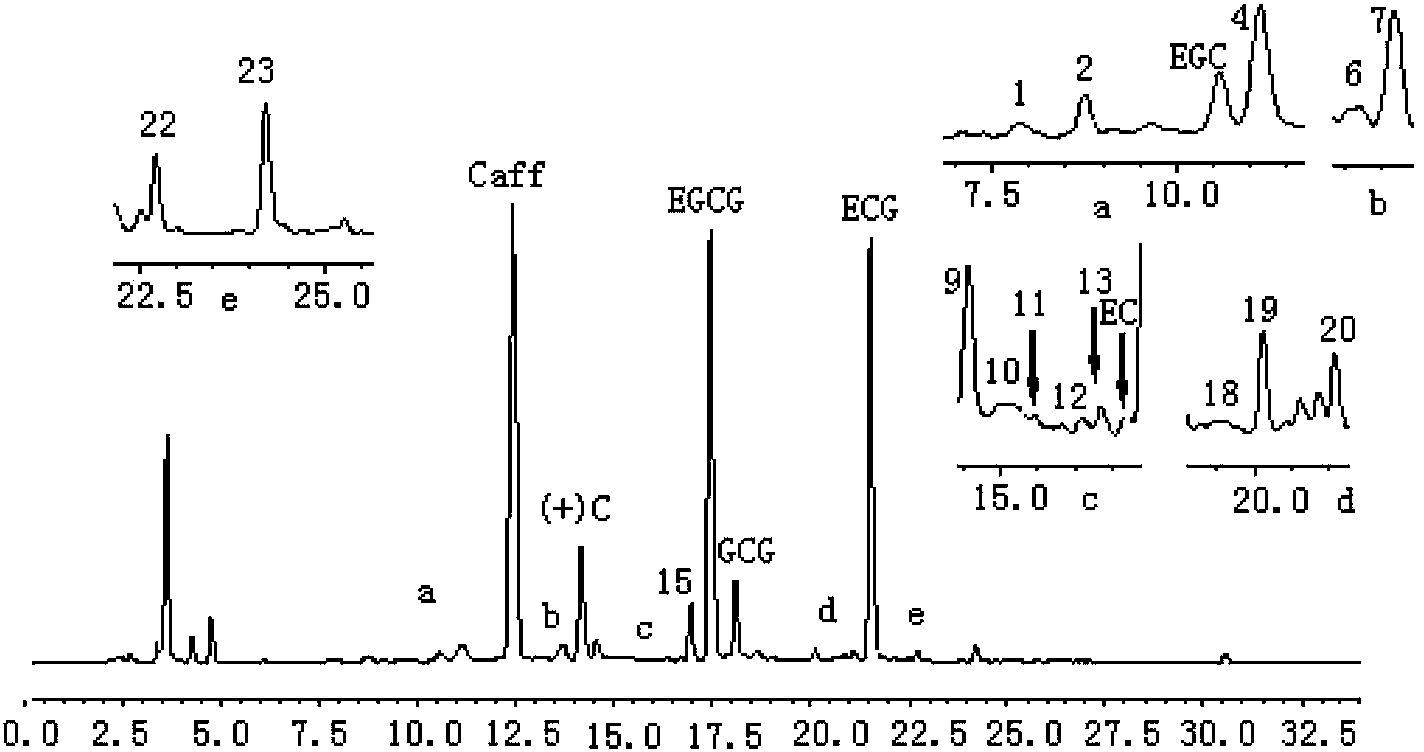
Fingerprint spectrum recognition method for Pu'er drying green raw tea and based on chemical components
InactiveCN103364503AEasy to handleGood stability and repeatabilityComponent separationFingerprintChemistry
The invention discloses a fingerprint spectrum recognition method for Pu'er drying green raw tea and based on chemical components. The fingerprint spectrum recognition method comprises the following steps: establishing a standard fingerprint spectrum of the Pu'er drying green raw tea; establishing a fingerprint spectrum of tea to be measured in the same way; and comparing the fingerprint spectrum of the tea to be measured and the standard fingerprint spectrum of the Pu'er drying green raw tea, and screening the tea to be measured in accordance with the standard fingerprint spectrum of the Pu'er drying green raw tea. The pretreatment of the samples is simple and convenient, so a lot of manpower labor is not required to be spent. The recognition method for the Pu'er drying green raw tea and based on the whole chemical components has relatively good precision, stability and repeatability, and has high recognition rate.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Distance error correction system for laser radar
