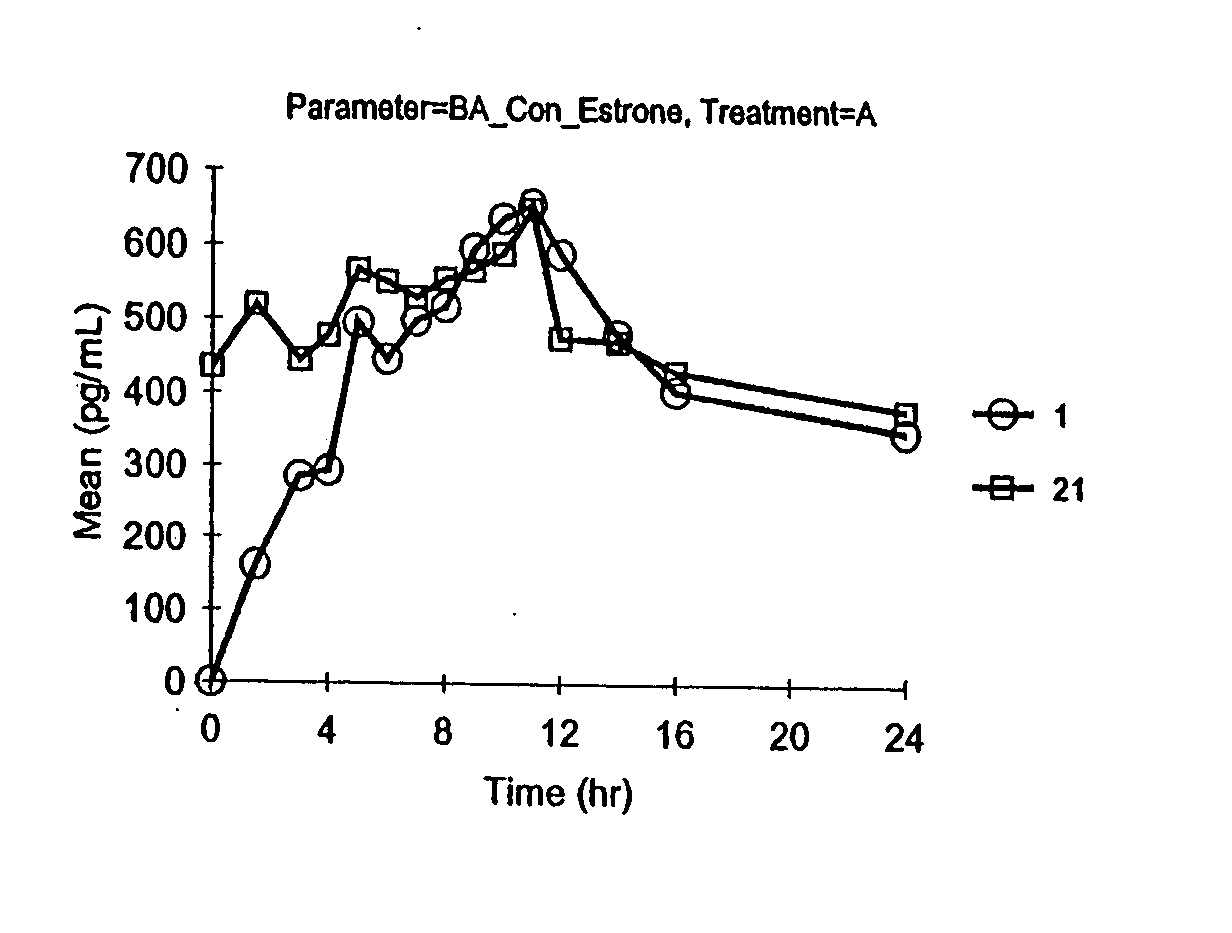
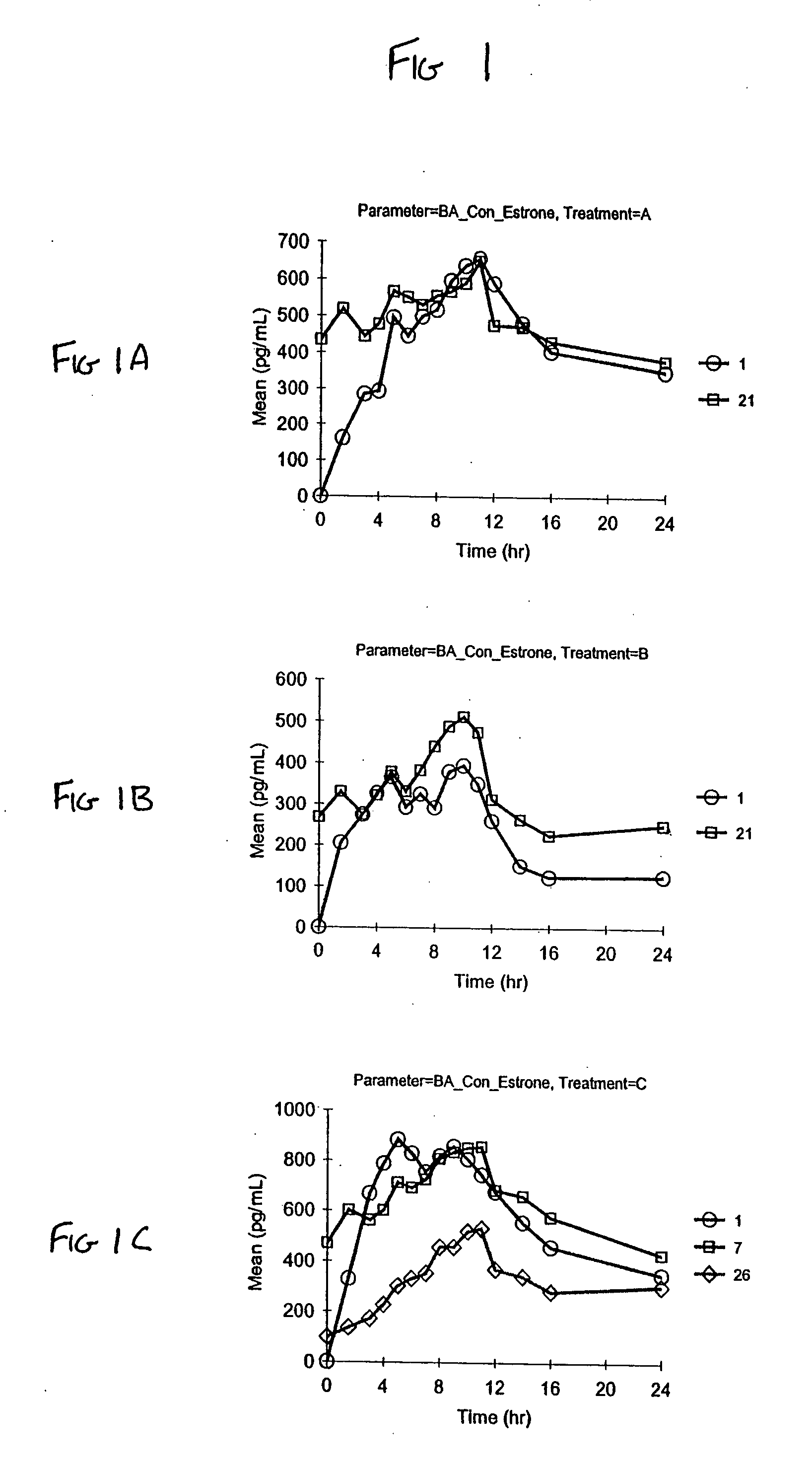
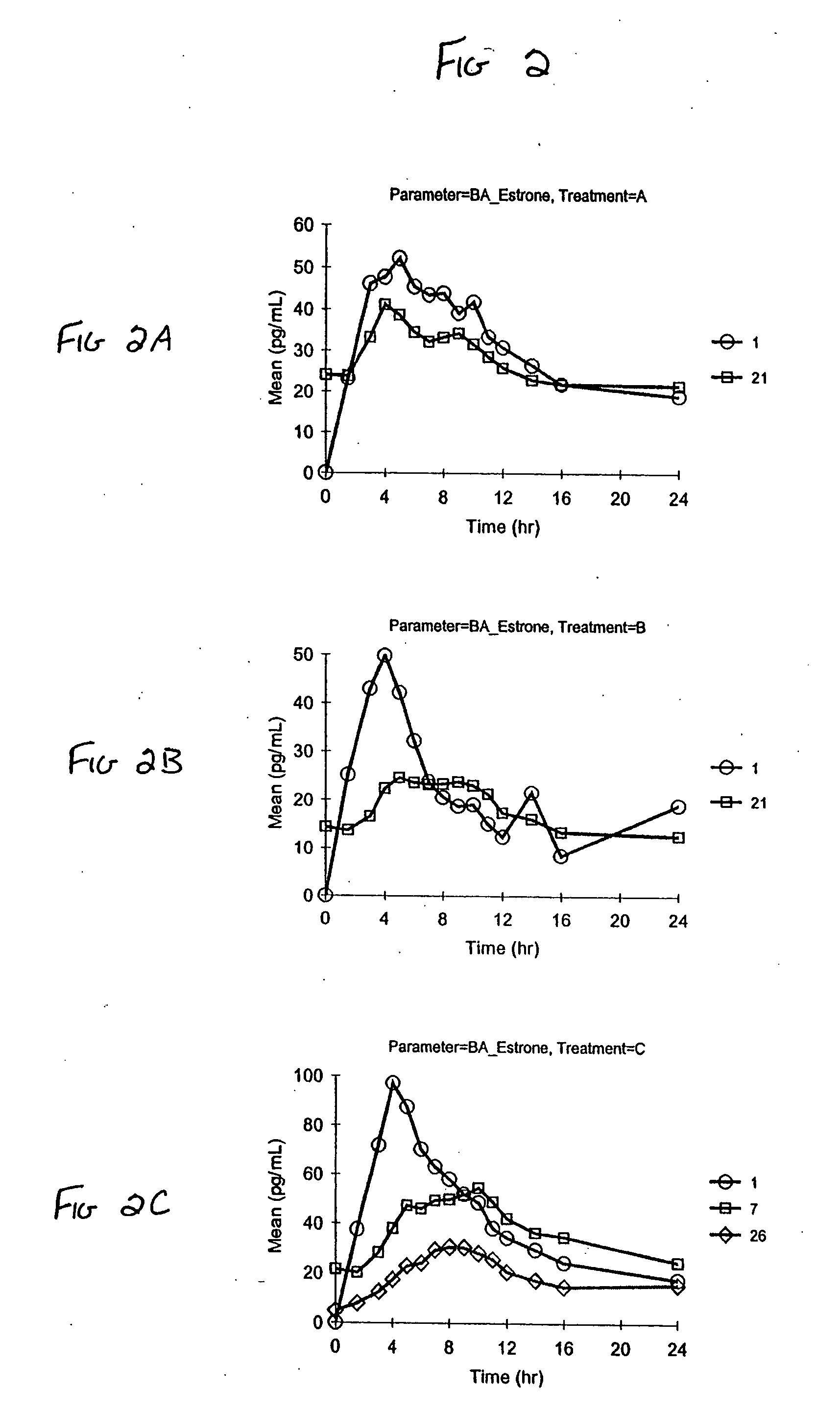
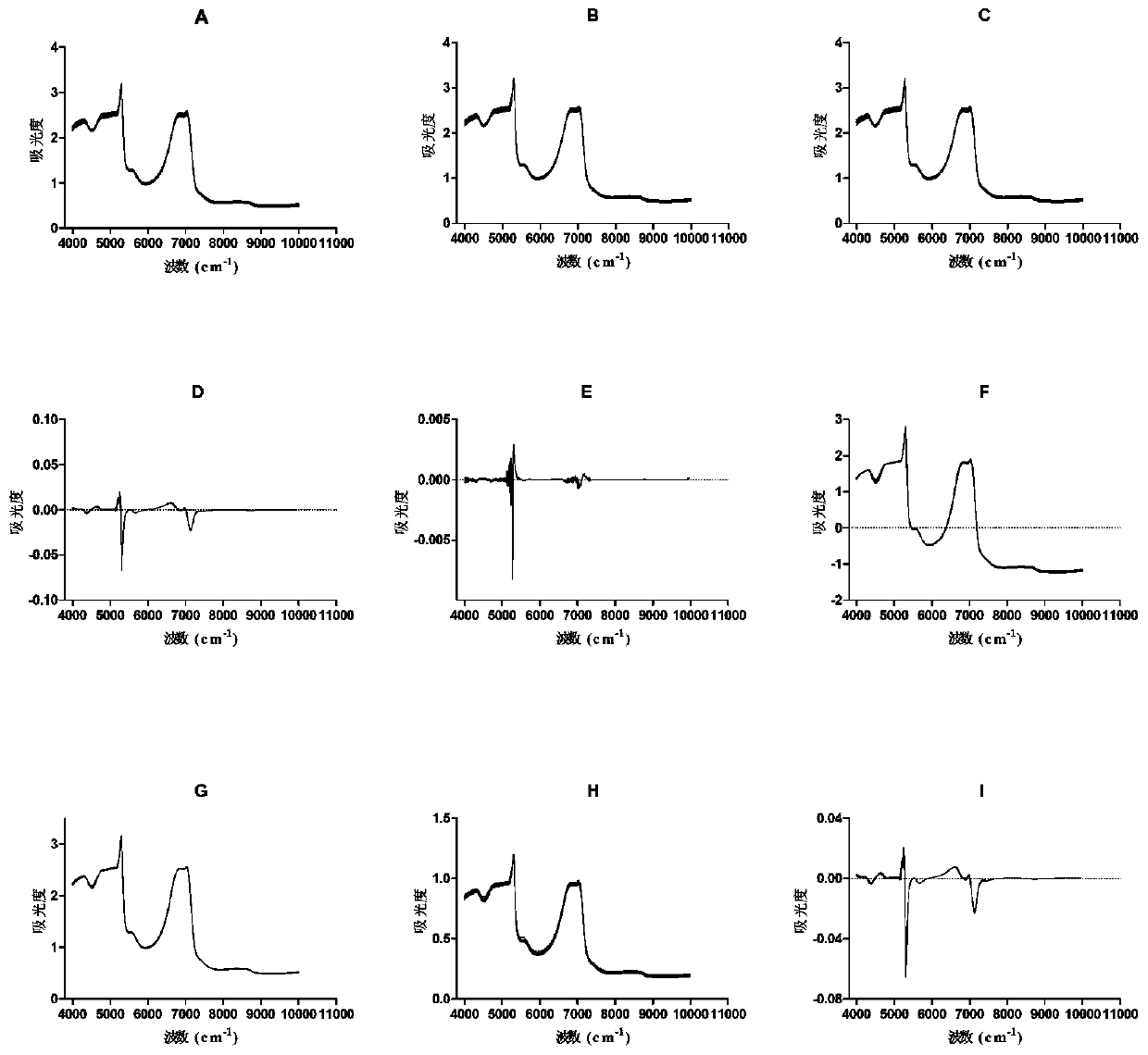
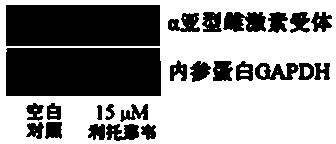Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Conjugated estrogen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
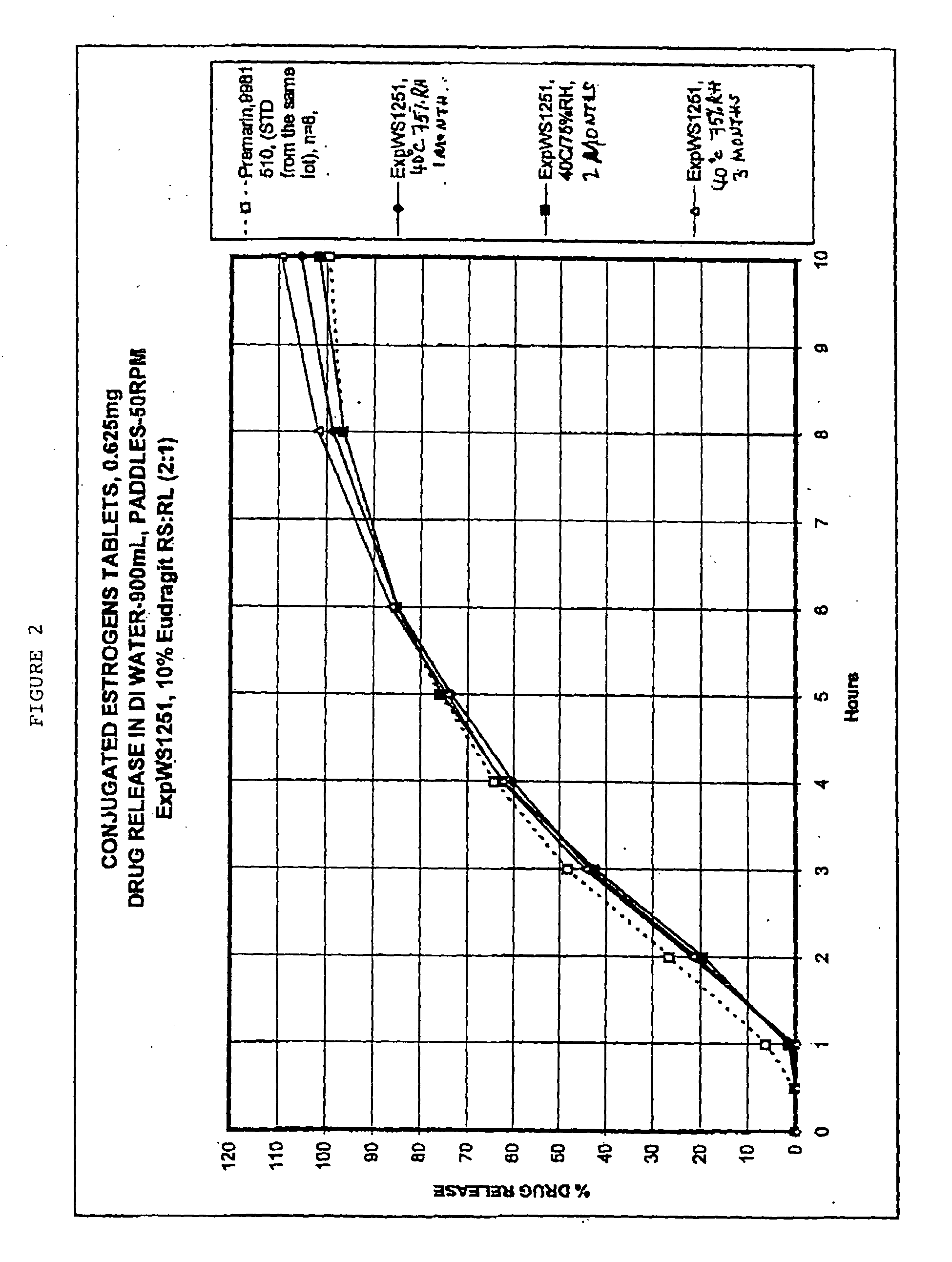
