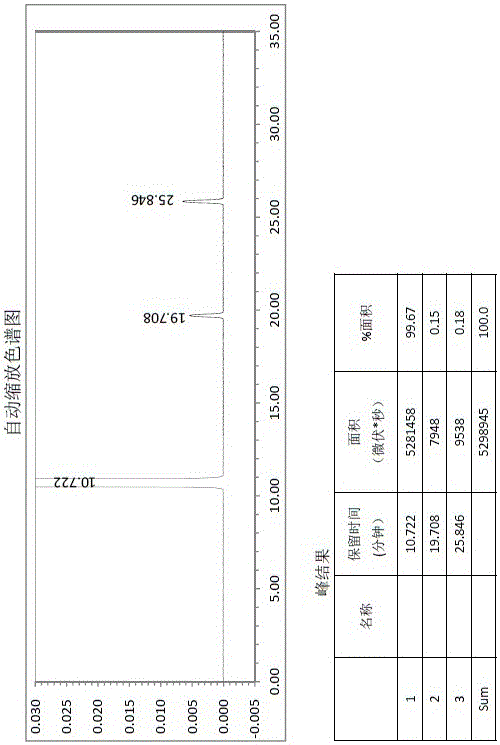
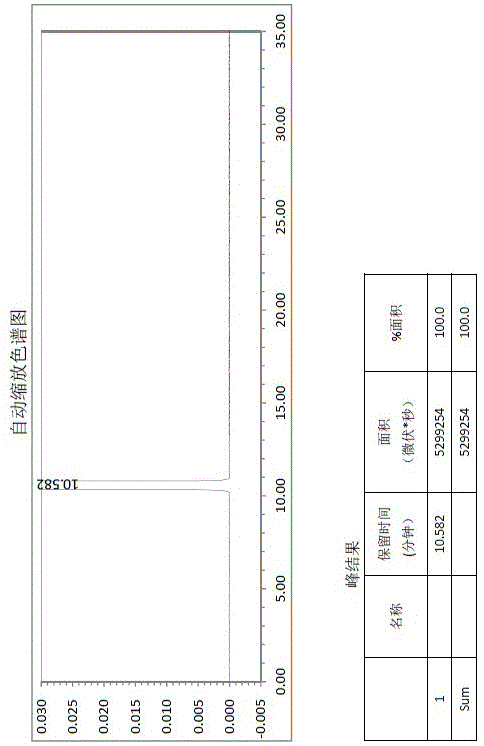
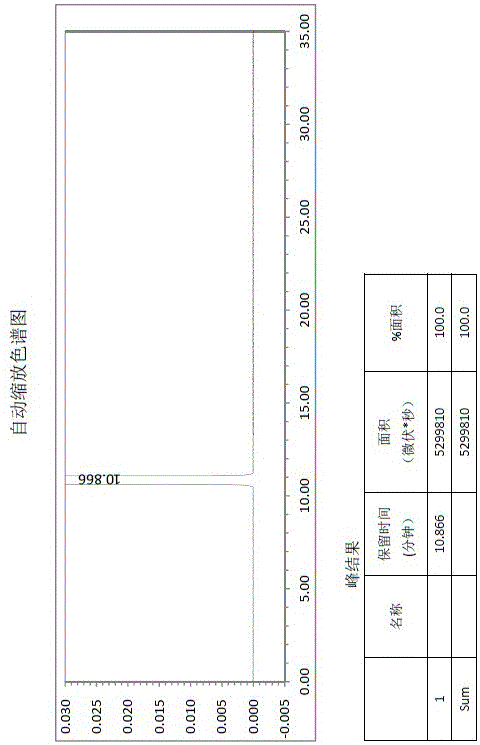
Butyl phthalide raw material drug product and preparation method thereof
A technology of butylphthalide and raw material medicine, which is applied in the field of medicine and can solve problems such as cumbersome operation, increased impurities, and low content purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0081] Preparation Example 1: Butylphthalide, a prior art raw material drug product, was prepared according to the preparation method disclosed by Li Shaobai et al. in "(±) Apigenin A Synthesis"
[0082] (1) Preparation of butylene phthalide
[0083] 148.0Kg of phthalic anhydride, 82.0Kg of anhydrous sodium acetate and 300.0L of n-valeric anhydride were heated and refluxed at 300°C for 4 hours, and the low-boiling point fraction was evaporated (controlled below 150°C), the residue was dissolved in hot water, and then NaHCO 3 Neutralize to pH=6~7, extract with 7×500L ether, combine organic layers, anhydrous Na 2 SO 4 Drying, filtering off the desiccant, distilling off diethyl ether, silica gel column chromatography, elution with chloroform-petroleum ether, to obtain 45.0Kg butylene phthalide
[0084] (2) Preparation of butylphthalide
[0085] Dissolve 45.0Kg of 3-butenephthalide in ether, add 4.5Kg10%Pd / C, and use H 2 Gas replacement 6 times, filled with H 2 , stirred, rea...
preparation example 2
[0086] Preparation example 2: Butylphthalide, a prior art raw material drug product, according to the preparation method disclosed in Chinese patent CN101962374
[0087] (1) Preparation of Bromobutane Grignard Reagent
[0088] Under the protection of nitrogen, add 200L of tetrahydrofuran, 6.0Kg of magnesium flakes and 0.1Kg of iodine in a reaction tank equipped with stirring, thermometer and reflux condensing device, raise the temperature to 50°C, and add 31.50Kg of bromobutyl dissolved in 40L of tetrahydrofuran dropwise Alkanes, the temperature is controlled not to exceed 70 ° C, after the dropwise addition, continue to stir for 1 hour to obtain the Grignard reagent of bromobutane
[0089] (2) Preparation of o-valerylbenzoic acid
[0090] Under the protection of nitrogen, add 300L tetrahydrofuran, 30Kg phthalic anhydride and 2.5Kg copper iodide, cool to -10°C, add the Grignard reagent of bromobutane obtained in step (1) dropwise for about 1 hour Complete; after the dropwise...
Embodiment 1
[0099] Embodiment 1: Butylphthalide bulk drug product
[0100] A. Preparation of potassium hydroxyamylbenzoate (ring-opening reaction): Add 8.0Kg potassium hydroxide to 18L methanol (density 0.79), heat to reflux until dissolved, add 20.2Kg crude butylphthalide (content: 94.9 %), heated to reflux for 1.5 hours; cooled to 26°C, slowly added 145L methyl tert-butyl ether (density 0.74), stirred for 2 hours, filtered to obtain a filter cake, which was washed with 4.5L methyl tert-butyl ether 1 time, dried to obtain 19.6Kg of potassium salt of hydroxyamylbenzoate
[0101] B. Add 92.0g of potassium hydroxyamylbenzoate obtained in step A to a mixed solvent of 450ml of water 1 and 225ml of dichloromethane (density 1.34), control the temperature at 35~40°C, slowly add dilute hydrochloric acid dropwise, Control the pH to 2.1~2.3, stir and react for 4 hours, stand still and separate the phases, take the dichloromethane phase, wash the dichloromethane phase with 150ml saturated sodium bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com