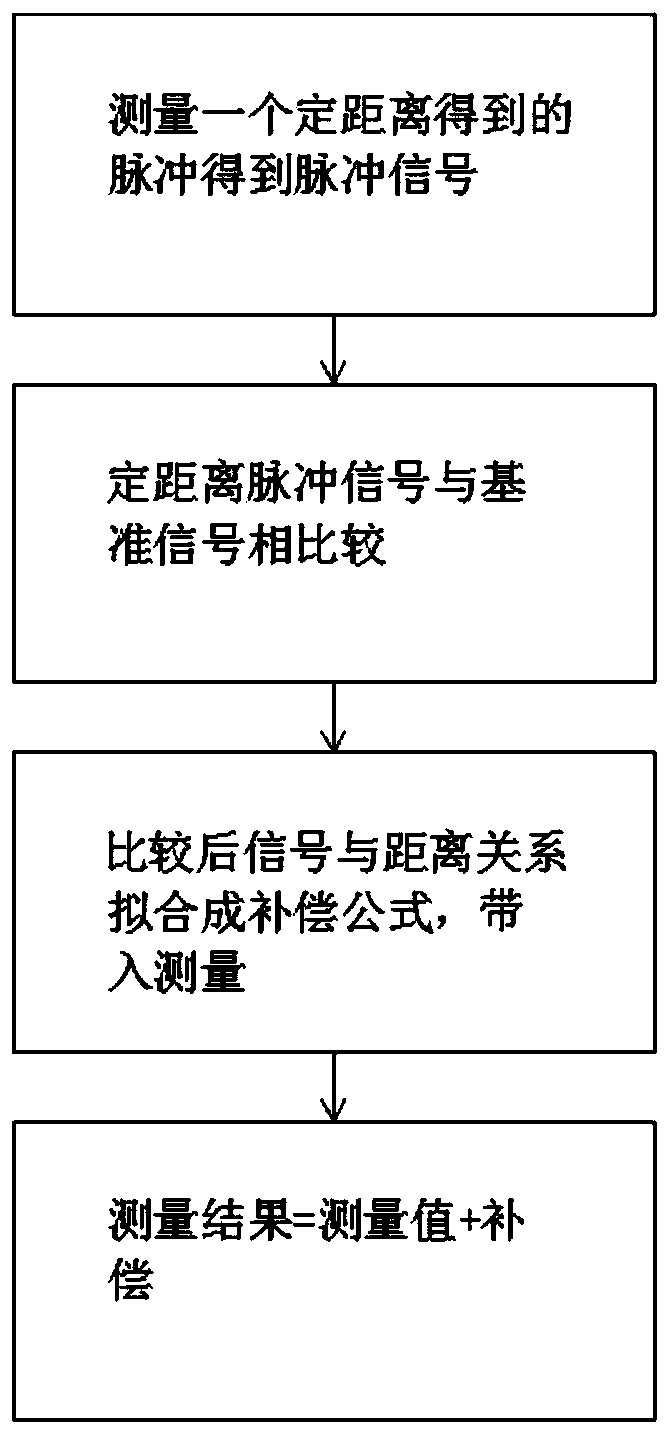
InactiveCN109917355AWide adaptabilityReduce measurement errorWave based measurement systemsMeasurement precisionCorrection method
The invention relates to an error correction method for the measuring distance accuracy of a laser radar by a laser radar echo signal and distance. The method comprise the following steps that: a laser radar emits a light pulse and the light pulse is detected and received by a photoelectric detector to obtain a Gaussian signal; a measuring error is corrected according to different signal strengthinformation obtained by different distances; and since the object measuring distances are different, the surface reflectivities of the measured objects are different and the tilt angles of the measures object are different, so that the energy change of the light pulse returning to the optical detector after irradiation on the object surface is affected. A problem that various factors, causing themeasured return power changes, of the object change in the laser radar measurement process and thus the accuracy of the measurement result is affected is solved. With the correction system, the measurement result is closer to the real distance and the measurement accuracy is improved.