Pharmaceutical compositions of conjugated estrogens and methods of analyzing mixtures containing estrogenic compounds
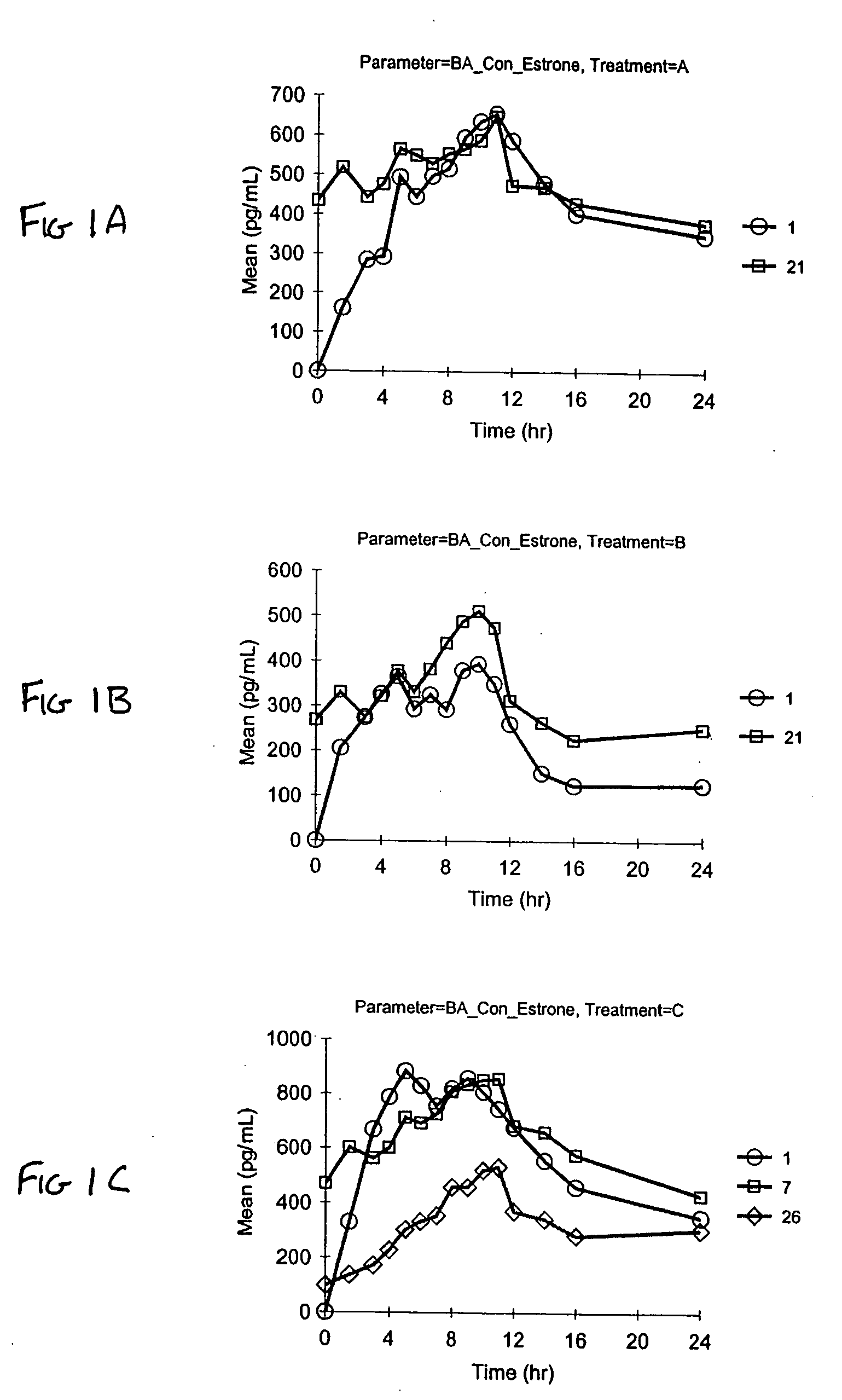
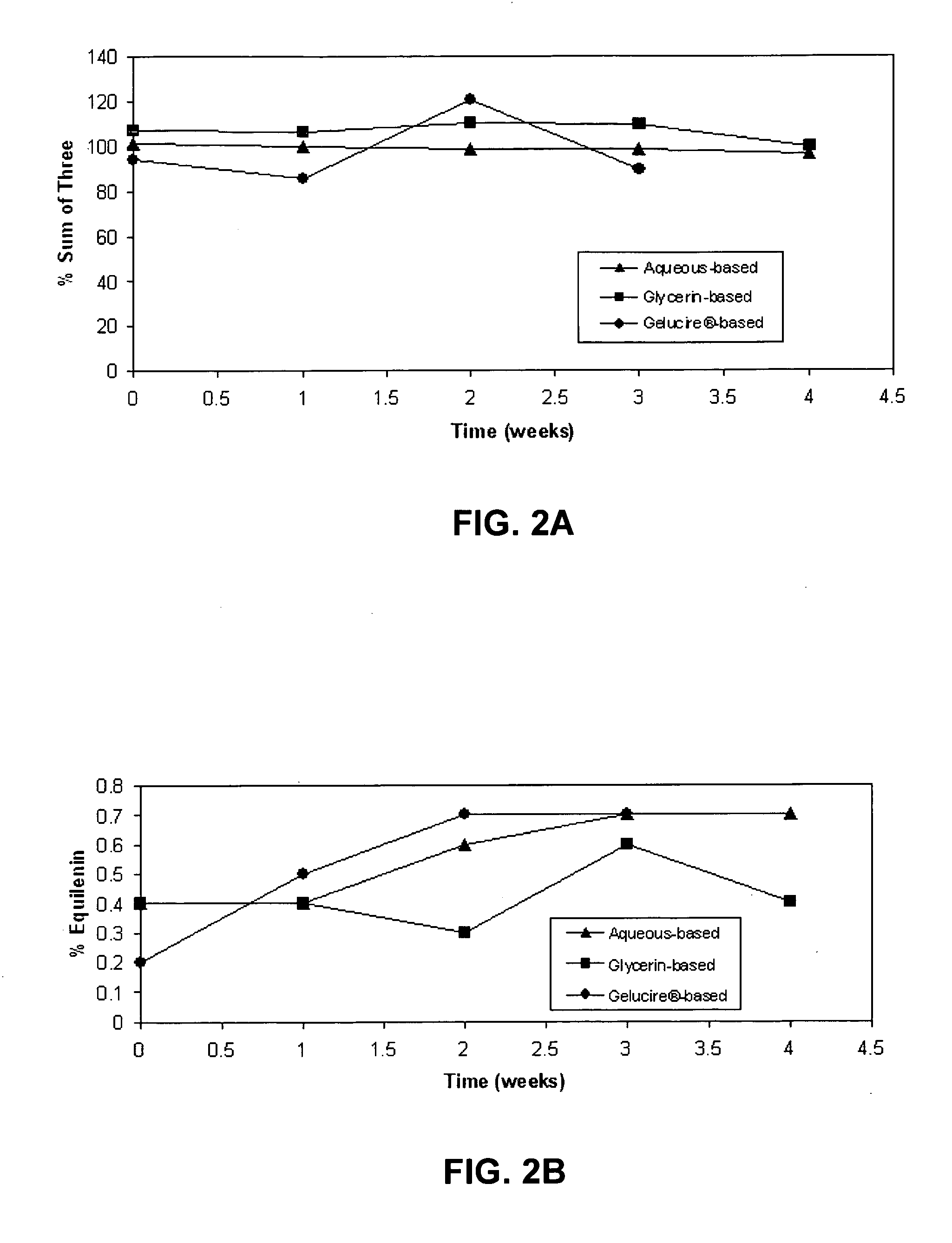
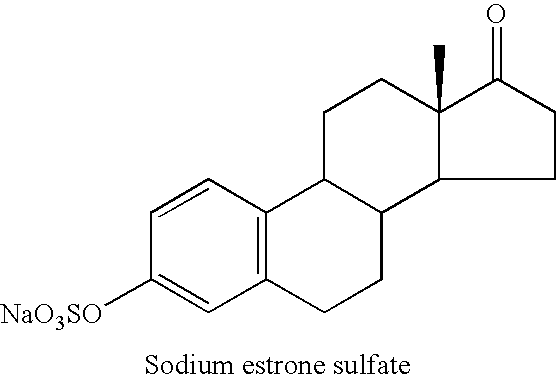
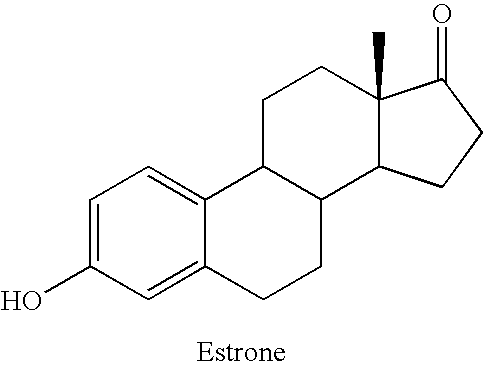
A composition of matter is provided having a mixture of active estrogenic compounds. The mixture is present in chemically pure form. The mixture includes salts of conjugated estrone, conjugated equilin, conjugated Δ8,9-dehydroestrone, conjugated 17α-estradiol, conjugated 17α-dihydroequilin, conjugated 17β-dihydroequilin, conjugated 17β-estradiol, conjugated equilenin, conjugated 17α-dihydroequilenin, and conjugated 17β-dihydroequilenin. The mixture also contains the same essential estrogenic compounds present in naturally derived equine conjugated estrogens. Drug products including the composition of matter are also provided, as are methods of using these drug products to treat mammals in need of treatment. Methods of analyzing mixtures containing conjugated estrogens are also provided.
