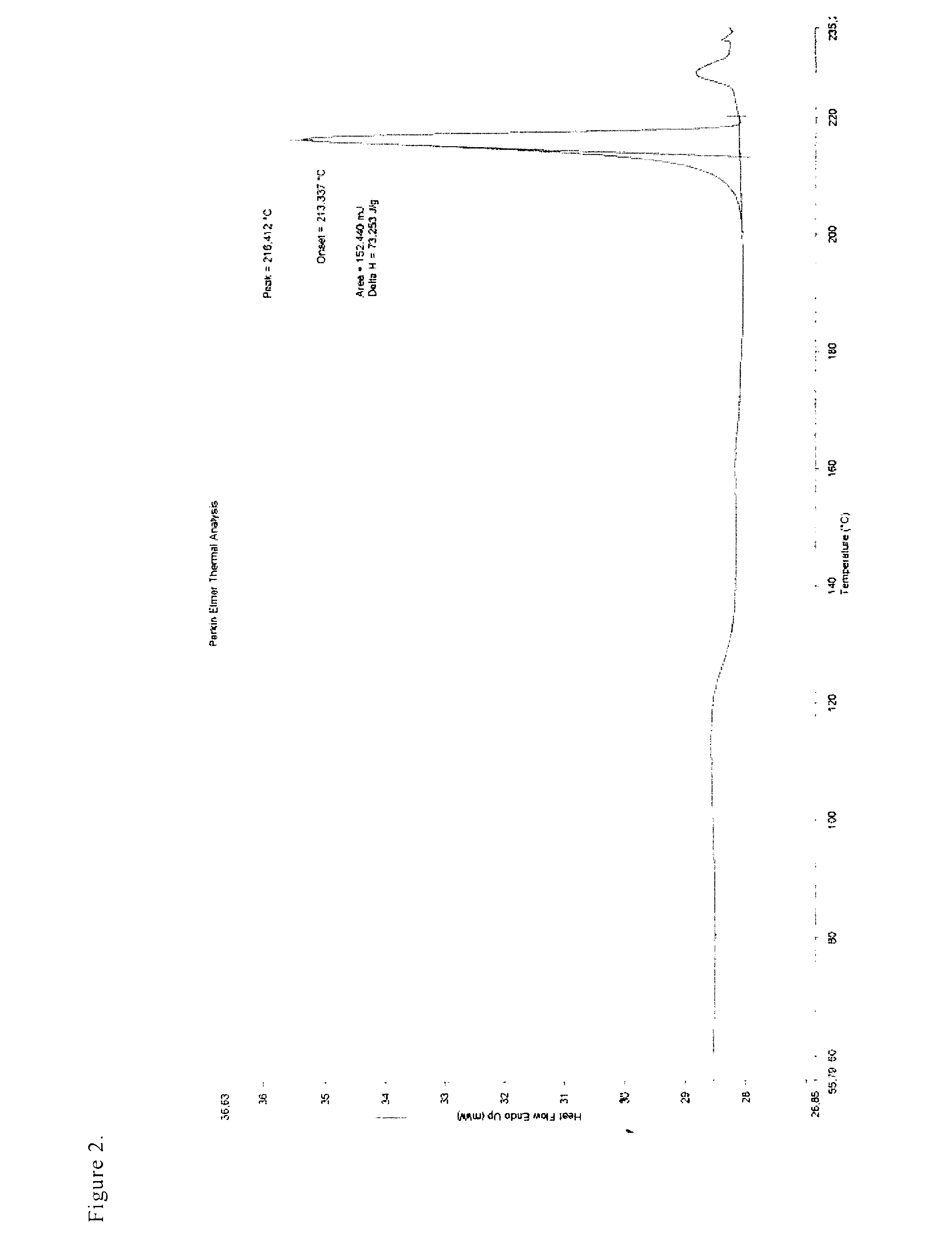
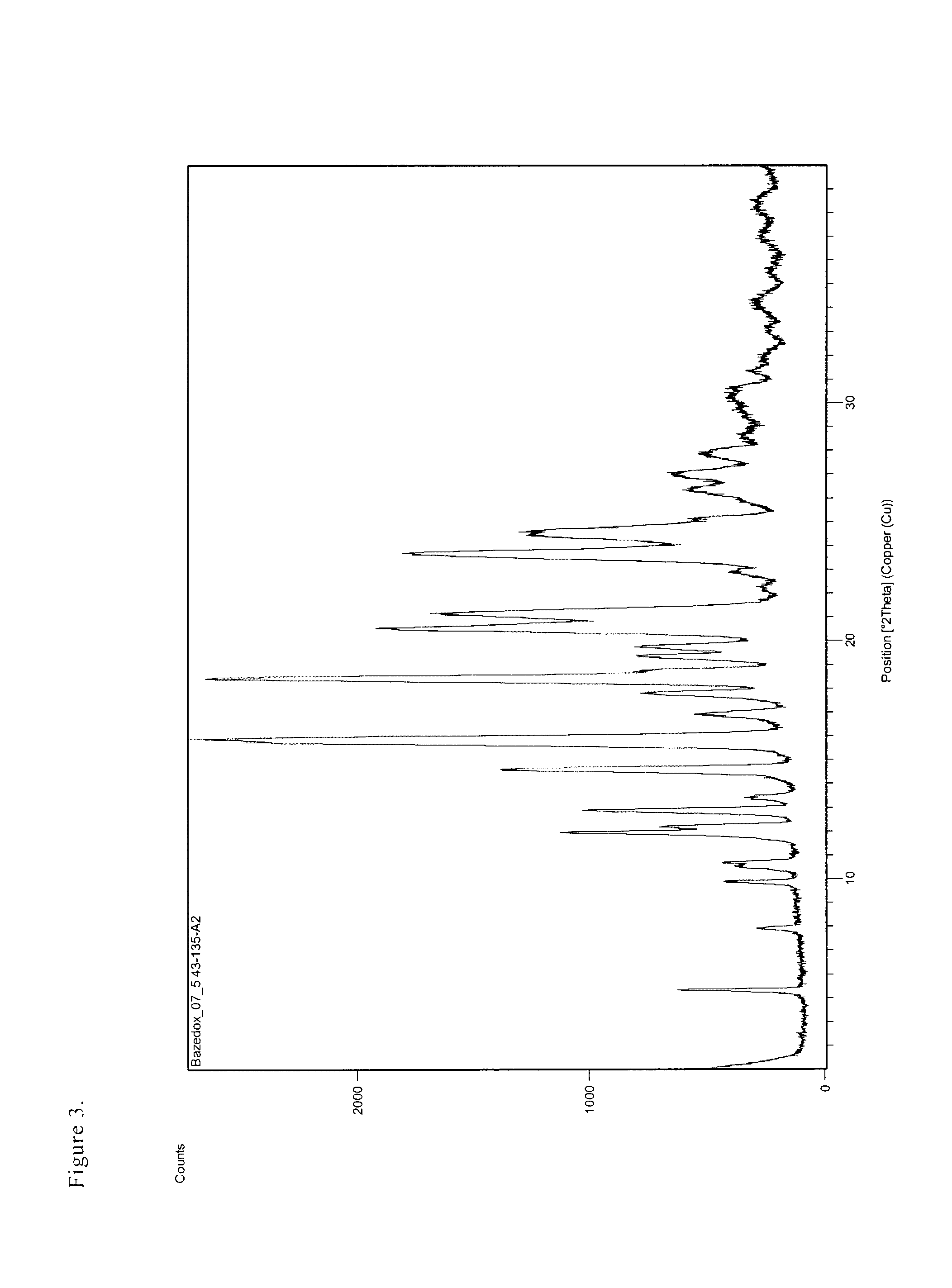
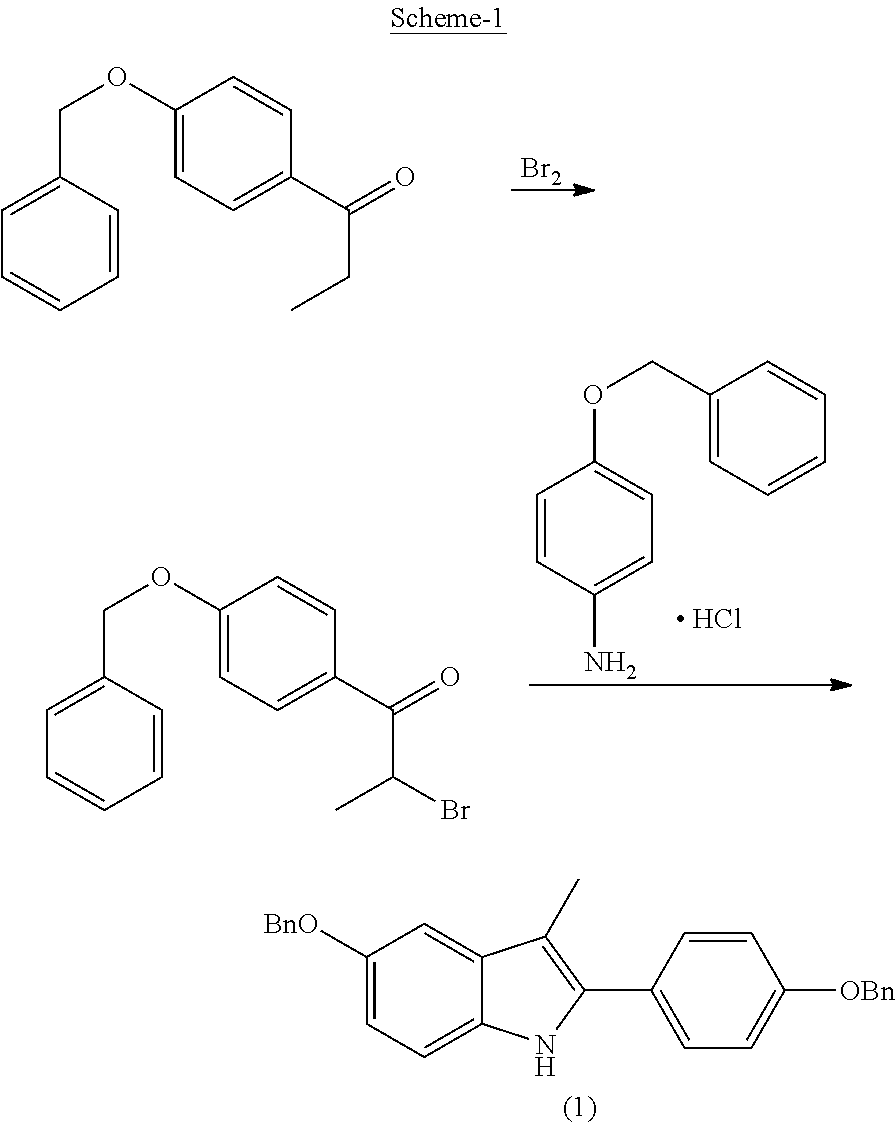
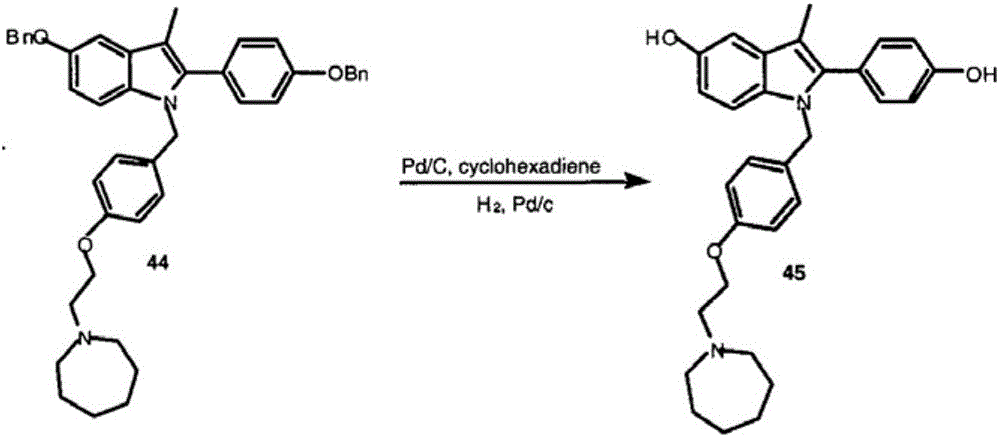
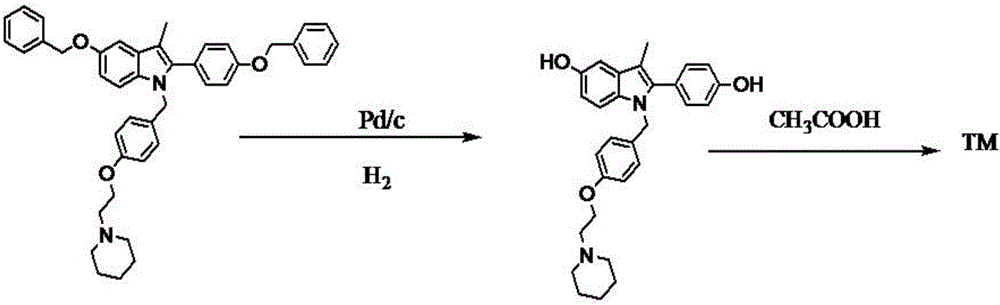
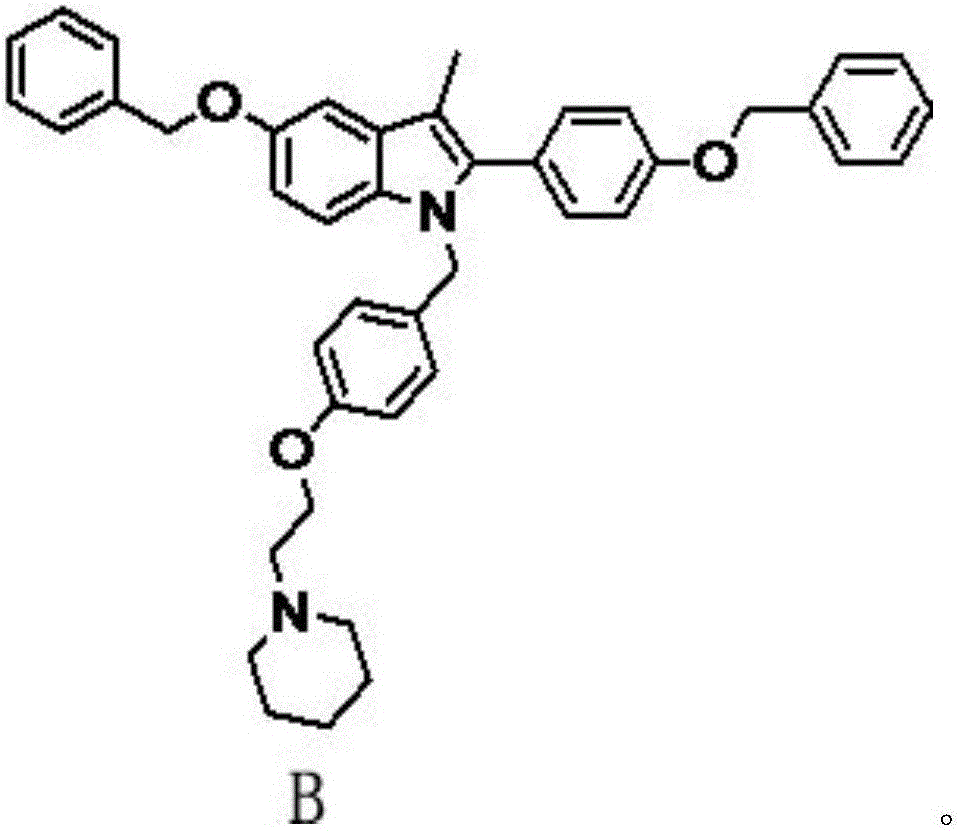
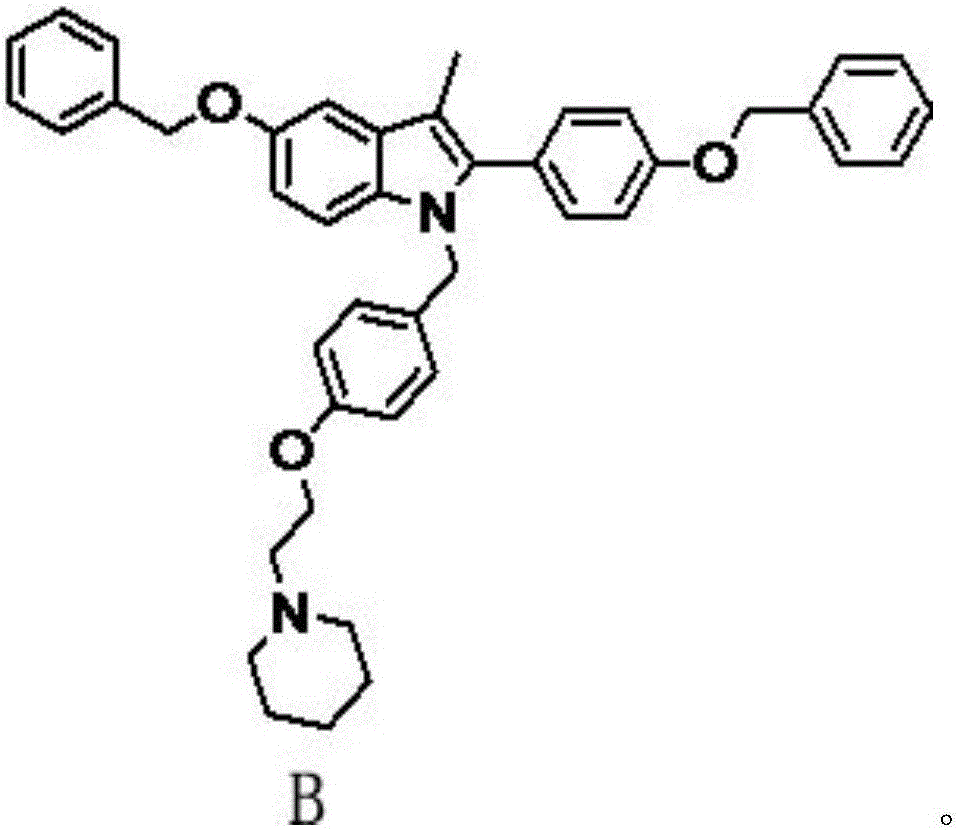
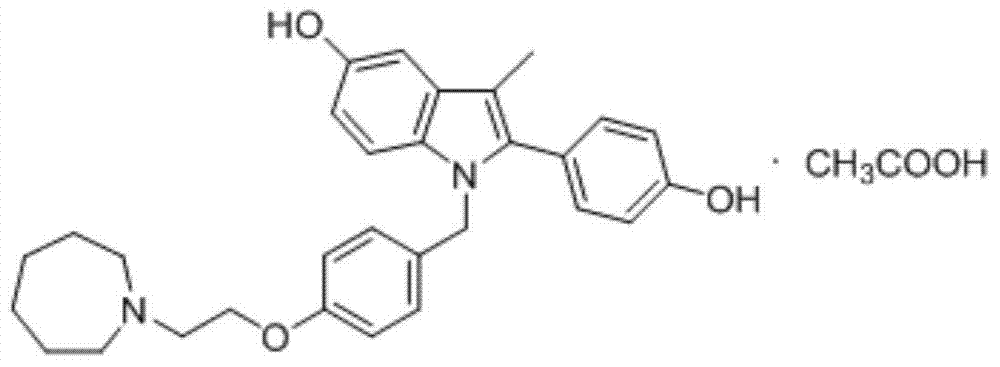
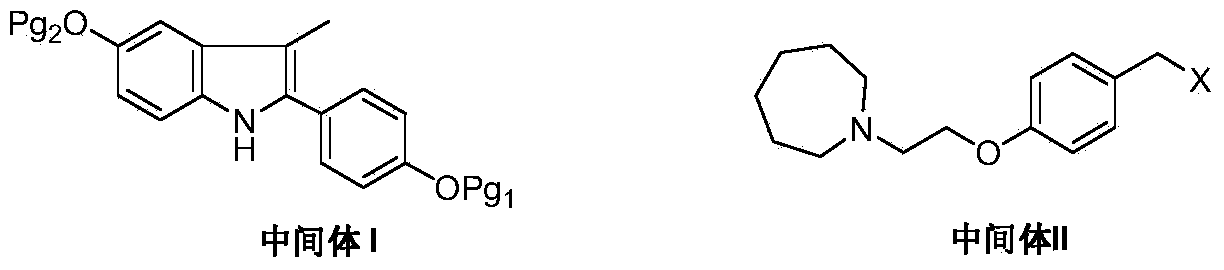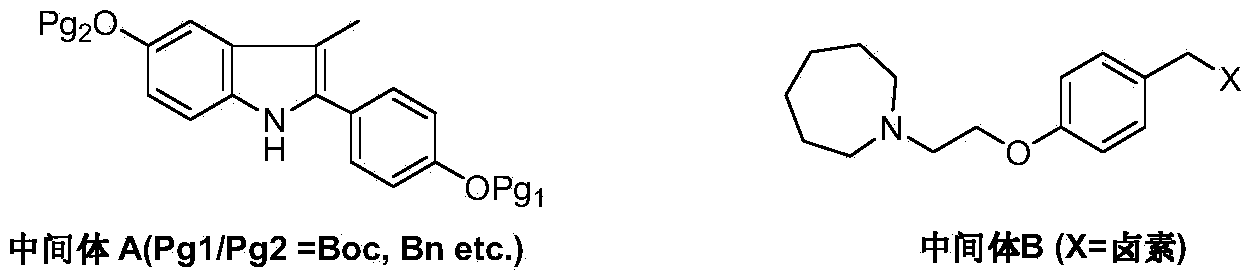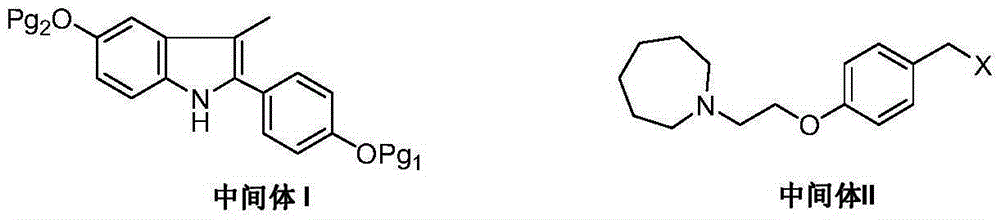Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
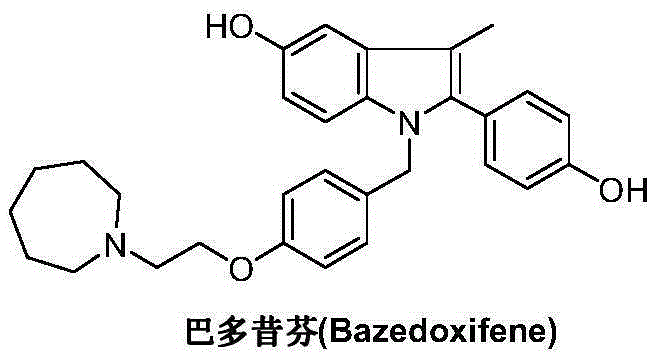
43 results about "Bazedoxifene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
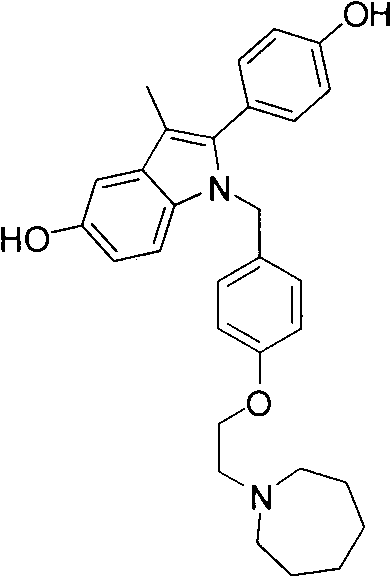
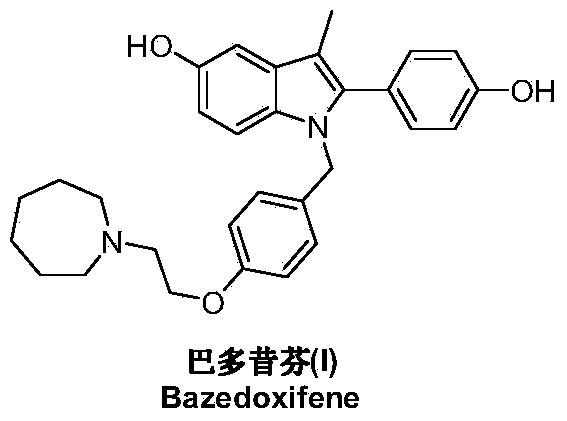
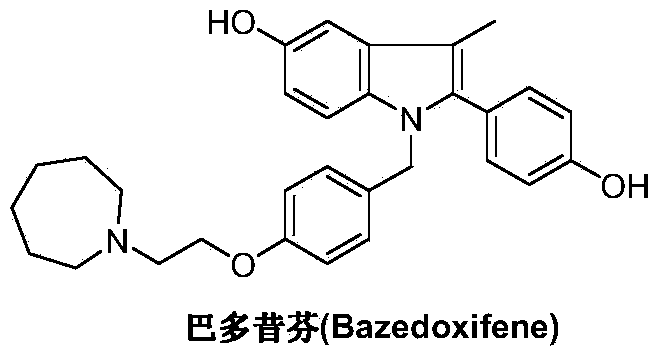