Owner:合肥嘉东光学股份有限公司
Specific chromatogram construction method and quality detection method of schizonepeta
ActiveCN109765322ACoexistence evaluationCoexistence Evaluation DetectionComponent separationThin-layer chromatographyChemistry
The invention relates to the technical field of traditional Chinese medicine detection, in particular to a specific chromatogram construction method and a quality detection method of schizonepeta. Liquid chromatography conditions, mobile phase compositions and an elution procedure are controlled, and a specific chromatogram of nonvolatile constituents of the schizonepeta is established; suitable gas chromatography conditions are selected, and a specific chromatogram of volatile constituents of the schizonepeta is established; specific peaks of the specific chromatograms are marked; the liquidchromatography elution procedure is adjusted, and the content of hesperidin in the schizonepeta is determined; and at the same time, a quality control method of the schizonepeta is established, the quality control method comprises the steps that the schizonepeta specific chromatograms are used for evaluating the schizonepeta, the content of the hesperidin is determined, the quality control methodfurther comprises the steps that thin layer chromatography identification is conducted, heavy metal and hazardous elements are examined, the pesticide residue amount is determined, extraction determination is conducted, and the quality of the schizonepeta is evaluated from the perspective of effectiveness and safety. The quality detection method has the advantages of being simple, fast, stable, reliable, high in precision, good in reproducibility, easy to master, and the like.
Owner:BEIJING ZHONGYAN TONGRENTANG CHINESE MEDICINE R & D +1
Method for producing ZnSO alloy film with adjustable sulfur-doped growth band gap
ActiveCN102226264AGood optical performanceGood stability and repeatabilityVacuum evaporation coatingSputtering coatingBand gapZinc sulfide
The invention discloses a method for producing ZnSO alloy film with an adjustable sulfur-doped growth band gap, which uses a pulse laser deposition method. A target material is produced by mixing pure zinc oxide and pure zinc sulfide powder for hydraulic forming to obtain a ceramic target, wherein mole content of zinc sulfide is 10 to 40%; the target material is arranged in a growth chamber of a pulsed-laser deposition device, wherein the degree of vacuum is 1*10(-4) to 1*10(-3)Pa, the laser frequency is 3 to 10Hz, the growth temperature is from 300 to 500 DEG C, a ZnSO alloy film with an adjustable band gap is grown on a substrate. A real time doping can be realized according to the method provided in the invention, the doping concentration can be controlled by regulating the growth temperature and mole content of sulfur in the target material. Method for producing the ZnSO alloy film with adjustable sulfur-doped growth band gap provided in the invention has the advantages of good optical performance, repeatability and stability.
Owner:ZHEJIANG UNIV
Fluorine-doped carbon covered lithium iron phosphate, and preparation method and application thereof
InactiveCN106207113AImprove material propertiesGood stability and repeatabilityCell electrodesSecondary cellsDoped carbonFluorine doping
The invention discloses a fluorine-doped carbon coated lithium iron phosphate, and preparation method and application thereof. The method comprises the steps of firstly mixing and grinding prepared pure lithium iron phosphate with organic matters including F for several hours by using an organic solvent and then drying in a vacuum chamber, and calcinating at high temperatures for several hours in the atmosphere of inert gas and thus obtaining the fluorine-doped carbon coated lithium iron phosphate. The fluorine-doped carbon coated lithium iron phosphate prepared by using the method can be significantly improved in electrochemical performance, and can reach a specific discharge capacity of 150mAh / g.