Owner:DURAMED PHARMA
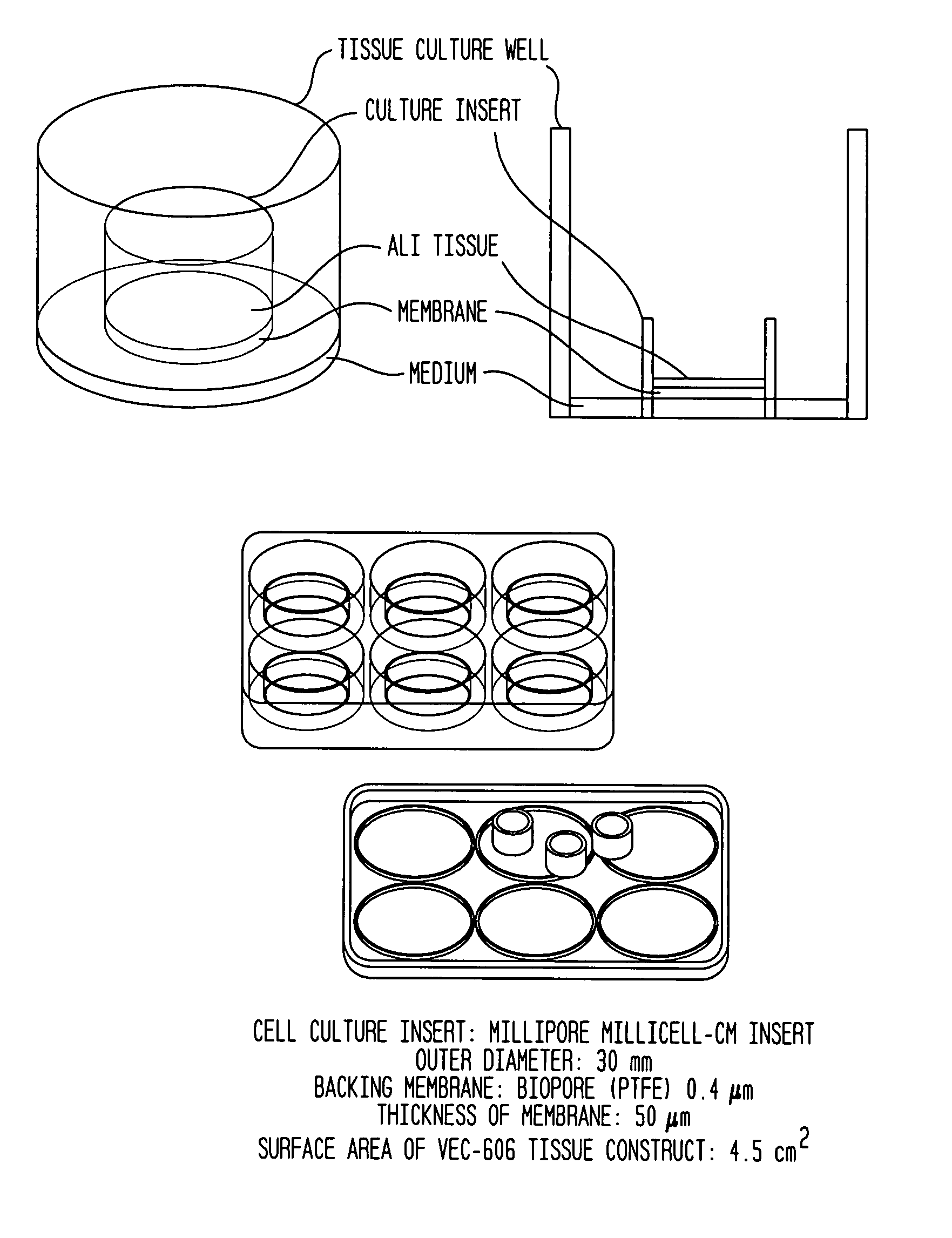
Preformulation for tabletting natural mixtures of conjugated estrogens
InactiveUS20050009800A1Easy to processLow hormone concentrationOrganic active ingredientsBiocideConjugated Equine EstrogensConjugated oestrogens
A pharmaceutical preformulation in the form of a solid, free-flowing dry extract of a natural mixture of conjugated equine estrogens, which is particularly suitable use in for solid galenic forms, e.g. tabletting. The conjugated estrogens are available for further galenic processing in a form which assures the chemical stability of the hormones and permits advantageous processing into solid galenic forms, for example a tablet. The invention furthermore relates to a method for producing these preformulations in the form of a dry extract.