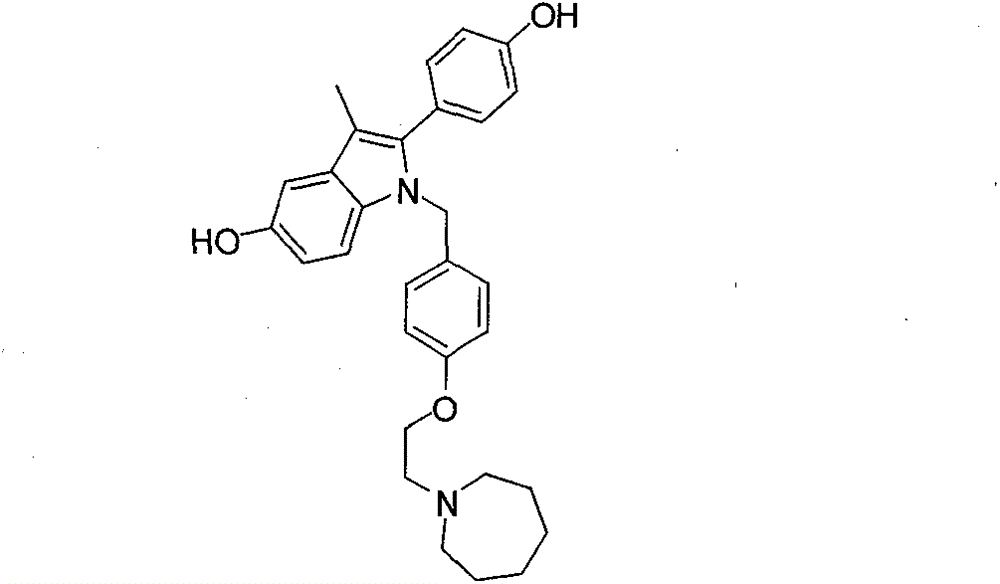
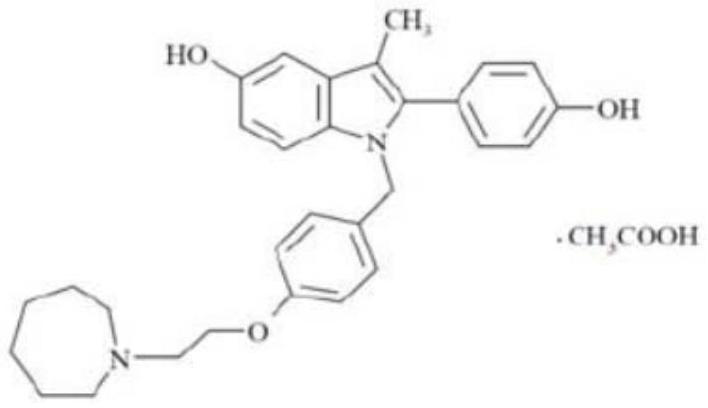
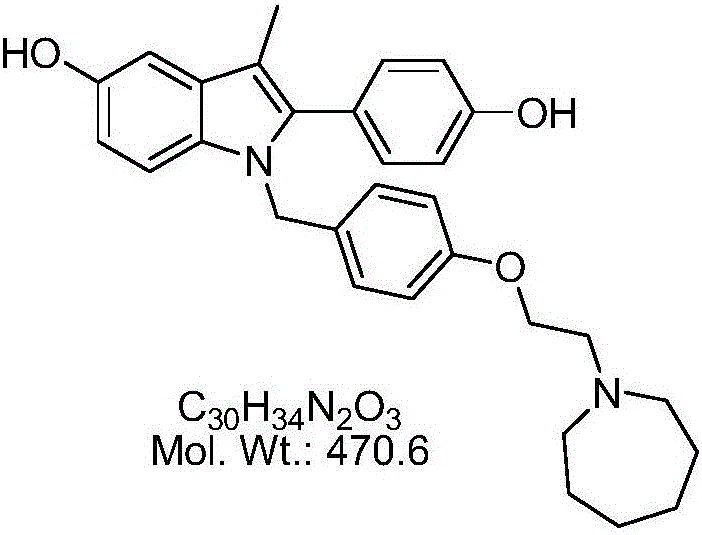
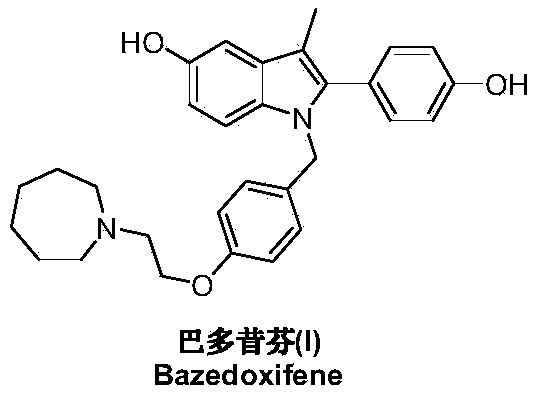
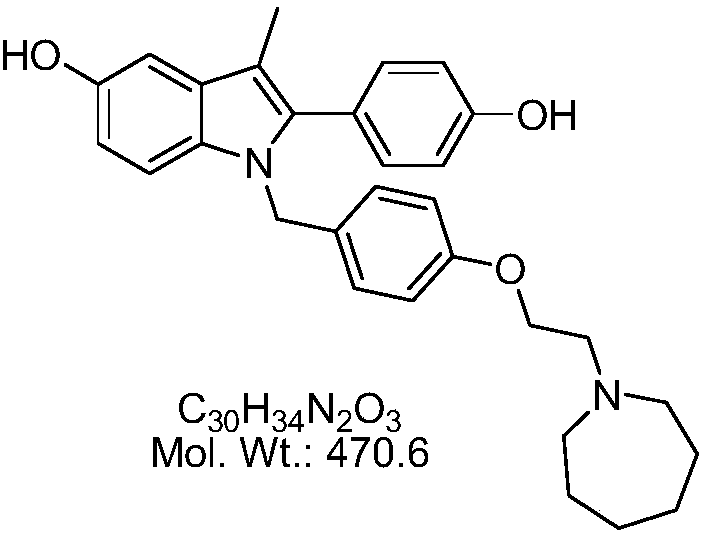
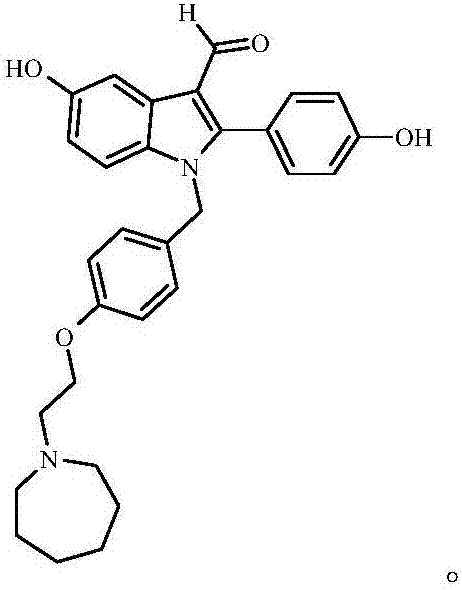
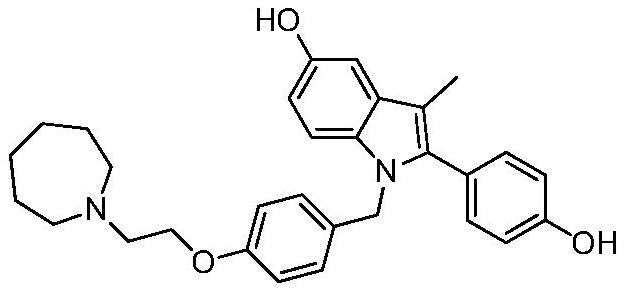
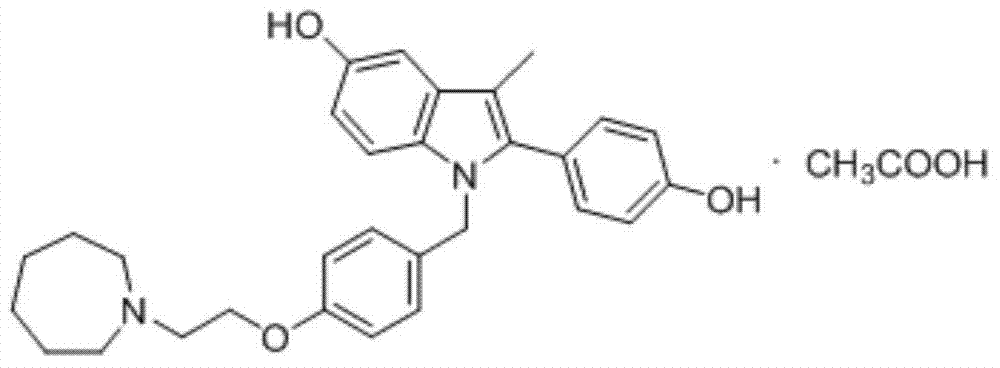
Bazedoxifene, used as bazedoxifene acetate, is a medication for bone problems and possibly (pending more study) for cancer. It is a third-generation selective estrogen receptor modulator (SERM). Since late 2013 it has had U.S. FDA approval for bazedoxifene as part of the combination drug Duavee in the prevention (not treatment) of postmenopausal osteoporosis. It is also being studied for possible treatment of breast cancer and pancreatic cancer.