Owner:CHENGDU UNIV
Method for analyzing contents of effective substances in Cordyceps martialis fruiting body and residue by HPLC
InactiveCN103512975AGood stability and repeatabilityEasy to useComponent separationChromatography columnChemistry
The invention discloses a method for analyzing the contents of adenosine and cordycepin in Cordyceps martialis fruiting body and solid culture medium residue thereof by HPLC. The method comprises the following steps of: firstly preparing standard solutions of adenosine and cordycepin and sample solutions of Cordyceps martialis fruiting body and solid culture medium residue thereof; and then detecting the sample solutions of Cordyceps martialis fruiting body and solid culture medium residue thereof respectively under the conditions that the chromatographic column is XBridge TMC 18 (5 mum, 4.6*150 mm), the mobile phase is methanol and ultra-pure water at a volume ratio of 10:90, the flow rate is 1.0 ml / min, the sample size is 10 muL (automatic sample injection), the column temperature is 30 DEG C, and the detection wavelength is 254 nm, wherein the sample solutions should appear chromatographic peaks having the same chromatographic retention time as the cordycepin standard solution. The method provided by the invention is convenient and effective, is particularly suitable for analyzing the contents of adenosine and cordycepin in Cordyceps martialis fruiting body and solid culture medium residue thereof, and has good reproducibility and stability.
Owner:SHANGHAI JIAO TONG UNIV
Structure for radiofrequency applications
ActiveUS10250282B2Suitable structureDrawback can be solvedElectrode carriers/collectorsSemiconductor/solid-state device manufacturingTrappingEngineering
A structure for radiofrequency applications includes: a semiconducting supporting substrate, and a trapping layer arranged on the supporting substrate. The trapping layer includes a higher defect density than a predetermined defect density. The predetermined defect density is the defect density beyond which the electric resistivity of the trapping layer is no lower than 10,000 ohm·cm over a temperature range extending from −20° C. to 120° C.
Owner:SOITEC SA
Primer, kit and method for identifying eel germplasm
InactiveCN107488736ASimple and fast operationGood stability and repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationGermplasmMicrobiology
The invention discloses a primer for identifying eel germplasm and also discloses a kit containing the primer, and a method for identifying eel germplasm by using the primer or the kit. According to the primer, kit or method disclosed by the invention, the time of detecting samples at a time is 2-3 hours only, many samples can be simultaneously detected, and the primer, kit or method disclosed by the invention has the characteristics of high efficiency and convenience. More conveniently, a large instrument is not needed by the method, field detection can be performed at any time in case of electrification, and the primer, the kit and the method are simple and convenient in operation, excellent in stability and repeatability and high in practicality.
Owner:JIMEI UNIV
Detection method for dose of ionizing radiation on human peripheral blood lymphocytes
InactiveCN103805683AGood stability and repeatabilityShorten the timeMicrobiological testing/measurementPeripheral blood lymphocyteStandard curve
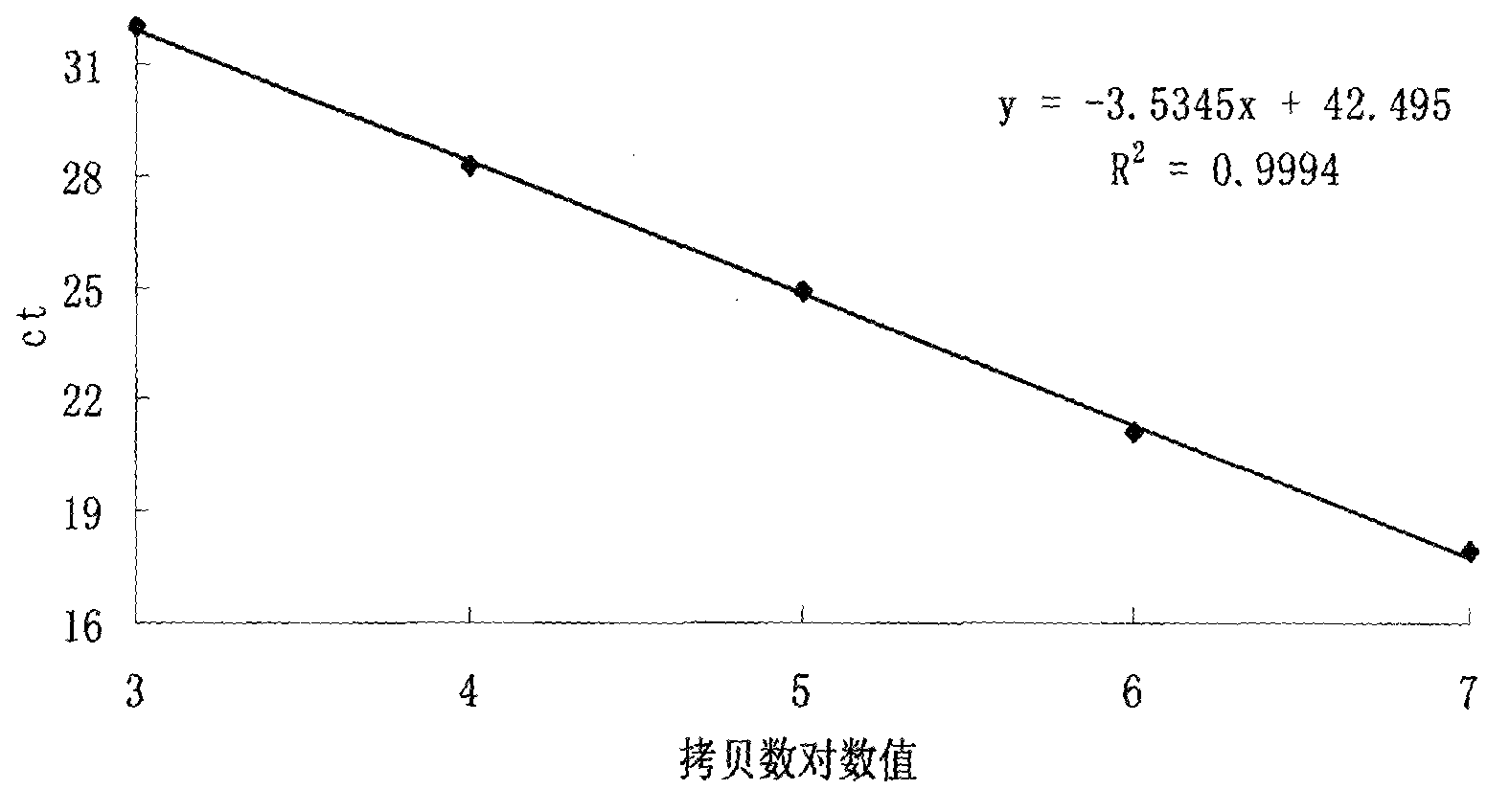
The present invention relates to a detection method for a dose of ionizing radiation on human peripheral blood lymphocytes. The detection method mainly comprises: designing primers and a Taqman-MGB probe according to a lymphocyte gdf15 gene expression sequence, and constructing a recombinant vector containing the lymphocyte gdf15 gene expression sequence as a standard substance; adopting the 10-fold serial diluted standard substance to carry out real-time fluorescence PCR, and drawing an absolute quantification standard curve; carrying out quantitative determination on the expression levels of the gdf15 gene of lymphocytes with different culture times after ionizing radiations with different doses to obtain a dose-effect fitting curve of the radiation dose and the gdf15 gene expression level; and carrying out quantitative determination on the expression level of the gdf15 gene of lymphocytes with the radiation dose requiring detection, and calculating the dose of the ionizing radiation on the lymphocytes requiring detection. According to the present invention, the dose of ionizing radiation on human peripheral blood lymphocytes can be rapidly and quantitatively detected so as to meet requirements of simpleness, rapid quantitation and high throughput, and the advantages can be provided when the large-scale radiation accident occurs.