Owner:ABBVIE PHARMA GMBH
Compositions for conjugated estrogens and associated methods
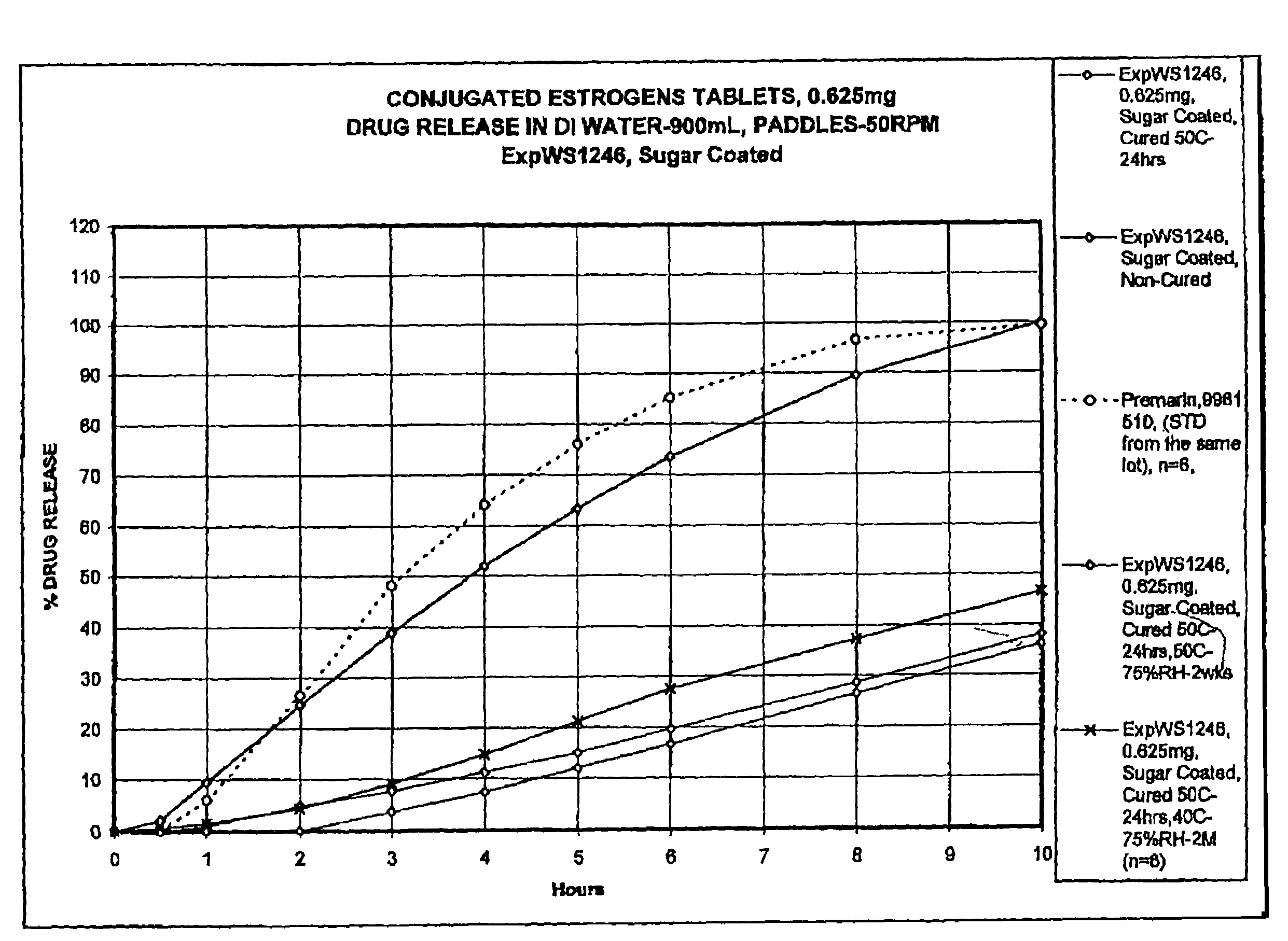
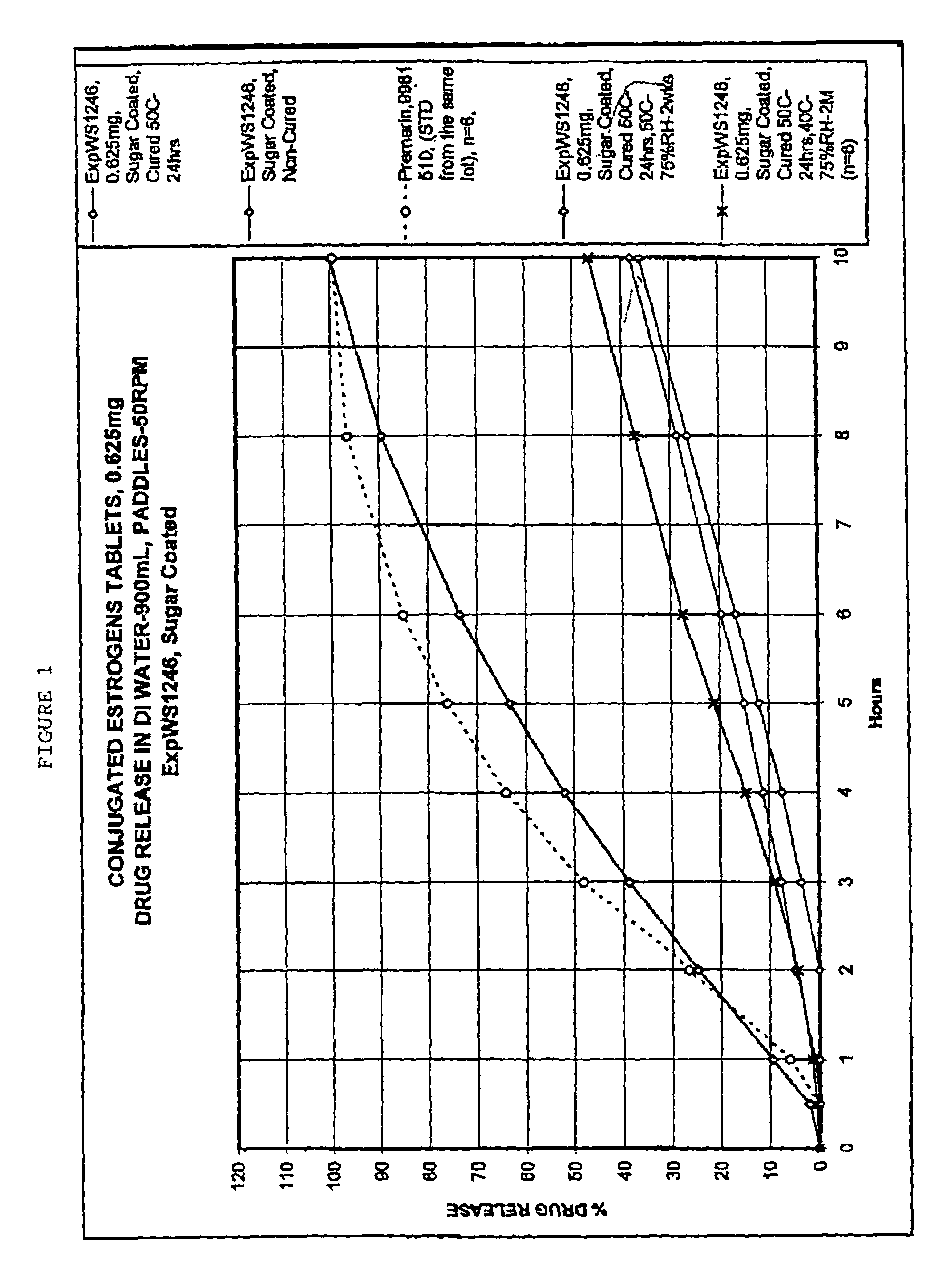
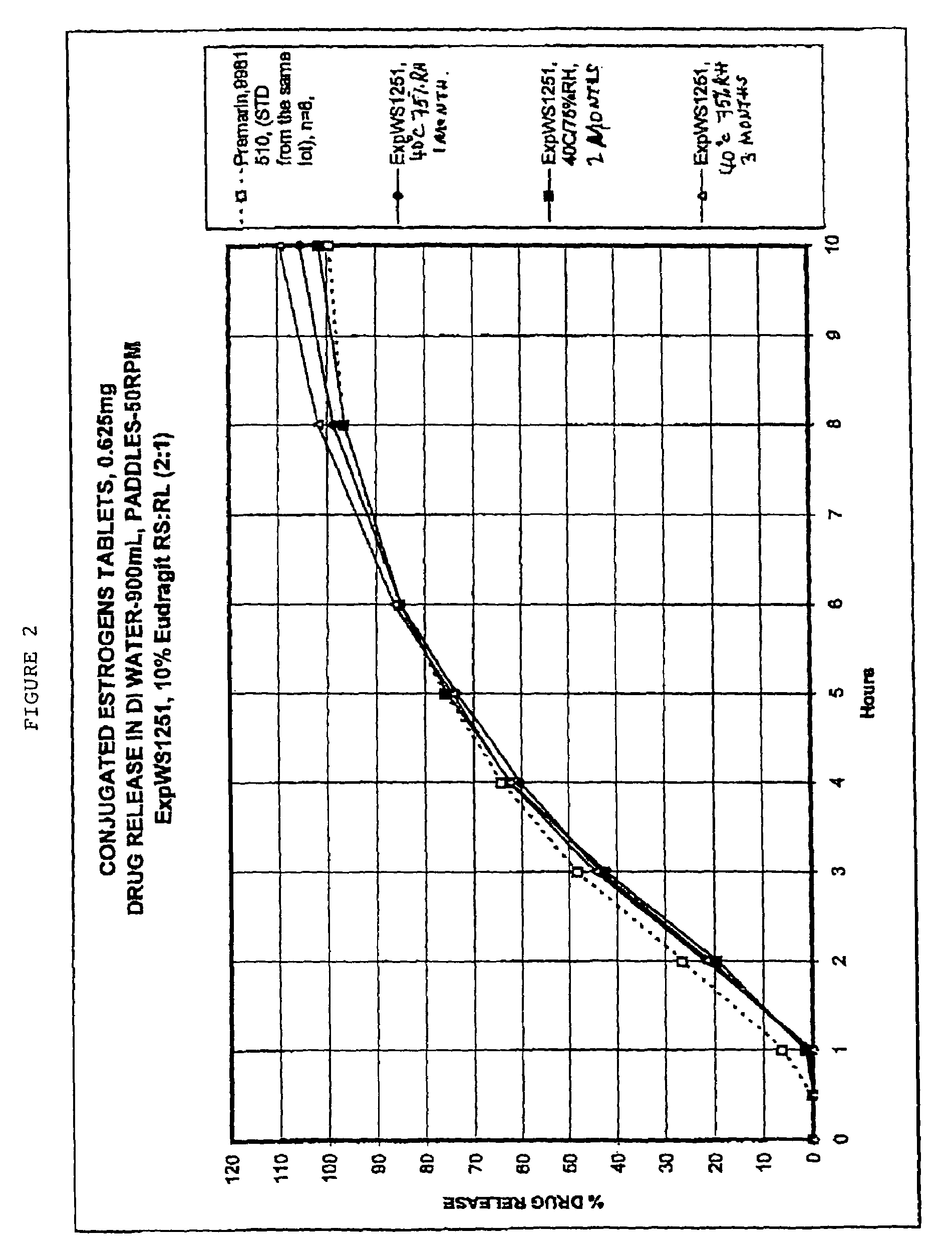
Oral conjugated estrogen formulations are disclosed and described. In one aspect, the oral formulation may be a tablet having a core and one or more coatings thereon. In addition to conjugated estrogen ingredients, the core may include one or more organic excipients and one or more inorganic excipients. In one aspect, the organic excipients may include less than about 20% w / w of a cellulose ingredient, and less than about 50% w / w of a sugar ingredient. In another aspect, the inorganic excipients may include less than about 10% w / w of a calcium phosphate tribasic ingredient. In yet another aspect, the formulation does not crack when stored at about 40° C. and about 75% relative humidity for about 2 months.
Owner:WATSON LAB INC
Vaginal cream compositions, kits thereof and methods of using thereof
The present invention is directed to pharmaceutical vaginal cream compositions comprising a conjugated estrogen and a stabilizer. The present invention is also directed to a method of treating a menopausal condition in a female in need thereof, said method comprising vaginally administering a pharmaceutical vaginal cream composition comprising a conjugated estrogen twice per week for at least 2 weeks.