Formulations of conjugated estrogens and bazedoxifene
InactiveUS20070003623A1Relieve vasomotor symptomEffective treatmentBiocideSkeletal disorderMedicineConjugated oestrogens
The present invention relates to solid dosage formulations containing conjugated estrogens and bazedoxifene, or a salt thereof. In some embodiments, the compositions include a core comprising conjugated estrogens, and at least one coating that comprises bazedoxifene, or a salt thereof.
Owner:WYETH LLC
New synthetic method of bazedoxifene
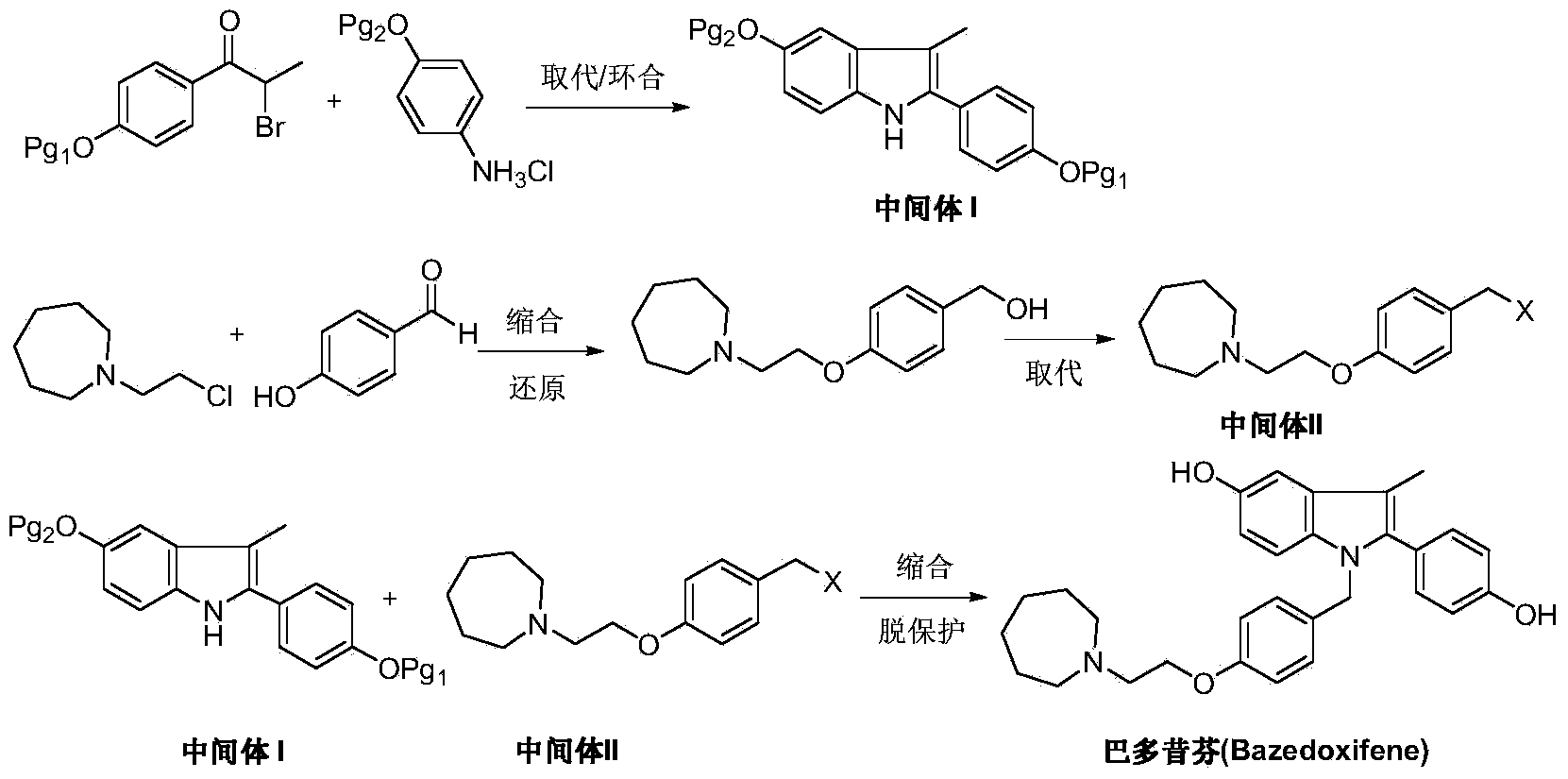
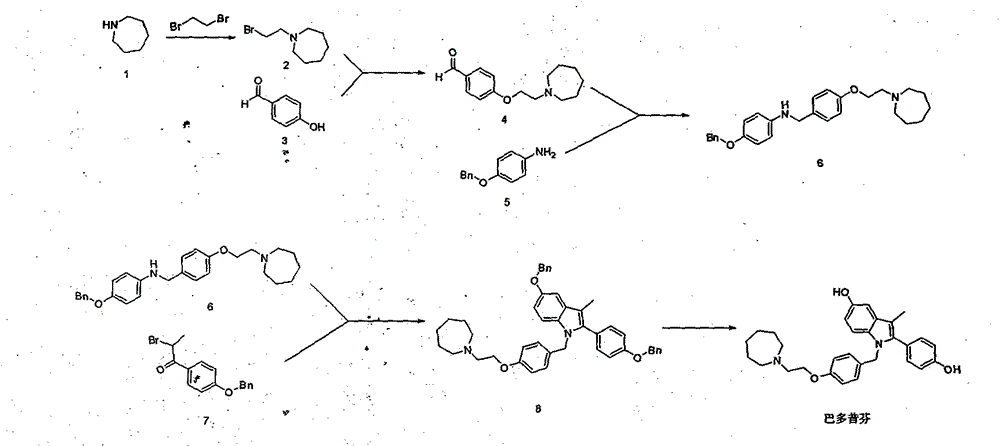
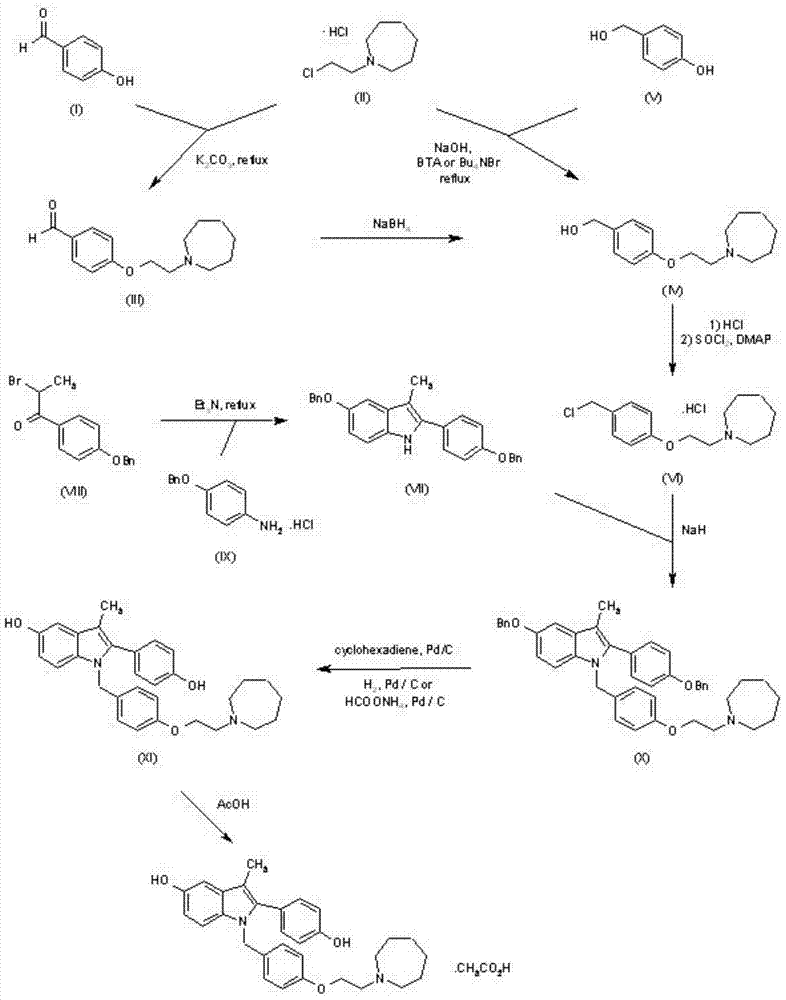
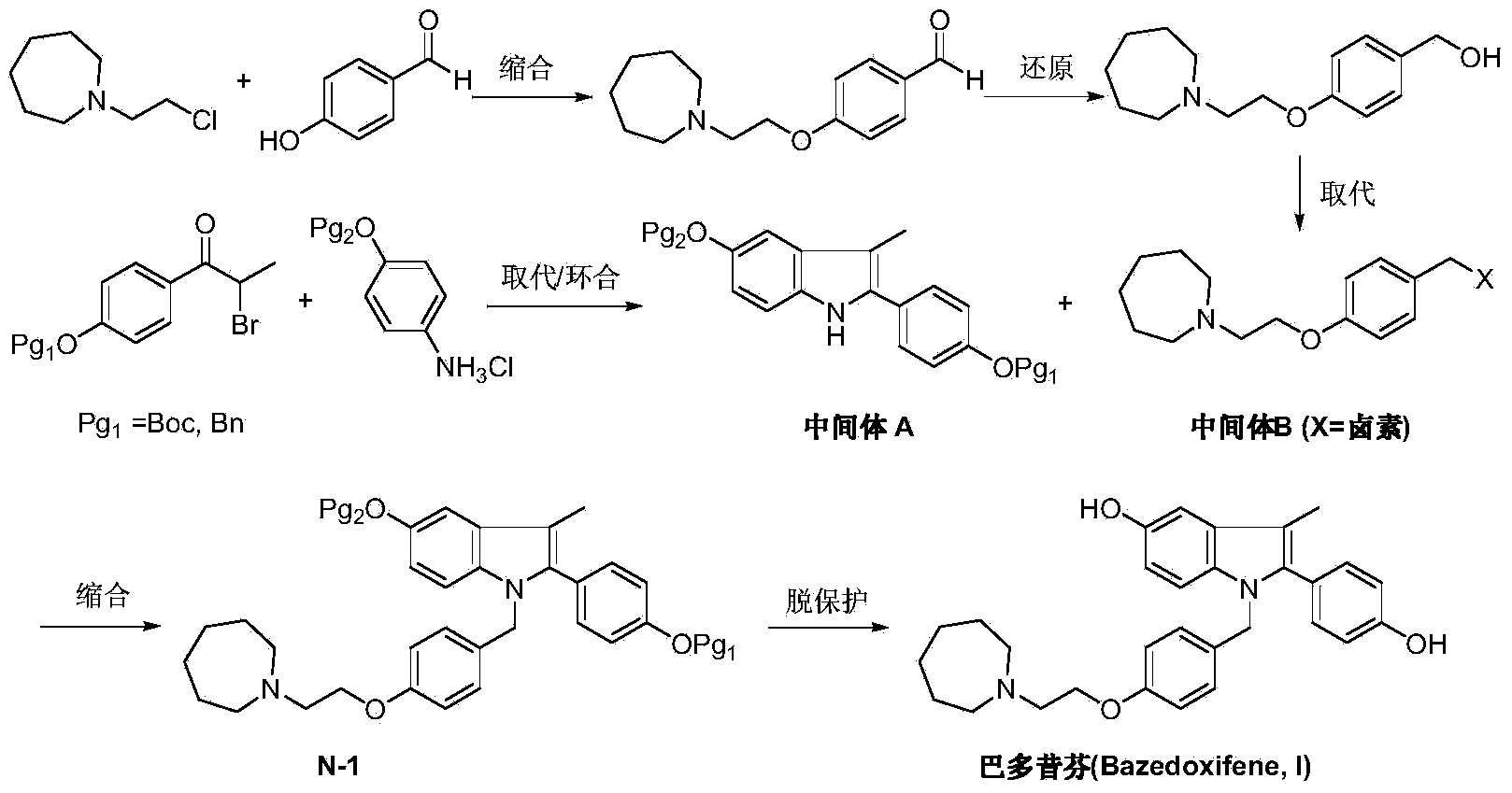
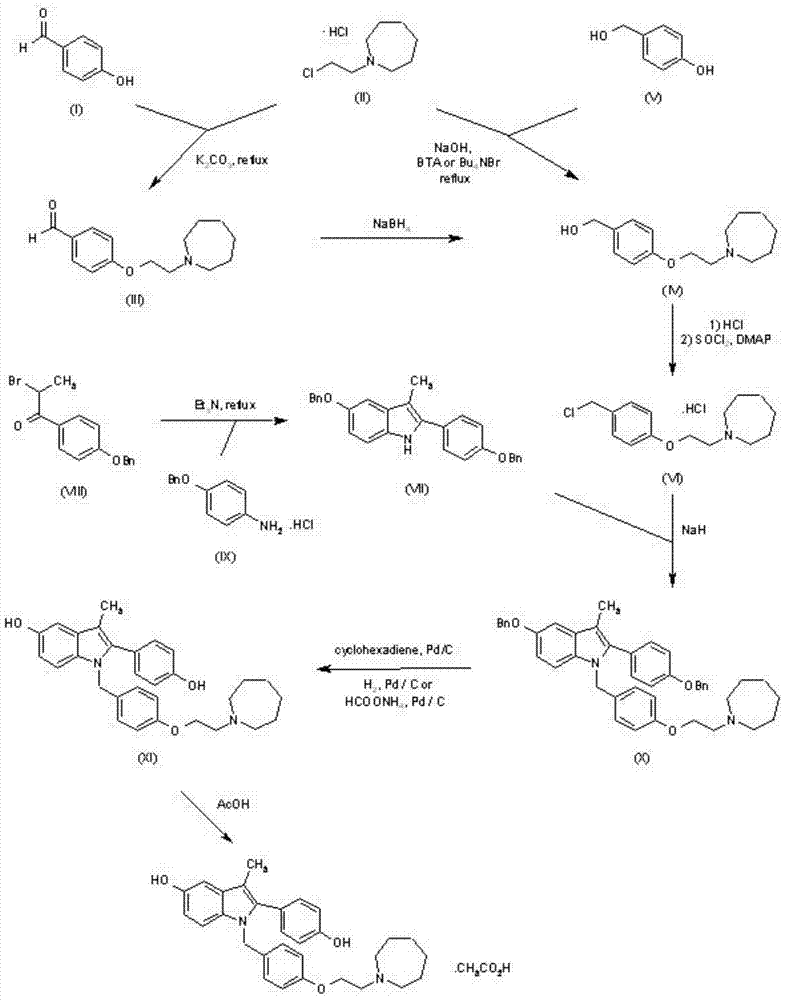
The invention discloses a new synthetic method of bazedoxifene. Bazedoxifene is prepared by the steps of bromination, substitution, reductive amination, substitution, cyclization, deprotection, and the like of azepane which is selected as a raw material. The synthetic method of the invention has the advantages of mild reaction conditions, simple and convenient operating process, easily obtained reagent, and low cost.
