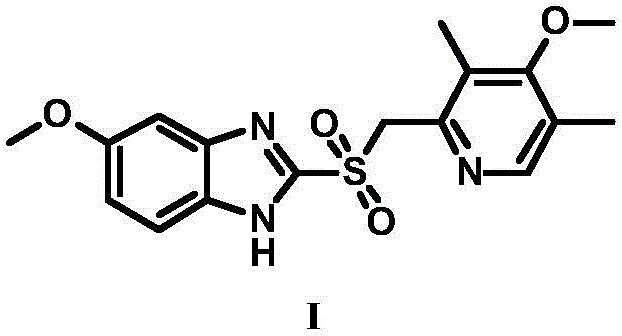
High-purity esomeprazole sodium preparation method
A technology for esomeprazole sodium and esomeprazole is applied in the field of preparation of high-purity esomeprazole sodium, can solve problems such as being unsuitable for industrial production, high equipment requirements, and many product impurities, and achieves operation Simple, reproducible, high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 esomeprazole sodium
[0052] Dissolve 100g of esomeprazole in 1800ml of acetonitrile and 200ml of methyl isobutyl ketone, and stir at 10°C until dissolved. Add 69.5ml of sodium hydroxide aqueous solution (13.9g of sodium hydroxide dissolved in 55.6ml of water) dropwise, control the drop rate, and finish dropping in 20 minutes. After dropping, stir and crystallize at 10°C for 8 hours. After filtering, the filter cake was stirred and washed twice with a mixed solvent of acetone / methyl tert-butyl ether (v / v=1 / 1), each time 200 ml, each time 10 minutes. After filtering, the filter cake was vacuum-dried at 40° C. to obtain 85 g of white solid, with a molar yield of 85%, a chromatographic purity of 99.92%, an optical purity of 99.89%, and a formula I peroxidation impurity content of 0.01%.
Embodiment 2
[0053] The preparation of embodiment 2 esomeprazole sodium
[0054] Dissolve 100g of esomeprazole in 3429ml of tetrahydrofuran and 571ml of acetone, and stir at 20°C until dissolved. 29ml of aqueous sodium hydroxide solution (11.6g of sodium hydroxide dissolved in 17.4ml of water) was added dropwise, the dropping speed was controlled, and the dropping was completed in 40 minutes. After the dropping, stirred and crystallized at 20°C for 9 hours. After filtering, the filter cake was stirred and washed twice with a mixed solvent of acetone / ether (v / v=1 / 3), each time 400 ml, each time 20 minutes. After filtration, the filter cake was vacuum-dried at 45° C. to obtain 87 g of a white solid with a molar yield of 87%, a chromatographic purity of 99.93%, and an optical purity of 99.91%. The peroxidized impurities of formula I were not detected.
Embodiment 3
[0055] The preparation of embodiment 3 esomeprazole sodium
[0056] Dissolve 100g of esomeprazole in 5455ml of dichloromethane and 545ml of butanone, and stir at 30°C until dissolved. 38.7ml of sodium hydroxide aqueous solution (23.2g of sodium hydroxide dissolved in 15.5ml of water) was added dropwise, the drop rate was controlled, and the drop was completed in 60 minutes. After the drop, stirred and crystallized at 30°C for 10 hours. Filter, and the filter cake is stirred and washed 4 times with a mixed solvent of acetone / n-heptane (v / v=1 / 5), each dosage is 600ml, and each time is 30 minutes. After filtration, the filter cake was vacuum-dried at 50° C. to obtain 84 g of a white solid with a molar yield of 84%, a chromatographic purity of 99.94%, an optical purity of 99.92%, and no peroxidized impurities of formula I were detected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com