Owner:DURAMED PHARMA
Conjugated estrogen compositions, applicators, kits, and methods of making and use thereof
InactiveUS20070191321A1Increased risk of infectionInability to controlBiocideOrganic active ingredientsMedicineExcipient
The present invention is directed to monophasic pharmaceutical compositions comprising a conjugated estrogen and a hydrophilic or lipophilic excipient. The present invention is also directed to kits and applicators comprising the pharmaceutical compositions. The invention is also directed to methods for treating menopausal conditions in a female comprising administration of the pharmaceutical compositions.
Owner:TEVA WOMENS HEALTH
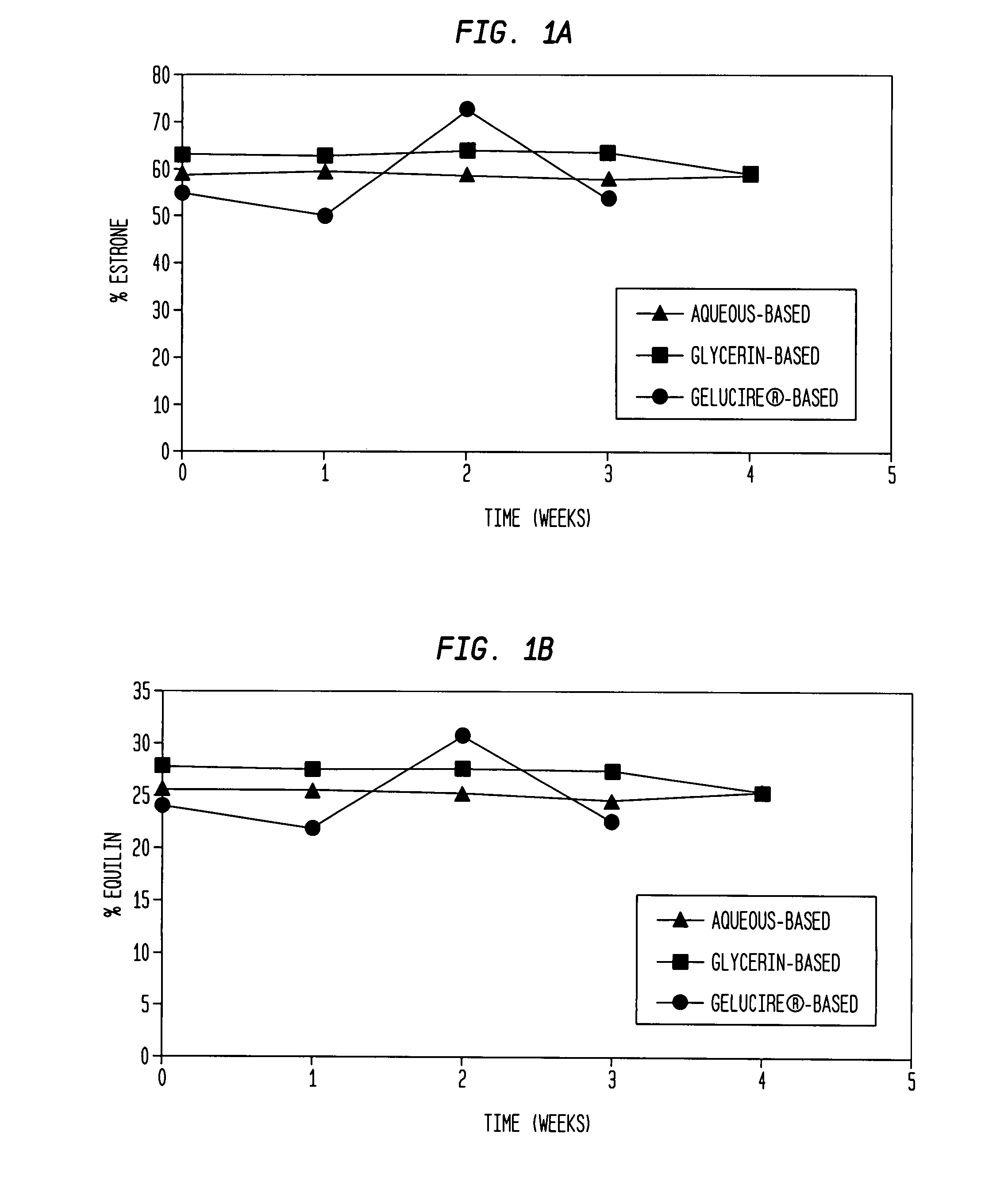
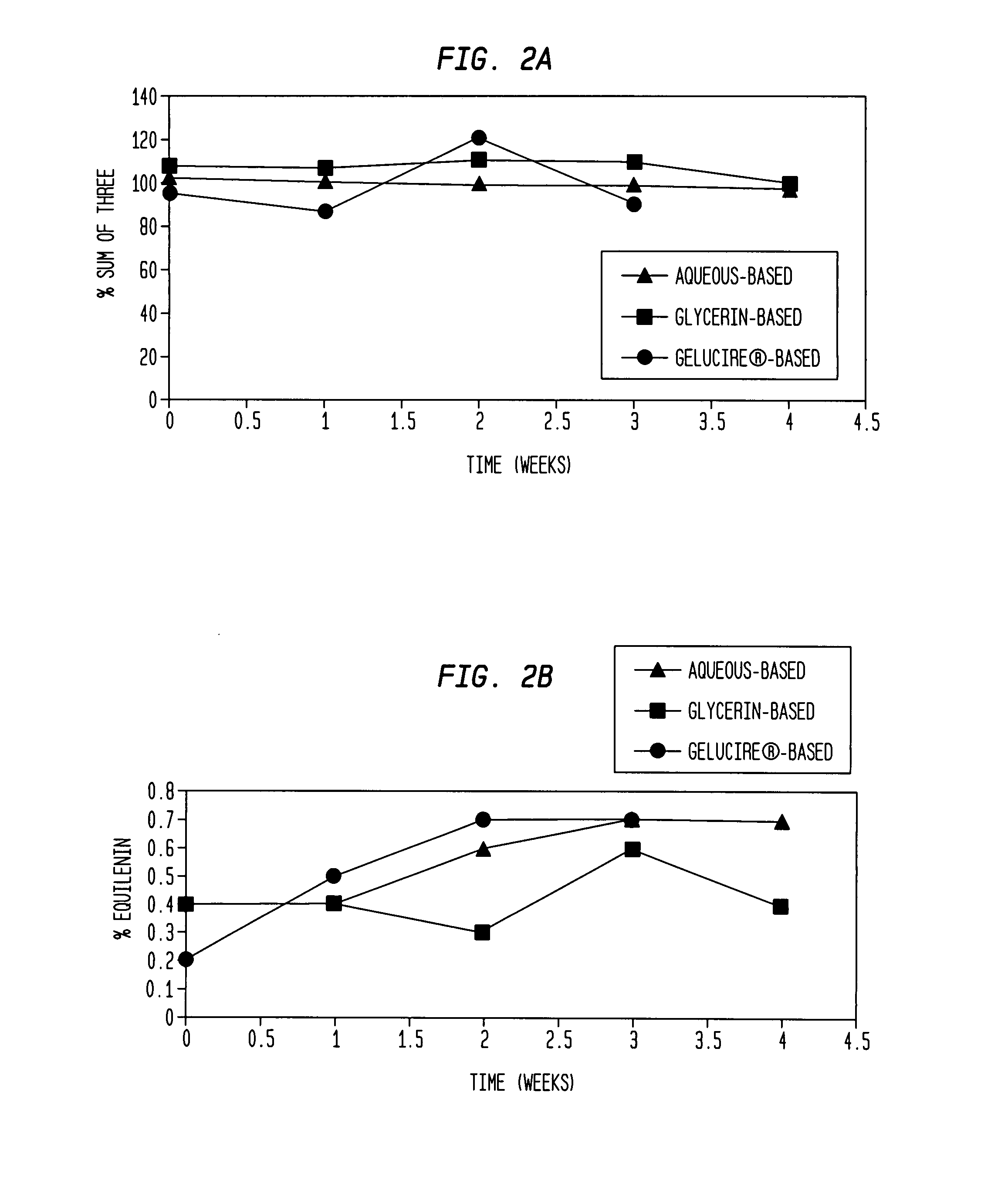
Compositions of unconjugated estrogens and methods for their use
InactiveUS20060183724A1Improve efficacyImprovement of side effectsOrganic active ingredientsBiocidePhysiologyOestrogen deficiency
The present invention relates to compositions containing unconjugated estrogens and methods of their use in the treatment of conditions associated with hypoestrogenism or reduced estrogen levels in females.
Owner:DURAMED PHARMA
Formulations of conjugated estrogens and bazedoxifene
InactiveUS20070003623A1Relieve vasomotor symptomEffective treatmentBiocideSkeletal disorderMedicineConjugated oestrogens