Owner:南京正济医药销售有限公司
O-desmethylvenlafaxine and bazedoxifene combination product and uses thereof
A combination product containing at least two active compounds, O-desmethylvenlafaxine or a pharmaceutically acceptable salt thereof and bazedoxifene or a pharmaceutically acceptable salt thereof is described. Also described are methods of making and using this combination product to treat a variety of conditions associated with low circulating estrogen levels or low estrogen receptor activity.
Owner:WYETH LLC
Preparation method of bazedoxifene
ActiveCN103772261AEasy to prepareHigh yieldOrganic chemistryBulk chemical productionMedicinal chemistryBazedoxifene
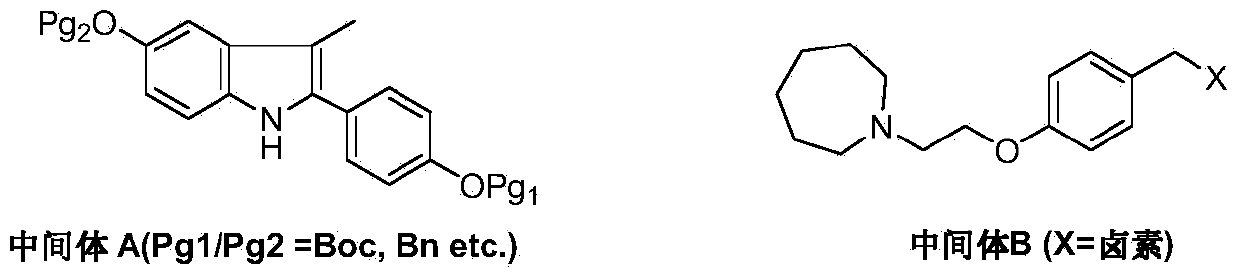
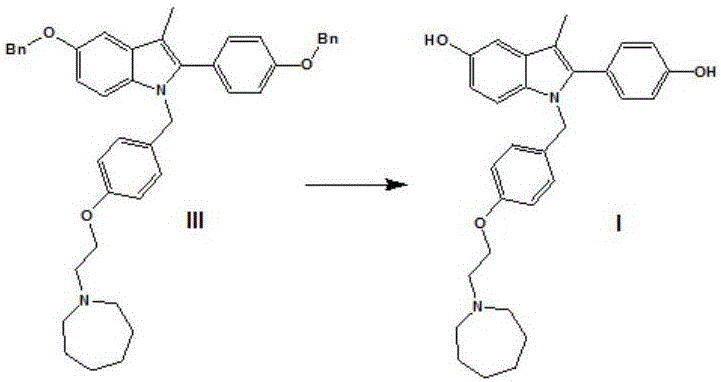
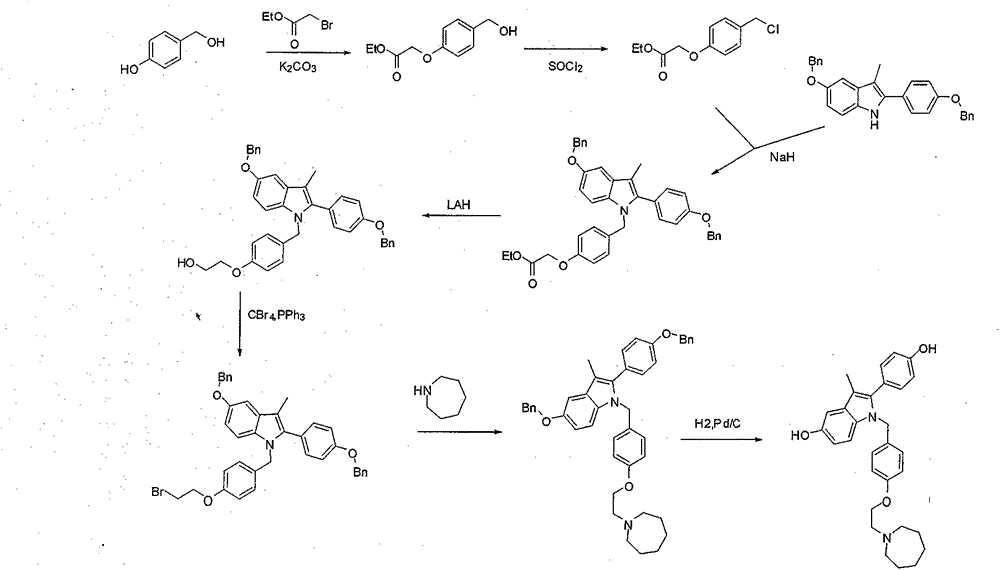
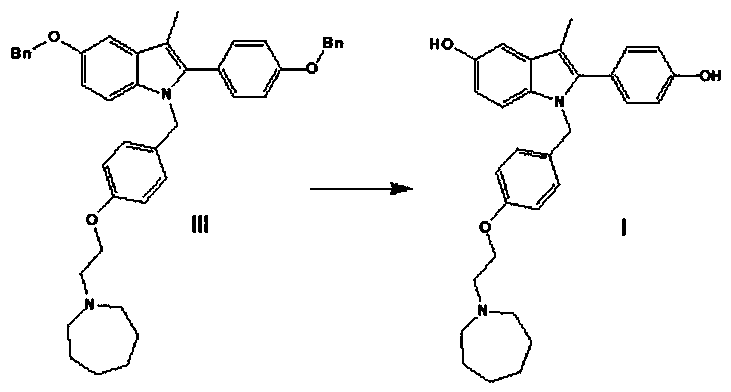
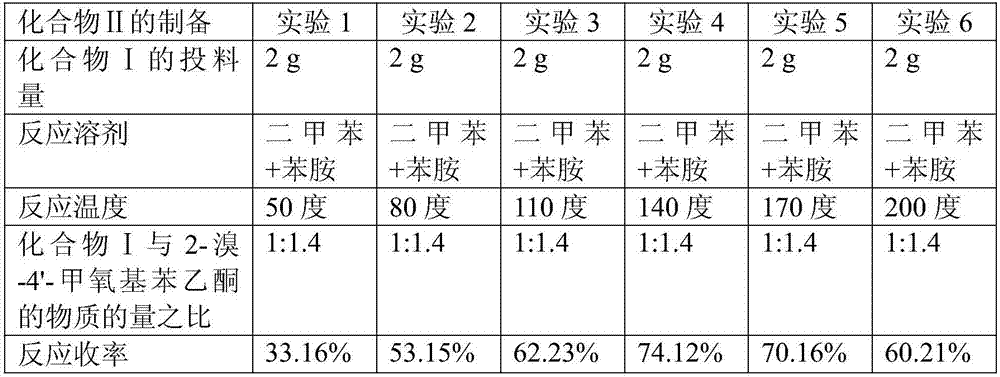
The invention discloses a preparation method of bazedoxifene. The preparation method of the bazedoxifene comprises the following steps: taking 1-(4-Pg1oxy-phenyl)-allylene (II) and N-[4-(2-azacycloheptane-1-yl- ethyoxyl-benzyl)]-N-[4-(Pg2 oxyphenyl)] hydrazine (III) to perform addition cyclization reaction and acquire 1-[4-(2-azacycloheptane-1-yl- ethyoxyl-benzyl)]-2-(4-Pg1oxy-phenyl)-3-methyl-5-(Pg2oxy)-1H-benzpyrole (IV); performing deprotection on an intermediate (IV) to prepare bazedoxifene (I). The preparation method is simple in process, high in yield, economical and environmental-friendly, so that a novel preparation method for industrial production of the bazedoxifene.
Owner:SUZHOU LIXIN PHARMA
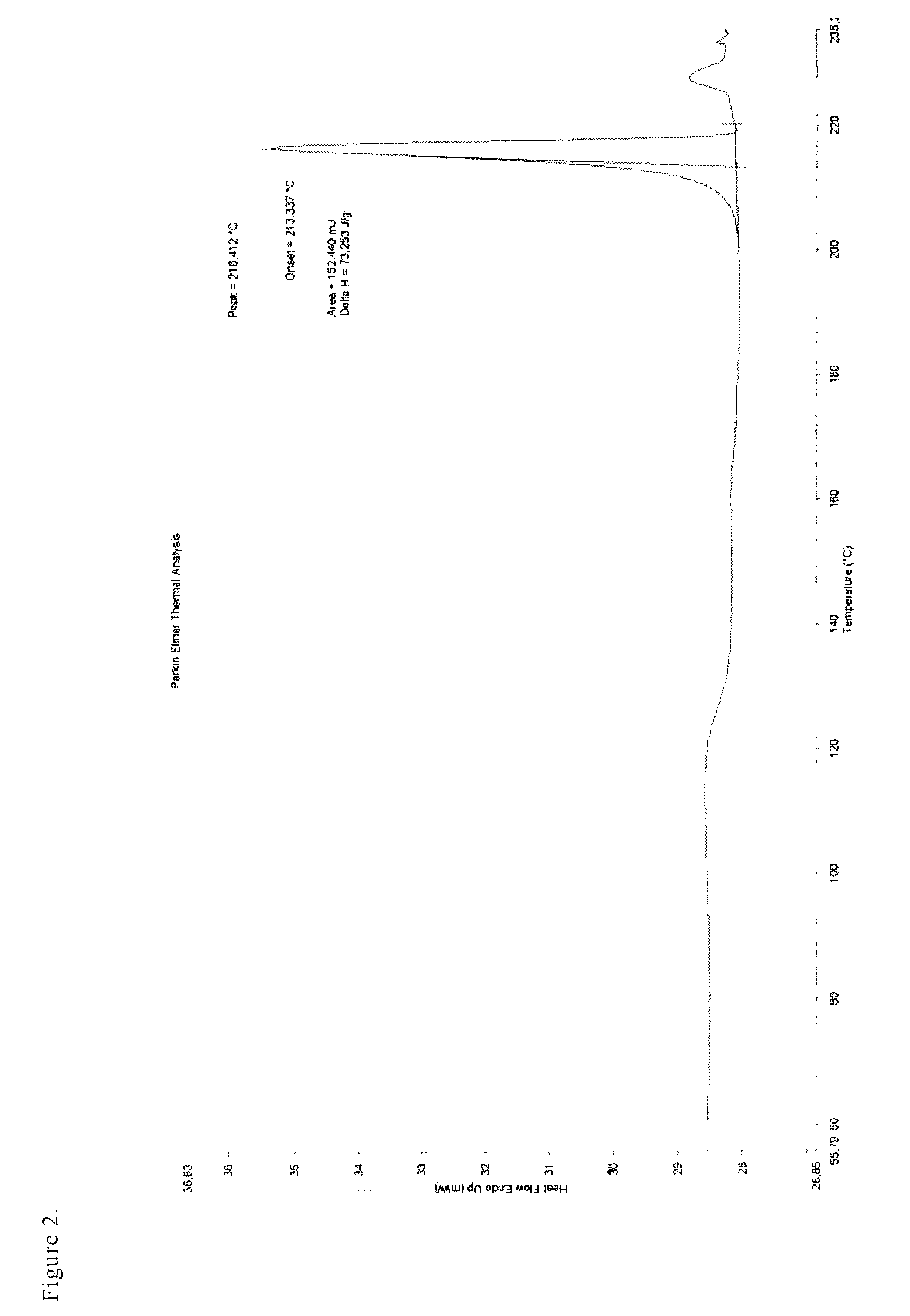
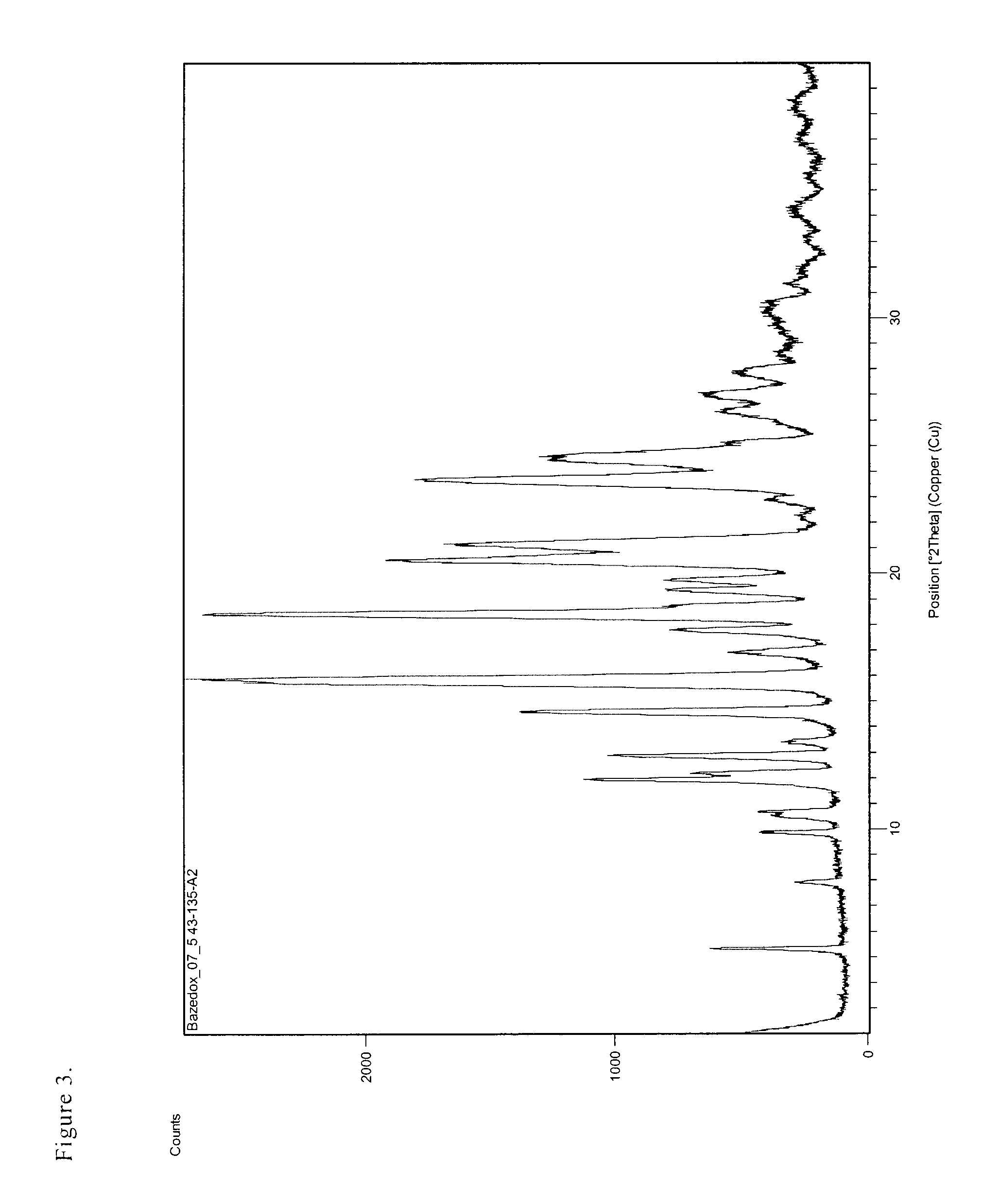
Salts of bazedoxifene
ActiveUS8034807B2Quality improvementHigh yieldBiocideOrganic chemistryMedicinal chemistryBazedoxifene
The invention deals with new crystalline salts of bazedoxifene, by means of which a high API quality can be achieved in a high yield.