Owner:NAT INST FOR RADIOLOGICAL PROTECTION & NUCLEAR SAFETY CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Detection method of Sanhuang granules
PendingCN113030364AHigh precisionGood stability and repeatabilityComponent separationSolventAloe emodin
The invention relates to the technical field of Sanhuang granule detection, in particular to a Sanhuang granule detection method, which comprises the following specific steps: S1, taking Sanhuang granules respectively, and performing thin-layer chromatography identification test on radix astragali preparata, prepared rhubarb and turmeric in the Sanhuang granules; S2, respectively detecting the durability of radix astragali preparata, prepared rhubarb and turmeric in the Sanhuang granules according to a thin-layer method under different temperatures, different humidity, thin-layer plates of different manufacturers, different sample application amounts and different developing solvent developing effects; and S3, determining the contents of aloe-emodin, rheinic acid, emodin, chrysophanol and physcion in the prepared rhubarb. According to the invention, the content and medicinal value of the radix astragali preparata, the prepared rhubarb and the turmeric in the Sanhuang granules can be effectively analyzed by adopting a thin-layer chromatography identification test on the radix astragali preparata, and the content and medicinal value of the radix astragali preparata, the prepared rhubarb and the turmeric in the Sanhuang granules can be effectively analyzed, so that the scheme is high in precision, good in repeatability and stability, simple and convenient to operate and high in detection efficiency so as to ensure the quality and the medicinal value of the Sanhuang granules.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
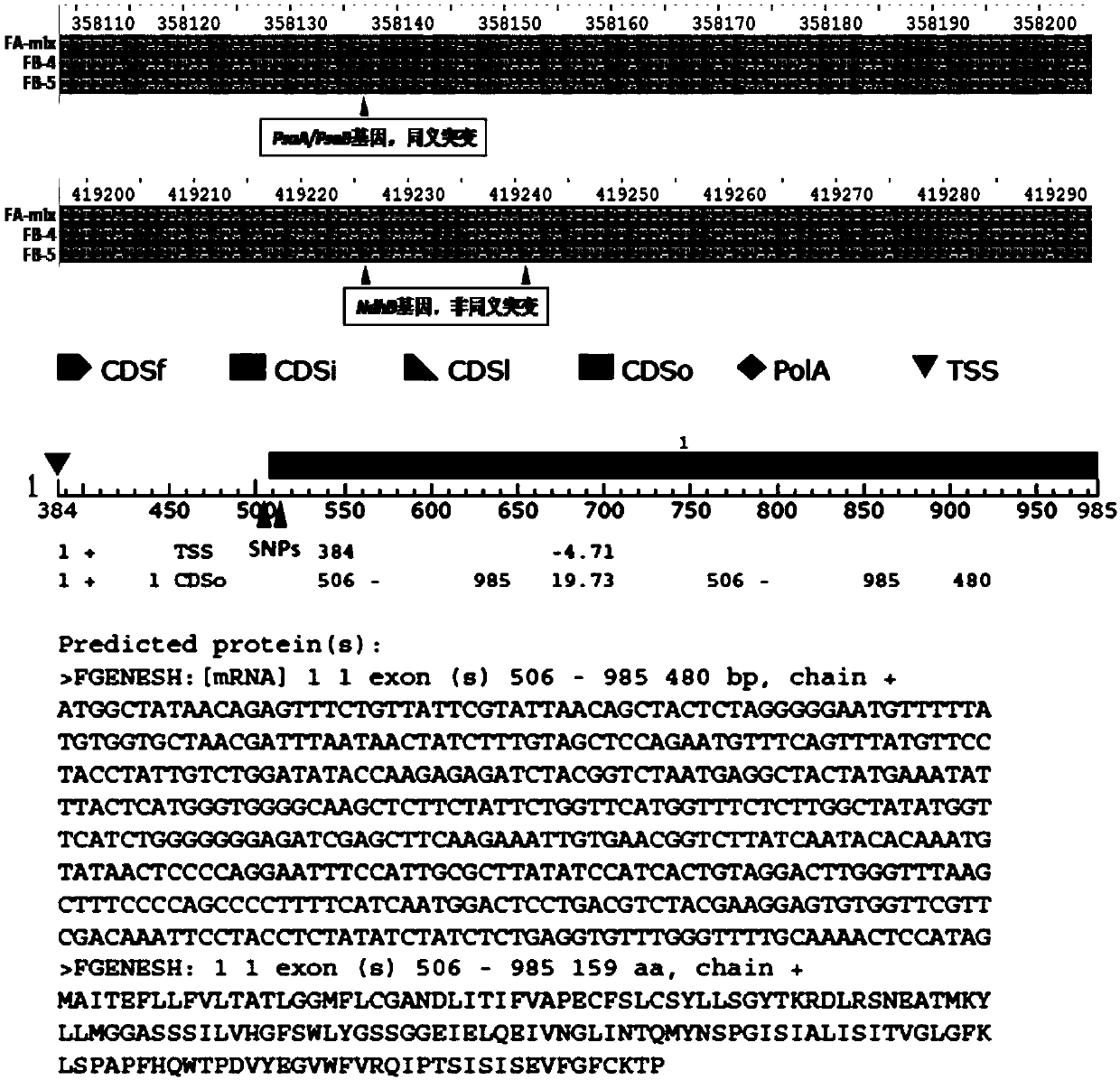
Development and application of molecular markers related to F-type three-line hybrid wheat male sterility gene
PendingCN110195100AStrong specificityGood stability and repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationAllele specificFertility
The invention discloses a method for development of specific PCR markers of SNP loci related to an F-type three-line hybrid wheat male sterility gene, wherein the method comprises the steps: (1) carrying out multi-generation sister crossing on wheat, dividing the obtained selfing lines into a maintainer line with high self-fruitful rate, a maintainer line with medium self-fertility rate, and a stable sterile line with self-fruitful rate low than 5%, wherein the three lines are identical in other biological