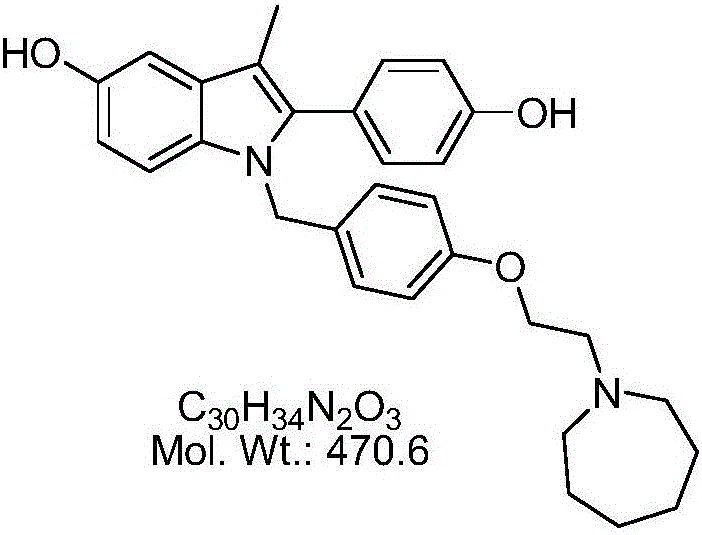
Preparation method of bazedoxifene acetate
A technology of bazedoxifene acetate and acetic acid, applied in the field of medicine, can solve the problems of high equipment requirements, industrialized amplification, complicated operation, etc., and achieve the effects of high reaction efficiency, short reaction time, safe and controllable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
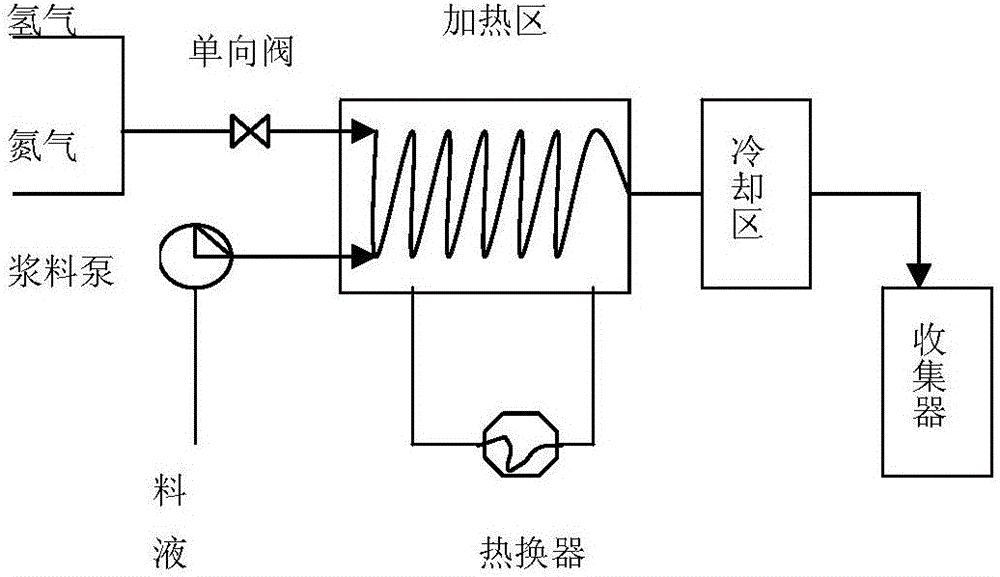
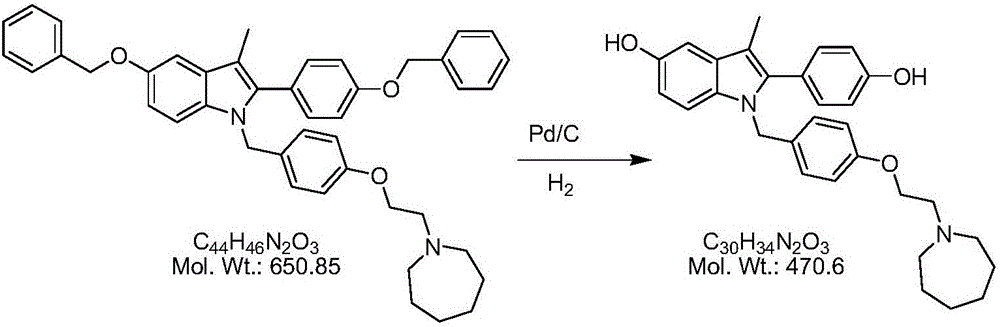
[0026] 1) Preparation of slurry: Dissolve 100 g of compound I completely in 500 ml of ethanol, add 3 g of Raney nickel at room temperature, stir evenly, and obtain a slurry for later use.
[0027] 2) Microreactor reaction: Use nitrogen to fully replace the air in the heating zone of the microreactor, then preheat the heating zone of the microreactor to an internal temperature of 30°C, then pass in hydrogen, and control the pressure at 0.2-0.3MPa to complete the step 1) The slurry is pumped into the heating zone of the microreactor through the slurry pump to control the temperature at 30°C and react for 3-8s; after the reaction is completed, pass through the cooling zone (the cooling zone is cooled by circulating water), and then collect the reaction solution;
[0028] 3) Generate bazedoxifene acetate: remove the insoluble matter by filtration, slowly add 10 g of acetic acid dropwise to the filtrate, and a white solid will slowly precipitate out. After the dropwise addition, coo...
Embodiment 2
[0030] 1) Preparation of slurry: Dissolve 500g of compound I completely in 4000ml of ethanol and ethyl acetate (3:1) mixture, add 20g of Raney nickel at room temperature, stir well, and set aside;
[0031] 2) Micro-reactor reaction: Use nitrogen to fully replace the air in the micro-reactor, preheat the micro-reactor to an internal temperature of 40°C, feed hydrogen, control the reaction pressure at 0.3-0.4MPa, and pump the slurry into the micro-reactor by the slurry pump In the reactor, control the temperature in the heating area at 40°C for 1-5s; after the reaction is completed, pass through the cooling area to cool (the cooling area is cooled by circulating water), and then collect the reaction liquid;
[0032] 3) Remove insoluble matter by filtration, slowly add 50g of acetic acid dropwise to the filtrate, and a white solid will slowly precipitate out. After the dropwise addition, cool down to 10°C-20°C, keep warm for crystallization for 1h, filter with suction, and air-dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com