Owner:ZENTIVA AS
Formulations of conjugated estrogens and bazedoxifene
The present invention relates to solid dosage formulations containing conjugated estrogens and bazedoxifene, or a salt thereof. In some embodiments, the compositions include a core comprising conjugated estrogens, and at least one coating that comprises bazedoxifene, or a salt thereof.
Owner:WYETH LLC
Bazedoxifene intermediate and preparation method thereof
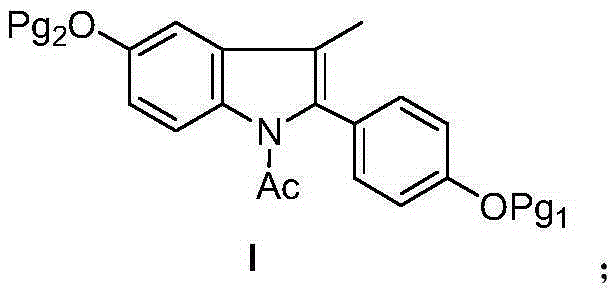
The invention discloses a bazedoxifene intermediate, namely 2-[4-(Pg1 oxyl) phenyl]-3-methyl-5-(Pg2 oxyl)-1-acetyl-indol (I) and a preparation method thereof. The preparation method of the bazedoxifene intermediate comprises the step that 1-(4-Pg1 oxyl-phenyl)-allylene (II) and N-[4-(Pg2 oxyl) phenyl] acetamide (III) are subjected to oxidation ring-closure reaction so as to obtain the bazedoxifene intermediate, namely the 2-[4-(Pg1 oxyl) phenyl]-3-methyl-5-(Pg1 oxyl)-1-acetyl-indol (I) through one step. The intermediate is stable in natural. The preparation method is concise, economical and environment-friendly and can be used for providing a new effective intermediate for industrial production of bazedoxifene bulk drugs.
Owner:XUZHOU LIXIN IRRIGATION & DRAINAGE EQUIP CO LTD
Separation preparation method of bazedoxifene acetate impurity A
InactiveCN104774170AEfficient separationOptimizing Chromatographic ConditionsOrganic chemistryMedicinal chemistryBazedoxifene
The invention relates to a method for preparing an impurity A having the purity of more than 97% from a bazedoxifene raw material, can be applied in research of reference substances and belongs to the field of medicine.
Owner:JIANGSU CAREFREE PHARM CO LTD
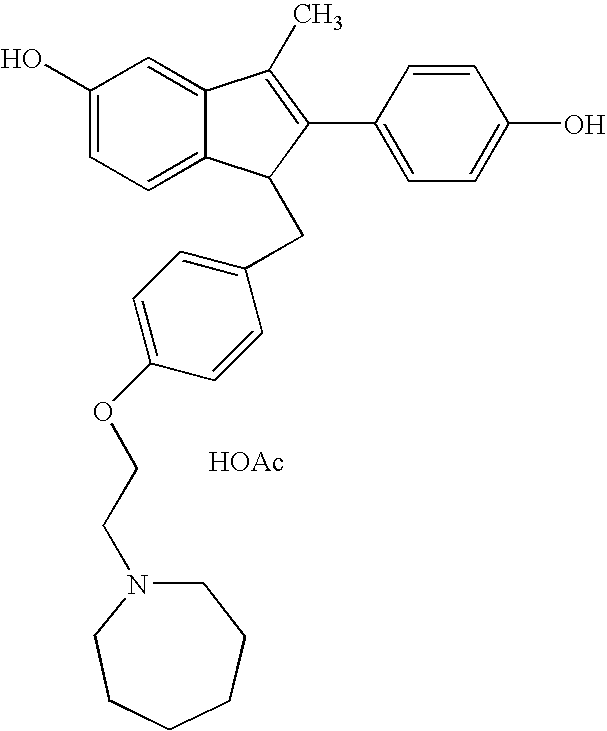
Preparation method of bazedoxifene acetate polycrystalline type B
ActiveCN104370796AEasy to prepareSuitable for industrial productionOrganic chemistry methodsAcetic acidAntioxidant
The invention discloses a preparation method of bazedoxifene acetate polycrystalline type B. Bazedoxifene free alkali and glacial acetic acid are taken as raw materials and salified to obtain the bazedoxifene acetate polycrystalline type B. The operation conditions of the preparation process are simple and do not need to be carried out under the protection of inert gas; no antioxidant is used in the preparation process; the yield and purity of the prepared product are high, and therefore the product is suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Salts of bazedoxifene
ActiveUS20100240888A1The implementation process is simpleQuality improvementOrganic chemistryUrinary disorderMedicinal chemistryBazedoxifene
The invention deals with new crystalline salts of bazedoxifene, by means of which a high API quality can be achieved in a high yield.
Owner:ZENTIVA AS
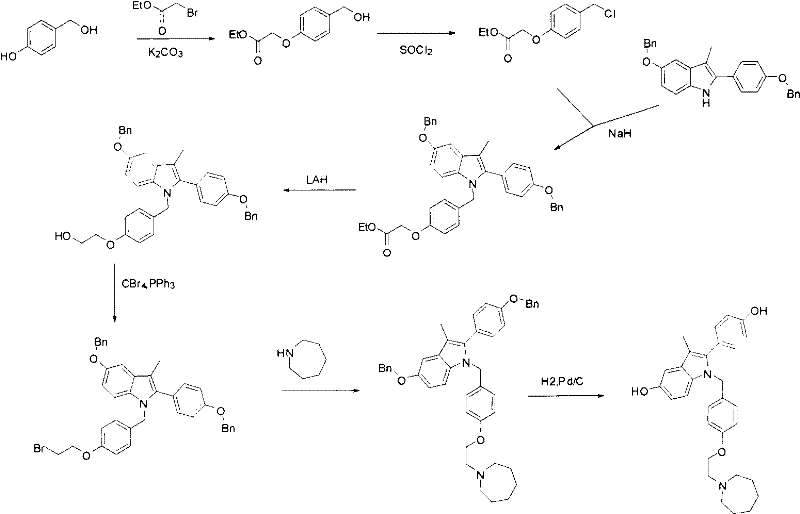
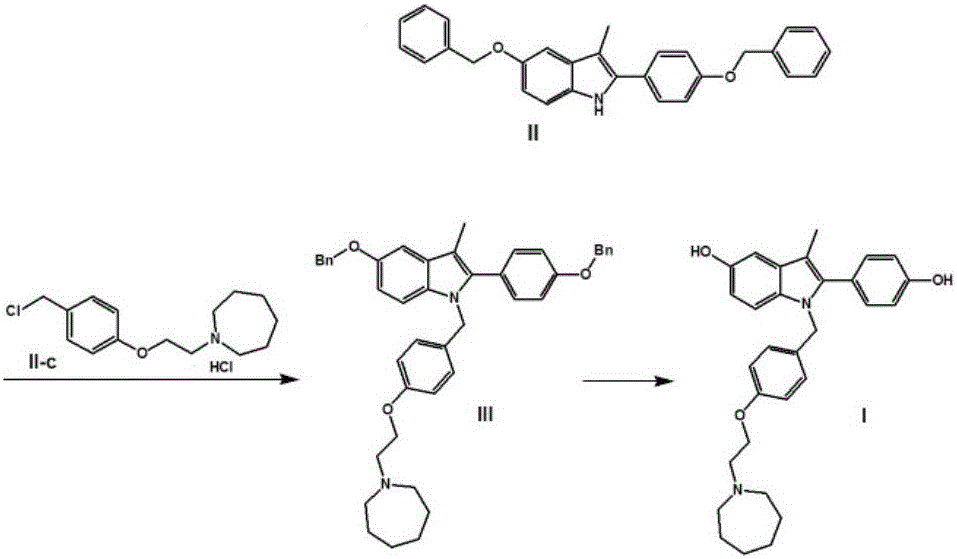
Process for the preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
The present invention is related to a process for the preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-Indole (formula-1, a useful intermediate for the synthesis of bazedoxifene) using 4-benzyloxy propiophenone and 4-benzyloxy phenyl hydrazine hydrochloride.
Owner:DIVI S LAB LTD
Efficient preparation method of bazedoxifene
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
New synthetic method of bazedoxifene
ActiveCN102690225BHigh activityMild reaction conditionsOrganic chemistryMedicinal chemistryBazedoxifene
The invention discloses a new synthetic method of bazedoxifene. Bazedoxifene is prepared by the steps of bromination, substitution, reductive amination, substitution, cyclization, deprotection, and the like of azepane which is selected as a raw material. The synthetic method of the invention has the advantages of mild reaction conditions, simple and convenient operating process, easily obtained reagent, and low cost.
Owner:南京正济医药销售有限公司
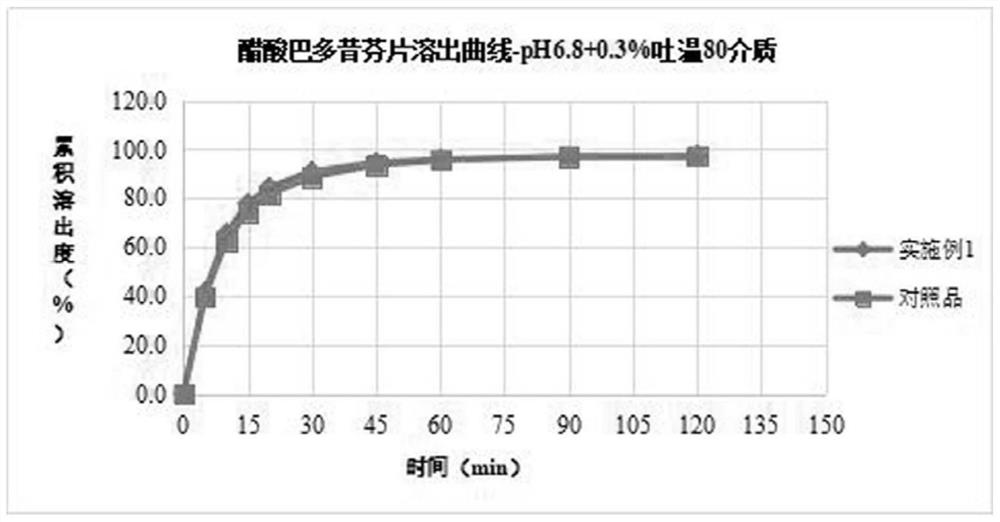
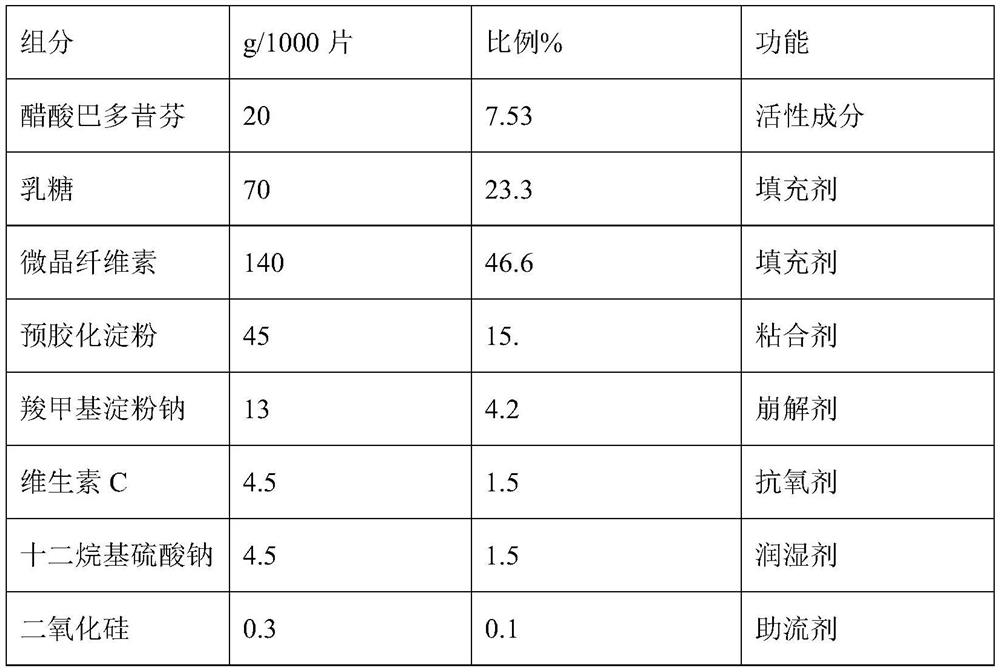
Bazedoxifene acetate composition and bazedoxifene acetate film-coated tablet preparation method
PendingCN112754999ASimple processImprove stabilitySkeletal disorderPharmaceutical non-active ingredientsCoated tabletsVitamin C
The invention discloses a bazedoxifene (Viviant) acetate composition and a bazedoxifene acetate film-coated tablet preparation method, and relates to the technical field of medicines. The bazedoxifene acetate composition comprises the following components by weight of 20-26 parts of bazedoxifene acetate, 250-350 parts of a filling agent, 30-50 parts of an adhesive, 8-15 parts of a disintegrating agent, 3-6 parts of an antioxidant, 2-6 parts of a flow aid, 0.1-0.5 part of a lubricant, 1-3 parts of a wetting agent, and a proper amount of a purified water solvent, wherein bazedoxifene acetate is a BCS II type medicine, and the particle size D90 is 30 microns; the antioxidant is vitamin C; and the filling agent is composed of lactose and microcrystalline cellulose, and the ratio of the lactose to the microcrystalline cellulose is 1: 2-2: 1 according to the weight fraction. The fluidized bed granulation technology adopting the process for preparation is simple in process, lower in cost and easy for industrial production.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Preparation method of pure acetic acid bazedoxifene crystal form A
InactiveCN106748959AIncrease productivitySimple processOrganic chemistry methodsCarboxylic acid salt preparationAcetic acidHydrogen
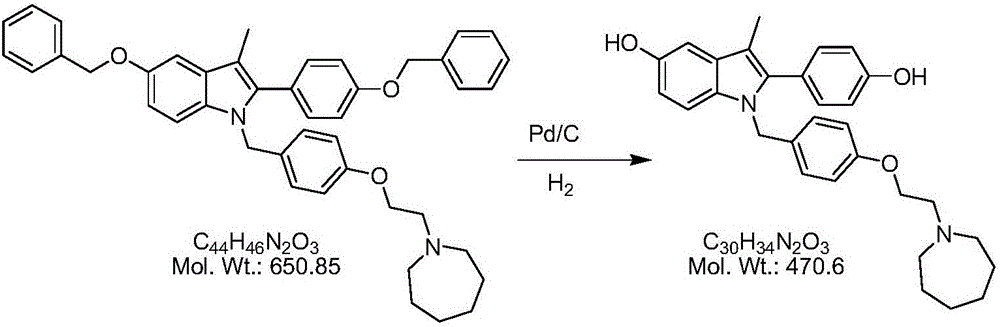
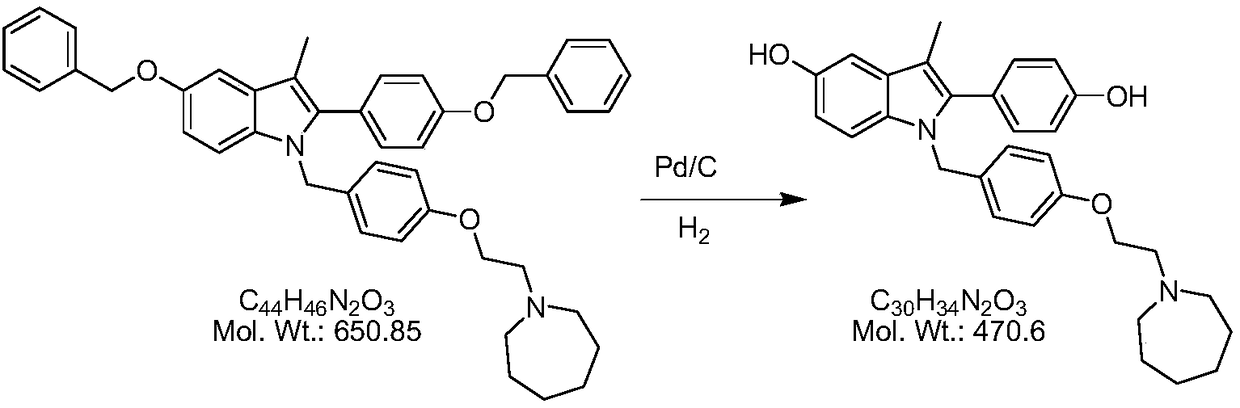
The invention discloses a preparation method of a pure acetic acid bazedoxifene crystal form A. The preparation method comprises the following process steps of firstly, under the condition of using palladium carbon as a catalyst, dissolving raw materials into a good organic solvent, and introducing hydrogen to react to a non-raw material point; after reaction is completed, filtering to remove the palladium carbon, cooling filtrate, adding acetic acid and a poor organic solvent, stirring, crystallizing, filtering, and drying, so as to obtain a crude product of the crystal form A. The preparation method has the advantages that the technology is simple, the production efficiency is high, and the preparation cost of the pure acetic acid bazedoxifene crystal form A is effectively reduced.