characteristics except having fertility difference; (2) carrying out library-building and sequencing of the three samples; (3) searching for SNP variations between mitochondrial and cell nucleus genomes of the three samples; and (4) aiming at mitochondrial and cell nucleus genome SNP loci obtained after identification of the sterile line and the maintainer lines with different fruitfulness rates, detecting, designing and developing restriction endonuclease amplified polymorphism sequence (CAPS) markers or competitive allele specific PCR (KASP) markers. The invention also discloses relevant SNP loci molecular markers and a detection method thereof.
Owner:BIOCENTURY TRANSGENE CHINA
Quality control method for rhizoma polygonatum medicinal material
ActiveCN110412196AGood stability and repeatabilityGood peak shape and resolutionComponent separationColor/spectral properties measurementsThin-layer chromatographyWater content
The invention relates to a quality control method for a rhizoma polygonatum medicinal material. The method comprises the following steps of a construction method of an HPLC fingerprint spectrum of therhizoma polygonatum medicinal material, a thin layer chromatography analysis method, a water content and total ash content measurement method and an extractum and total saponin measurement method; the construction method of the HPLC fingerprint spectrum of the rhizoma polygonatum medicinal material comprises the step of preparation of a test solution, wherein fine rhizoma polygonatum powder is collected, water is added for ultrasonic treatment, centrifugation is conducted, and a supernate is collected; after evaporation to dryness is conducted, hydrochloric acid is added for dissolution, andwater bath treatment is conducted; methyl alcohol is dropwise added to take residual hydrochloric acid away; residues after being evaporated to dryness are dissolved with methyl alcohol, centrifugation and filtration through a microfiltration membrane are conducted, and the test solution is obtained; according to the construction method of the HPLC fingerprint spectrum of the rhizoma polygonatum medicinal material, the same peak value exists for different rhizoma polygonatum medicinal materials, the precision, repeatability and stability are good, the similarity, to a contrast spectrum, of thefingerprint spectrum is 0.99 or greater, and the HPLC fingerprint spectrum is good in separation degree and peak shape, the quality of three kinds of rhizoma polygonatum can be comprehensively reflected, and counterfeit medicinal products are identified based on the reason.