Owner:成都归合科技有限公司
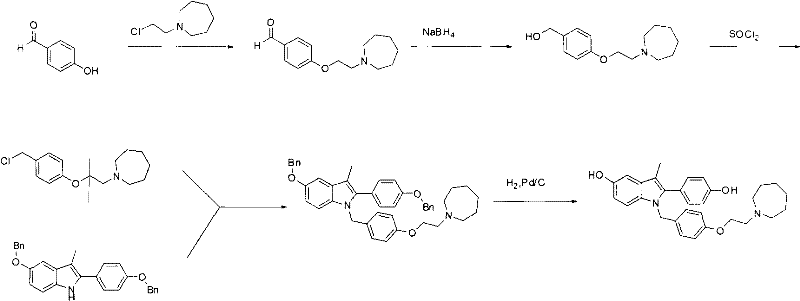
Preparation method of novel azacycloheptane derivative
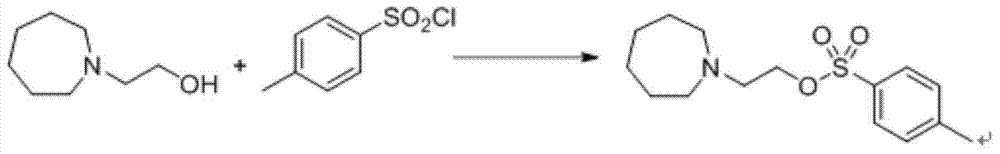
The invention provides a preparation method of 1-(2-haloethyl)azacycloheptane base ligand and its acid salt. The preparation method is suitable for industrial production. The preparation method is characterized in that 1,6-hexanediol as an initial raw material undergoes three-step reactions to produce the 1-(2-haloethyl)azacycloheptane base ligand and its acid salt. The 1-(2-haloethyl)azacycloheptane base ligand and its acid salt are key intermediates for bazedoxifene synthesis. The preparation method has the advantages of cheap raw material, operation simpleness, mild conditions, simple after-treatment and high yield and is a preparation route suitable for industrialization.
Owner:BEIJING LABWORLD BIO MEDICINE TECH
Preparation method of bazedoxifene acetate
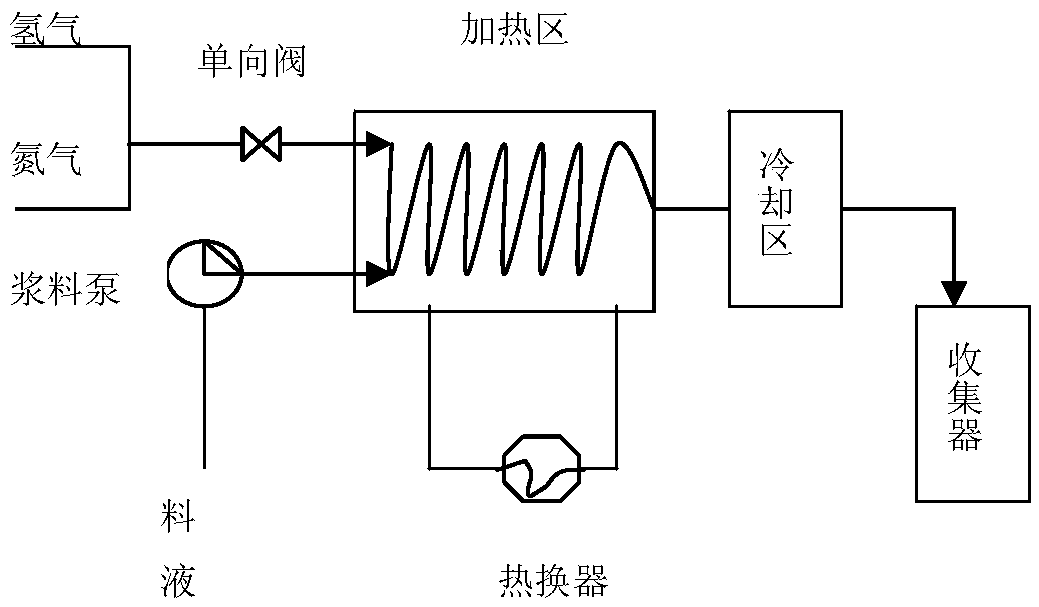
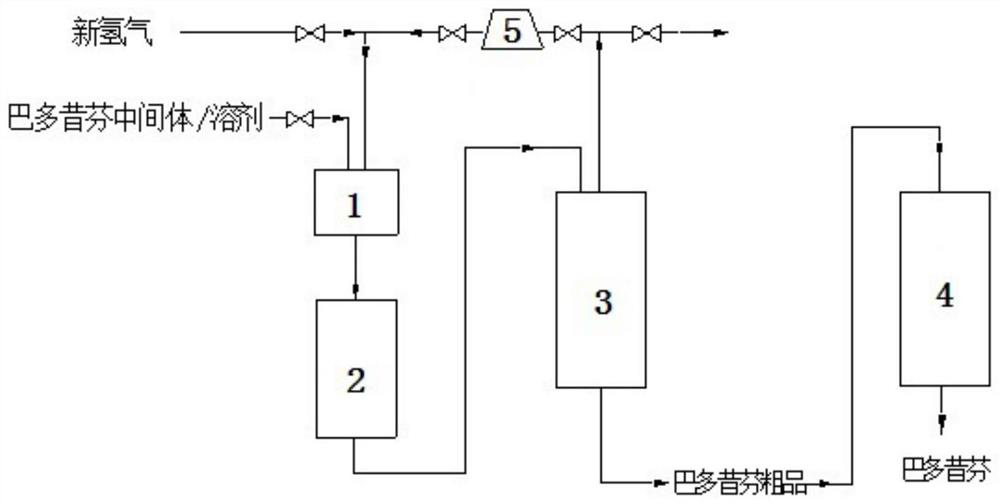
The invention discloses a preparation method of bazedoxifene acetate. The method is characterized in that raney nickel is used as a catalyst, and a compound I and hydrogen react in a microreactor to obtain bazedoxifene under the conditions that the temperature is 20 to 40DEG C and the hydrogen pressure is controlled to be 0.1 to 0.5Mpa. According to the method, the raney nickel with lower price is used to replace expensive palladium carbon, so that the production cost is effectively reduced, the microreactor is used and the production is in streamline operation; an obtained product is high in yield and good in quality; the preparation method has the advantages of high reaction efficiency, mild reaction conditions, safe and controllable operation, short reaction time and low cost; the method is easier for industrial production (The formula is shown in the description).
Owner:山东安信制药有限公司
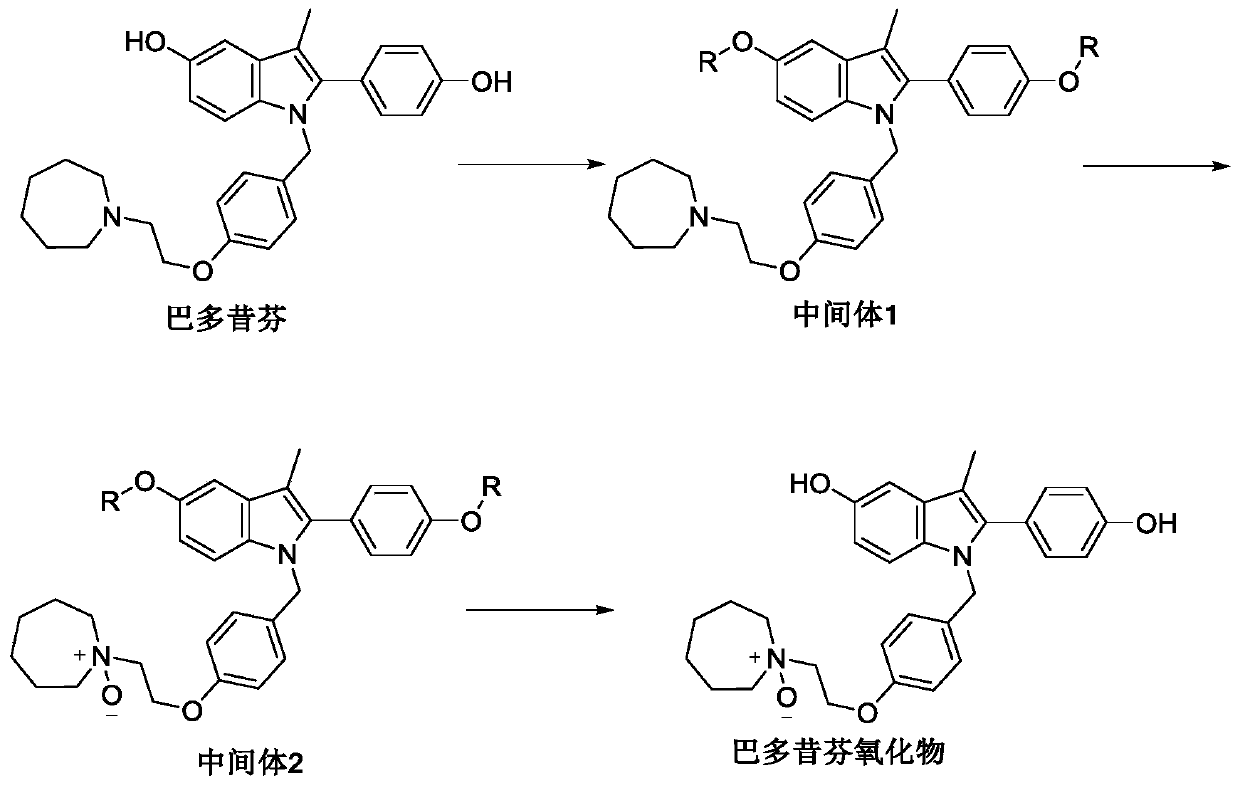
Preparation method of bazedoxifene oxide
InactiveCN111018770AEffective quality controlSolve the problem of quality controlOrganic chemistryBulk chemical productionPhenyl groupPhenol
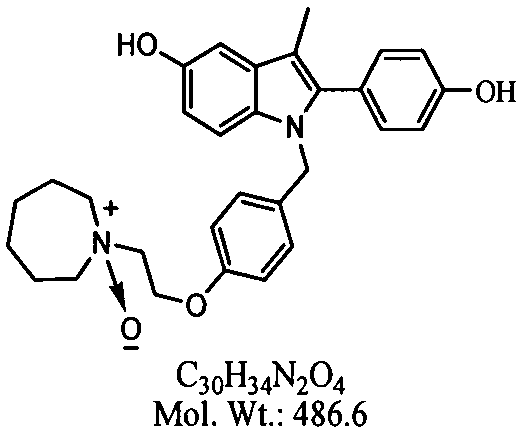
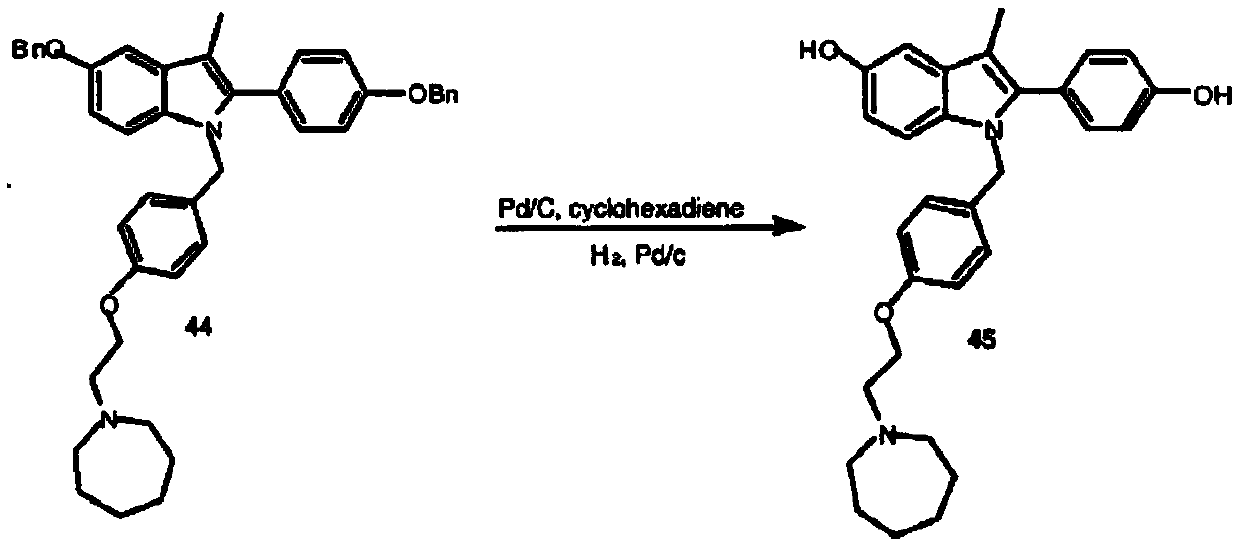
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of bazedoxifene oxide, wherein the preparation method comprises the steps: by using bazedoxifene free alkali as a raw material, dissolving in a first benign solvent, adding an organic alkali, and adding a silane protecting group to protect two phenolic hydroxyl groups, and thus obtaining anintermediate 1; dissolving the intermediate 1 in a second benign solvent, and adding an oxidant for oxidation to obtain an intermediate 2; and dissolving the intermediate 2 in a third benign solvent,and adding a protecting group removing reagent to remove two molecular silane protecting groups, and thus obtaining the bazedoxifene oxide. According to the preparation method of the bazedoxifene oxide provided by the invention, a preparation method of 1-[4-[2-(azacycloheptan-1-yl)ethoxy]benzyl]-2-(4-hydroxyphenyl)-3-methyl-1H-indole-5-phenol-N-oxide is provided, and the preparation method has important significance in effectively controlling the quality of bazedoxifene.
Owner:北京鑫开元医药科技有限公司
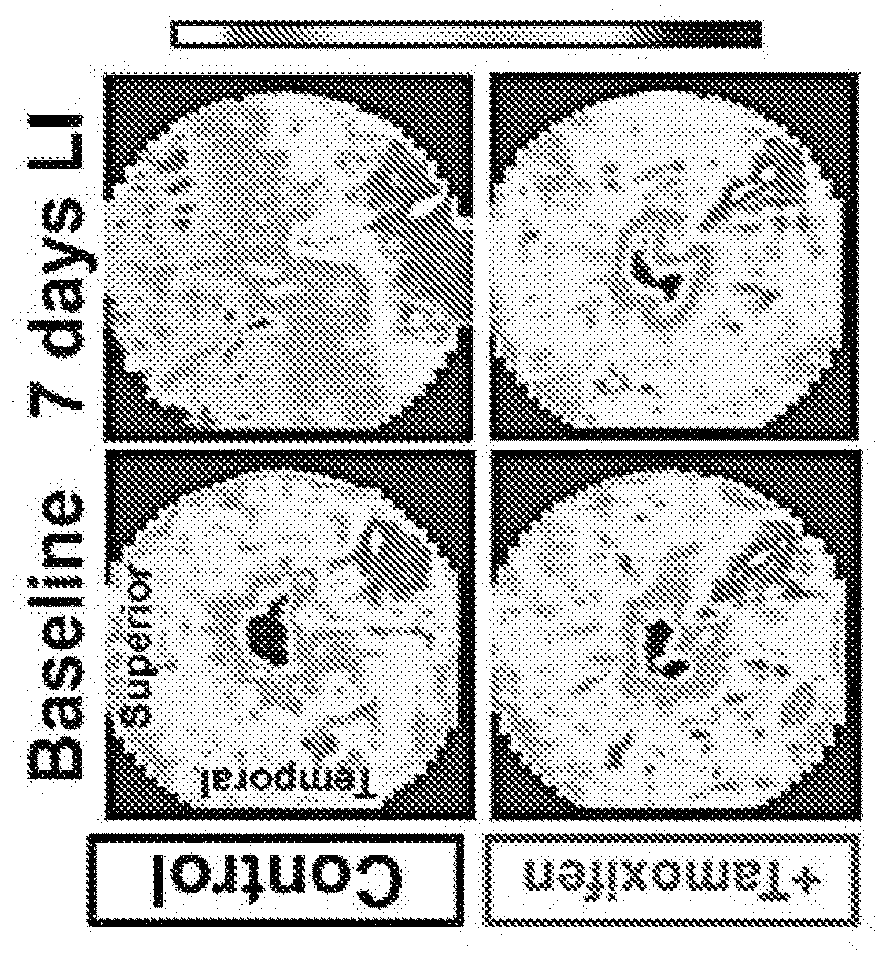
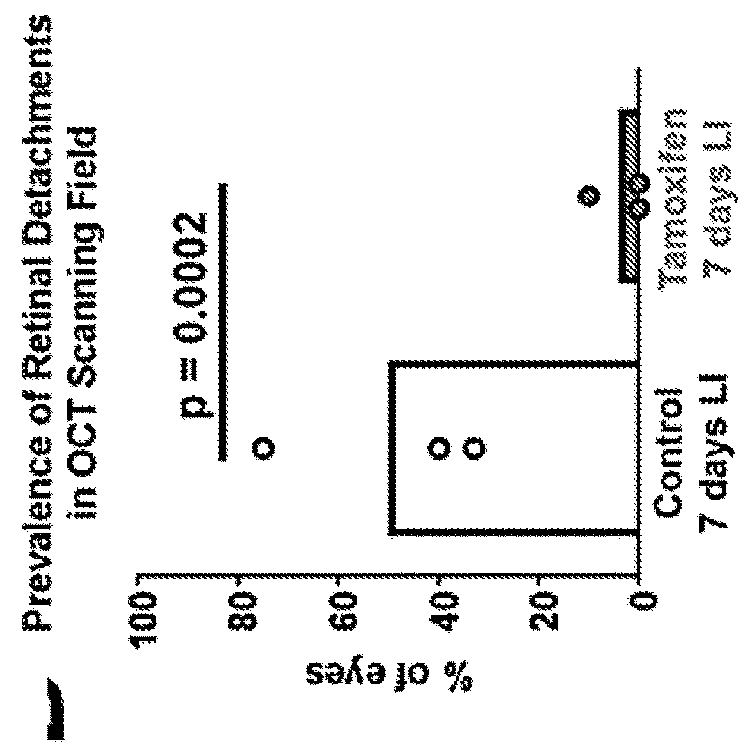
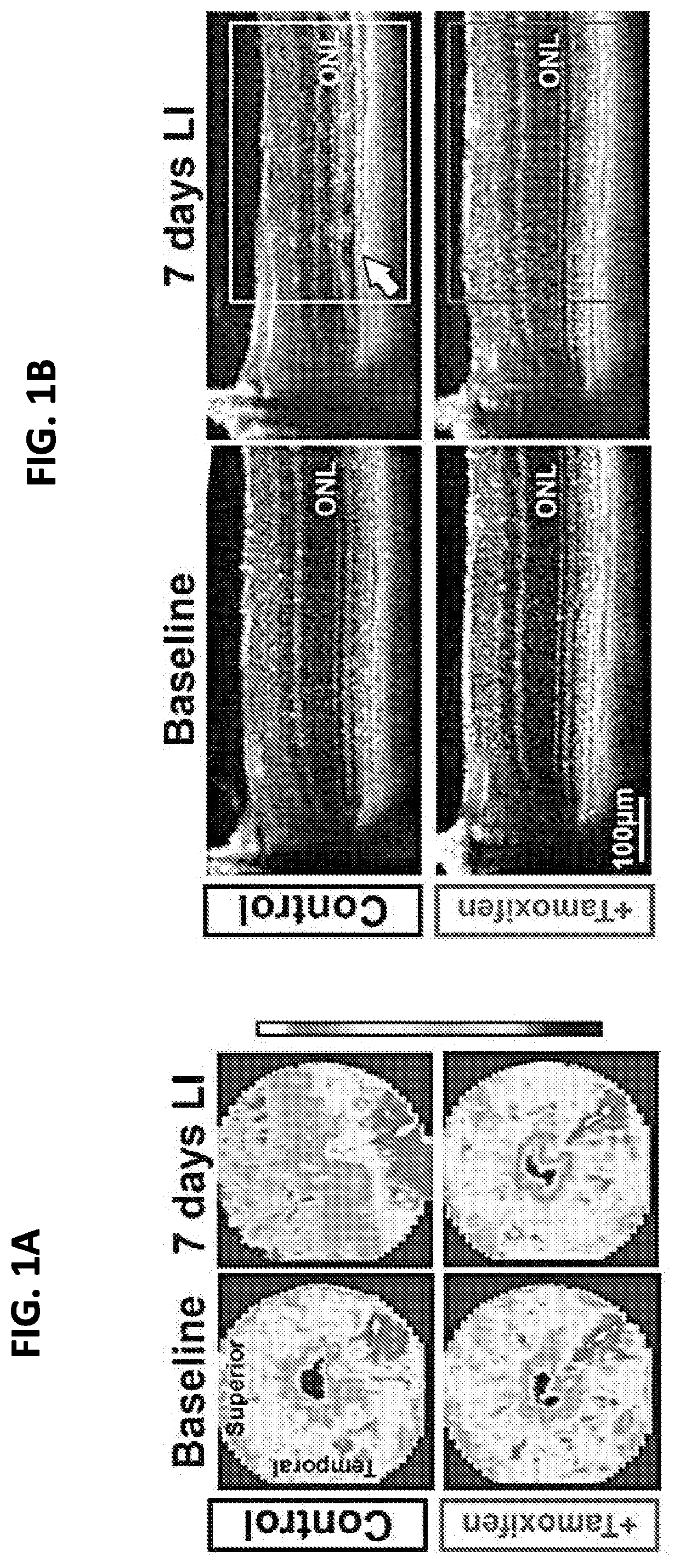
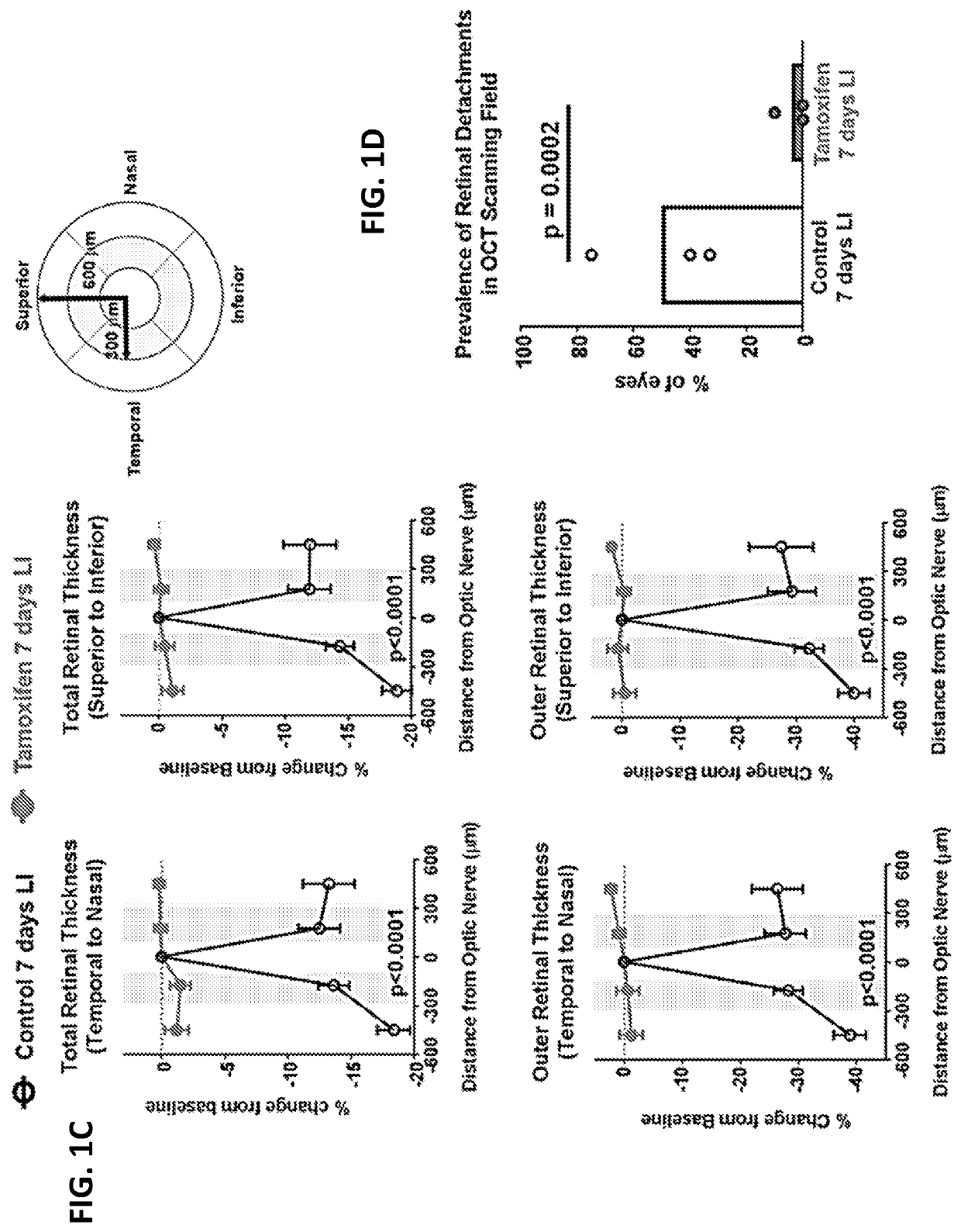
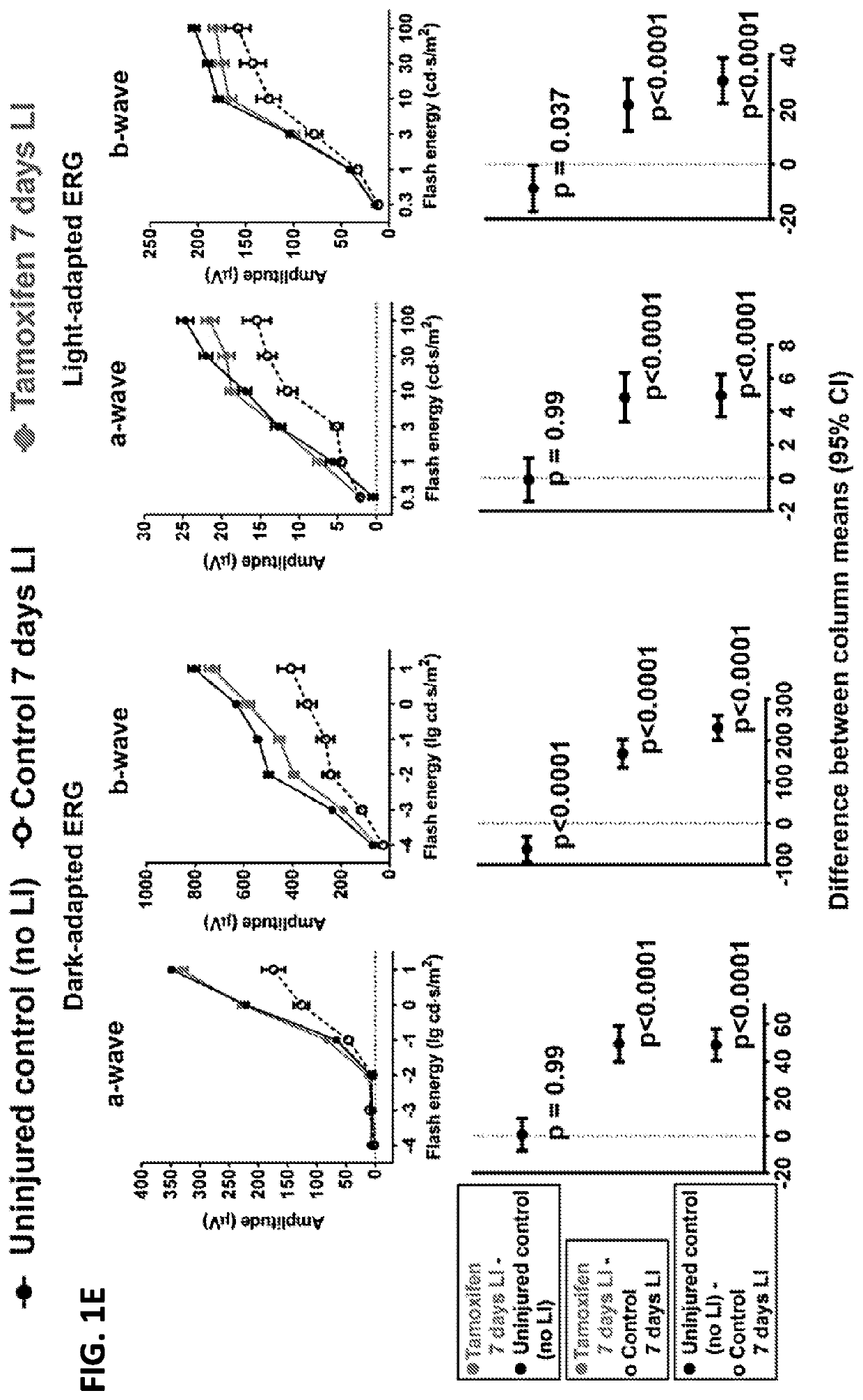
Selective estrogen-receptor modulators (SERMS) confer protection against photoreceptor degeneration
ActiveUS20190209497A1Treating and preventing degenerationMaximum protectionSenses disorderElectro-oculographyRaloxifeneMedicine
Methods are disclosed for treating and / or preventing retinal degeneration is a subject. In some embodiments, the method includes administering to the subject a therapeutically effective amount of a selective estrogen receptor modulator (SERM) to treat the retinal degeneration in the subject. In other embodiments, the SERM is administered orally. In some examples, the SERM is tamoxifen, afimoxifene, raloxifene, bazedoxifene, arzoxifene, desmethylarzoxifene, or a salt or derivative thereof, or combinations thereof.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
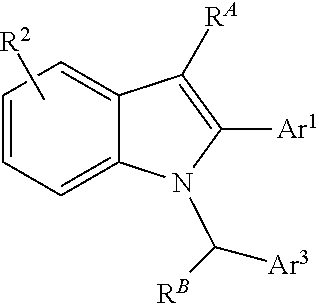
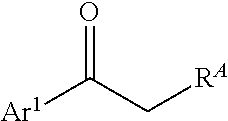
Processes and intermediates for preparing indole pharmaceuticals
ActiveUS9751835B2Low costImprove efficiencyHydrazone preparationMetal/metal-oxides/metal-hydroxide catalystsMedicinal chemistrySelective estrogen receptor modulator
The invention described herein pertains to processes and intermediates for preparing indole containing pharmaceuticals, particularly to processes and intermediates for preparing selective estrogen receptor modulators, such as bazedoxifene.