Owner:HUNAN XINHUI PHARMA +1
Polarization insensitivity liquid crystal photon crystal filter and making method thereof
InactiveCN101382684BReduce the driving voltageGood stability and repeatabilityNon-linear opticsPhotonic crystalRefractive index
The invention provides a polarization insensitive liquid crystal photonic crystal filter and a manufacturing method thereof. The filter comprises three glass substrates, wherein, one internal glass substrate is positioned in the middle and two external glass substrates are positioned at the two sides of the internal glass substrate; the inner sides of the two external glass substrates and the twosides of the internal glass substrate are plated with ITO electrode films which are alternately plated with multilayer films of silicon dioxide and titanium dioxide; the multilayer films are printed with oriented films which are subject to directional friction treatment and cause the friction directions of the opposite glass substrates to be antiparallel and overlaid; isolation pads used for controlling the thickness of the liquid crystal layers are arranged among all glass substrates and pure nematic phase liquid crystals with larger anisotropism difference of refractive index are injected among all glass substrates; the two liquid crystal layers are arranged in an antiparallel manner respectively and the arrangement directions of the two layers are vertical; and the peripheries of the glass substrates are provided with a sealing structure. The crystal filter and the manufacturing method have the advantages of low cost, low driving voltage, large tuning range, high tuning precision and good repeatability, being easy to control, and the like.
Owner:HARBIN ENG UNIV
Method for quantitatively detecting quail-derived components based on ddPCR technology
ActiveCN112980972AGood stability and repeatabilityThe measurement process is convenient and quickMicrobiological testing/measurementStandard curveFreeze dry
The invention discloses a method for quantitatively detecting a quail-derived component based on a ddPCR technology, and belongs to the field of molecular biological analysis. A sample to be detected is subjected to vacuum freeze drying to remove moisture and then ground into powder, a dried and uniform powder sample is obtained, DNA of the powder sample is extracted to serve as a template, the ddPCR primer pair and the probe of quails are added into an amplification reaction system, a microdroplet generator is used for generating microdroplets, a ddPCR instrument is used for amplification, after amplification is finished, the ddPCR instrument reads signals and analyzes data, and a quantitative detection result is obtained by contrasting a standard curve. According to the method, the sample freeze-drying treatment method can improve the uniformity of the sample, and meanwhile, the interference of the moisture content on quail-derived component quantification is removed; the ddPCR method is high in specificity and sensitivity, the quantitation limit is 5 mu g / mg, the detection limit is 1 mu g / mg, and quantitative detection work of quail-derived components in complex samples can be met.
Owner:南京市食品药品监督检验院
A method for preparing high-purity ethylhexylglycerin
The invention provides a method for preparing high-purity ethylhexylglycerin. According to the method, an intermediate 4-alkoxymethyl-1,3-dioxoane is generated from 2-ethylhexylglycidyl ether and acetone under the action of boron trifluoride diethyl etherate, a terminator is added in good time before hydrolysis, and after liquid separation, an oil-phase substance is neutralized by use of sodium hydrogen carbonate and then washed, next, a stabilizer is added, and finally, the high-purity ethylhexylglycerin is obtained by use of short-path distillation. The method is suitable for cosmetic additives and suitable for large-scale industrial production, and has the advantages of simple process, small energy consumption, product yield of greater than 88%, purity of 99.3%, no color and no taste, and the like.
Owner:SHAANXI RES DESIGN INST OF PETROLEUM CHEM IND
Dynamic positioning precision detection device and detection method thereof
PendingCN113917498AMeet the requirements of dynamic positioning accuracy testingHigh factor of motion trajectory accuracySatellite radio beaconingControl systemThree degrees of freedom
The invention discloses a dynamic positioning precision detection device. The device comprises a circular workbench assembly, wherein a rotary arm assembly is arranged on the circular workbench assembly, a precision detection assembly is arranged on the rotary arm assembly, a three-degree-of-freedom loading platform assembly is arranged at the end part of the rotary arm assembly, the circular workbench assembly, the precision detection assembly and the three-degree-of-freedom carrying platform assembly are all connected with a drive control system. The GNSS dynamic precision detection device is simple in structure, high in motion track precision, good in stability, good in repeatability, convenient to control, low in cost and good in practical value, and factors influencing the motion track precision of a receiver to be detected are few; and measurement errors caused by space-time desynchrony are effectively compensated through forward and reverse measurement, and the GNSS dynamic precision detection device has good practical value.
Owner:XIAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com