Owner:INDIANA UNIV RES & TECH CORP
A kind of efficient preparation method of bazedoxifene
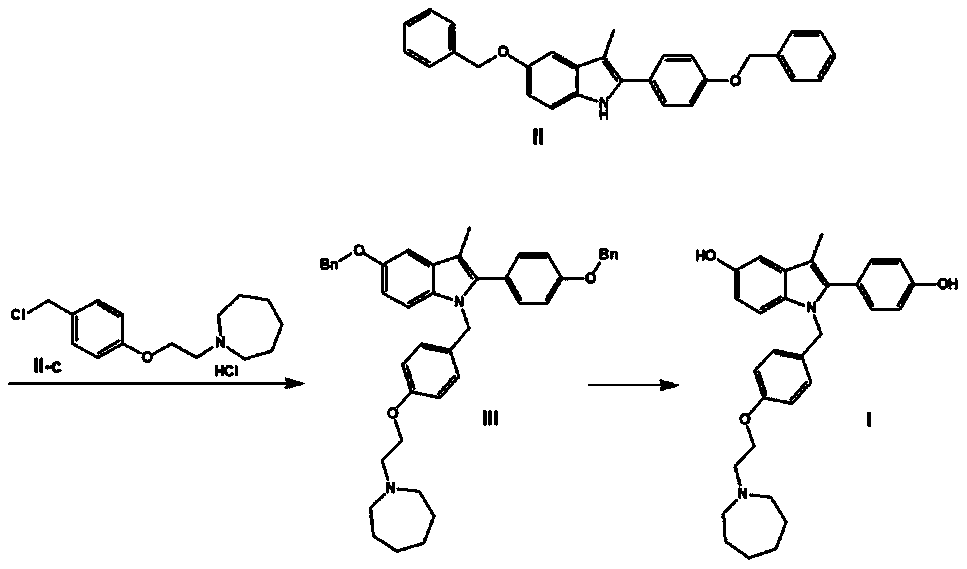
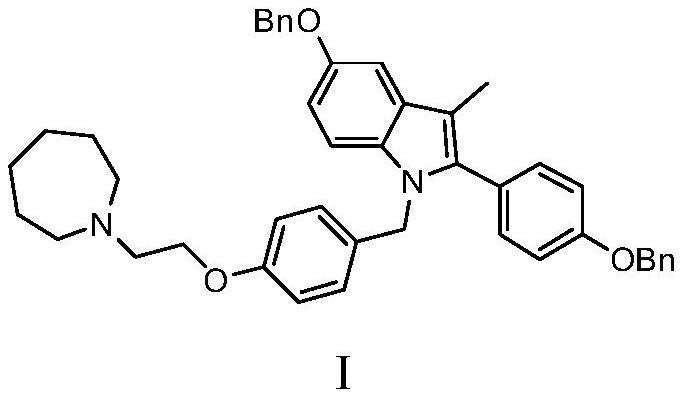
The invention discloses a preparation method of bazedoxifene. The preparation method comprises the following steps: (a) taking 1-(4-(2-(azepane-1-yl)ethoxy)benzyl)-5-(benzyloxy)-2-(4-(benzyloxy)phenyl)-3-methyl-1H-indole, adding a polar organic solvent, an aprotic lewis acid organic solution and a hydrogen ion provider, and stirring at 0-40 DEG C to obtain a reaction liquid; and (b) separating and purifying the reaction liquid obtained in the step (a) to obtain bazedoxifene. In the method disclosed by the invention, the low-cost and easily available aprotic lewis acid such as boron trifluoride is adopted as a catalyst for preparing bazedoxifene, the reaction conditions are mild, the operation is convenient, the safety is high, and the energy consumption is low; the obtained bazedoxifene has high yield and high purity, and the preparation difficulty and production cost of bazedoxifene are lowered; and the method brings a remarkable positive effect and is very suitable for industrialized use.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Preparation method of bazedoxifene
ActiveCN103772261BEase of industrial productionPromote the development of economy and technologyOrganic chemistryBulk chemical productionMethyl groupPhenyl group
The invention discloses a preparation method of bazedoxifene. The preparation method of the bazedoxifene comprises the following steps: taking 1-(4-Pg1oxy-phenyl)-allylene (II) and N-[4-(2-azacycloheptane-1-yl- ethyoxyl-benzyl)]-N-[4-(Pg2 oxyphenyl)] hydrazine (III) to perform addition cyclization reaction and acquire 1-[4-(2-azacycloheptane-1-yl- ethyoxyl-benzyl)]-2-(4-Pg1oxy-phenyl)-3-methyl-5-(Pg2oxy)-1H-benzpyrole (IV); performing deprotection on an intermediate (IV) to prepare bazedoxifene (I). The preparation method is simple in process, high in yield, economical and environmental-friendly, so that a novel preparation method for industrial production of the bazedoxifene.
Owner:SUZHOU LIXIN PHARMA
Selective estrogen-receptor modulators (SERMs) confer protection against photoreceptor degeneration
Owner:UNITED STATES OF AMERICA
Methods and compositions for the treatment of Shiga Toxicosis
Methods for treating Shiga toxicosis, caused by infection with Shiga toxin bacteria, are provided. The methods include administering to a subject in need thereof an effective amount of one or more active agents selected from tamoxifen, 4-hydroxytamoxifen, endoxifen, toremifene, raloxifene, bazedoxifene, and pharmaceutically acceptable salts thereof. In some embodiments, the methods further include the administration of an antibiotic or a manganese compound to the subject. Pharmaceutical compositions for the treatment of Shiga toxicosis are also described.
Owner:BOARD OF REGENTS
The preparation method of bazedoxifene acetate polymorph a
The present invention discloses a Bazedoxifene Acetate polymorphism A preparation method, wherein a raw material Bazedoxifene free alkali is dissolved in a single solvent methanol, and salt forming and crystallization are performed to directly obtain the high-purity Bazedoxifene Acetate polymorphism A. According to the present invention, the preparation method has characteristics of simple process, economy and environmental protection, and is suitable for industrial production.
Owner:SUZHOU LIXIN PHARMA
A kind of preparation method of bazedoxifene acetate
The invention discloses a preparation method of bazedoxifene acetate. The method is characterized in that raney nickel is used as a catalyst, and a compound I and hydrogen react in a microreactor to obtain bazedoxifene under the conditions that the temperature is 20 to 40DEG C and the hydrogen pressure is controlled to be 0.1 to 0.5Mpa. According to the method, the raney nickel with lower price is used to replace expensive palladium carbon, so that the production cost is effectively reduced, the microreactor is used and the production is in streamline operation; an obtained product is high in yield and good in quality; the preparation method has the advantages of high reaction efficiency, mild reaction conditions, safe and controllable operation, short reaction time and low cost; the method is easier for industrial production (The formula is shown in the description).
Owner:山东安信制药有限公司
Preparing method of bazedoxifene derivative
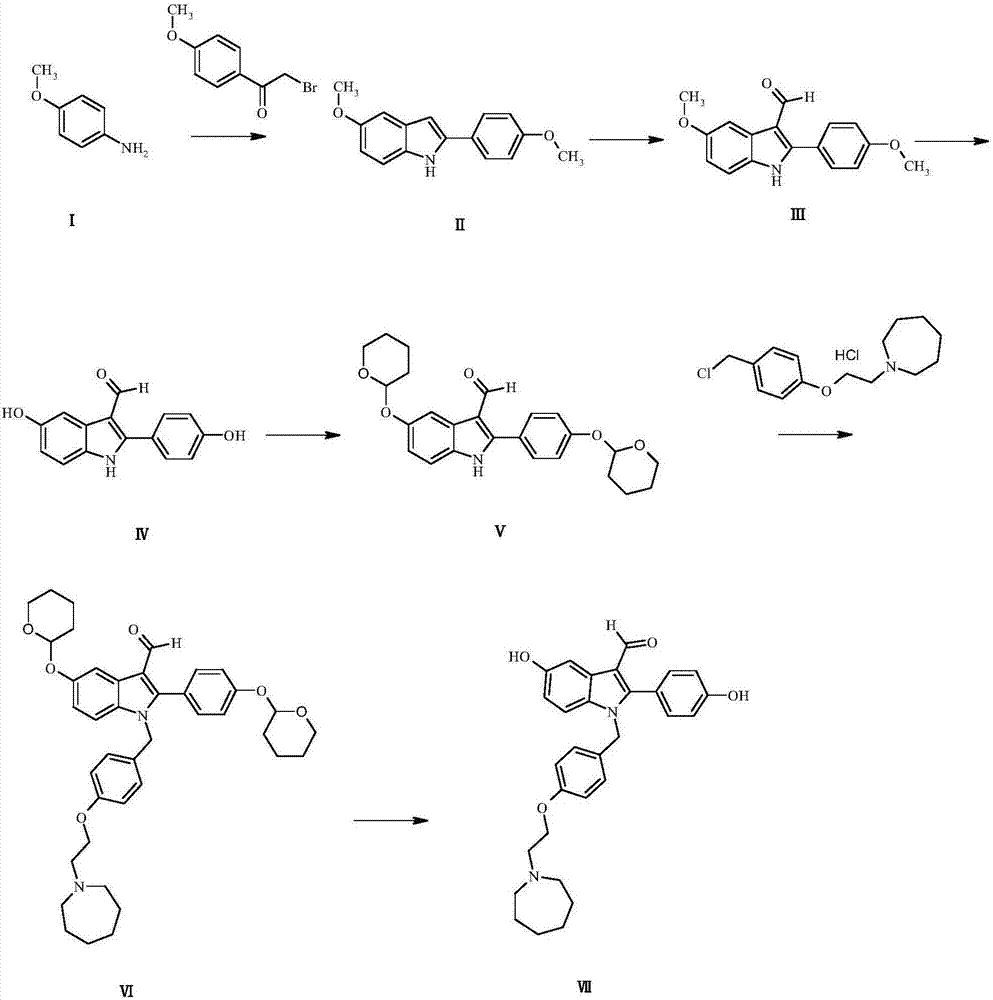
ActiveCN107033062AAdvantages of preparation methodImprove protectionOrganic chemistrySynthesis methodsP-Anisidine
The invention discloses a synthesis method of a bazedoxifene derivative. According to the preparing method of the bazedoxifene derivative, p-anisidine is adopted as a raw material, and through a six-step reaction, the synthesis of the bazedoxifene derivative is achieved. The preparing method of the bazedoxifene derivative is reasonable in technological design and high in operability, the reaction condition is mild, the yield is high, and thus industrialized production can be achieved. The prepared bazedoxifene derivativ bazedoxifene provides an important basis for scientific evaluation of quality, safety and efficiency of bazedoxifene; meanwhile, the bazedoxifene derivative is good in pharmacological activity, can be developed into medicines used for treating osteoporosis, and thus the bazedoxifene derivative has important application value.
Owner:TLC NANJING PHARMA RANDD CO LTD
Preparation method of bazedoxifene
The invention discloses a preparation method of bazedoxifene. The preparation method comprises the following steps: (1) reaction materials are mixed, and the reaction materials comprise a bazedoxifeneintermediate of formula I; (2) fixed bed catalytic reaction; (3) post-treatment. The preparation method has the advantages of realization of continuous reaction, fast reaction speed, repeated and continuous use of the catalyst and hydrogen, high reaction safety, no discharge of waste water and waste gas, environmental protection, saving, and easy realization of automation.
Owner:JIANGSU LONG HEALTHCARE
Bazedoxifene intermediate and preparation method thereof
The invention discloses a bazedoxifene intermediate, namely 2-[4-(Pg1 oxyl) phenyl]-3-methyl-5-(Pg2 oxyl)-1-acetyl-indol (I) and a preparation method thereof. The preparation method of the bazedoxifene intermediate comprises the step that 1-(4-Pg1 oxyl-phenyl)-allylene (II) and N-[4-(Pg2 oxyl) phenyl] acetamide (III) are subjected to oxidation ring-closure reaction so as to obtain the bazedoxifene intermediate, namely the 2-[4-(Pg1 oxyl) phenyl]-3-methyl-5-(Pg1 oxyl)-1-acetyl-indol (I) through one step. The intermediate is stable in natural. The preparation method is concise, economical and environment-friendly and can be used for providing a new effective intermediate for industrial production of bazedoxifene bulk drugs.
Owner:XUZHOU LIXIN IRRIGATION & DRAINAGE EQUIP CO LTD
Preparation method of (4-(2-azacycloheptane-1-yl)ethoxy)phenyl)methanol
InactiveCN104725334ASolve the problem of high toxicityImprove product qualityOrganic chemistryCombinatorial chemistryBazedoxifene
The invention provides a preparation method suitable for industrial production of (4-(2-azacycloheptane-1-yl)ethoxy)phenyl)methanol. The method is characterized in that a bazedoxifene synthesis key intermediate (4-(2-azacycloheptane-1-yl)ethoxy)phenyl)methanol is obtained through a three step reaction of low-toxicity 2-(azacycloheptane)-1-ethanol as an initial raw material. The method has the advantages of low-toxicity cheap raw material, simple operation, mild condition, simple post-treatment and high yield, and is a preparation route very suitable for industrialization.
Owner:BEIJING LABWORLD BIO MEDICINE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com