The present invention relates to solid dosage formulations containing conjugated estrogens and bazedoxifene, or a salt thereof. In some embodiments, the compositions include a core comprising conjugated estrogens, and at least one coating that comprises bazedoxifene, or a salt thereof.
Owner:WYETH LLC
Matrix film tablet with controlled release of a natural mixture of conjugated estrogens
InactiveUS20050019408A1Simple wayQuality improvementPowder deliveryOrganic active ingredientsControlled releaseConjugated oestrogens
A pharmaceutical matrix film tablet with controlled release of natural mixtures of conjugated estrogens which have been obtained from the urine of pregnant mares.
Owner:ABBVIE PHARMA GMBH
Slow-releasing composition of estrogen medicine and its preparing process
InactiveCN1378840ASolve the problem of being released slowly on a regular basisReduce physiological effectsOrganic active ingredientsNervous disorderPhysiologyCoronary heart disease
A slowly releasing conjugated oestrogen composition for preventing and treating femal climacteric syndrome, osteoporosis and coronary heart disease and male senile dementia is composed of slow-releasing layer and core containing conjugated oestrogen and progestogen.
Owner:XINJIANG NUZILINE BIO PHARMA CO LTD
Pharmaceutical composition for treating polycystic ovary syndrome (PCOS)
InactiveCN102302668ADecreased androgen levelsIncrease ovulation rateHeavy metal active ingredientsOrganic active ingredientsIn vivoPolycystic ovary
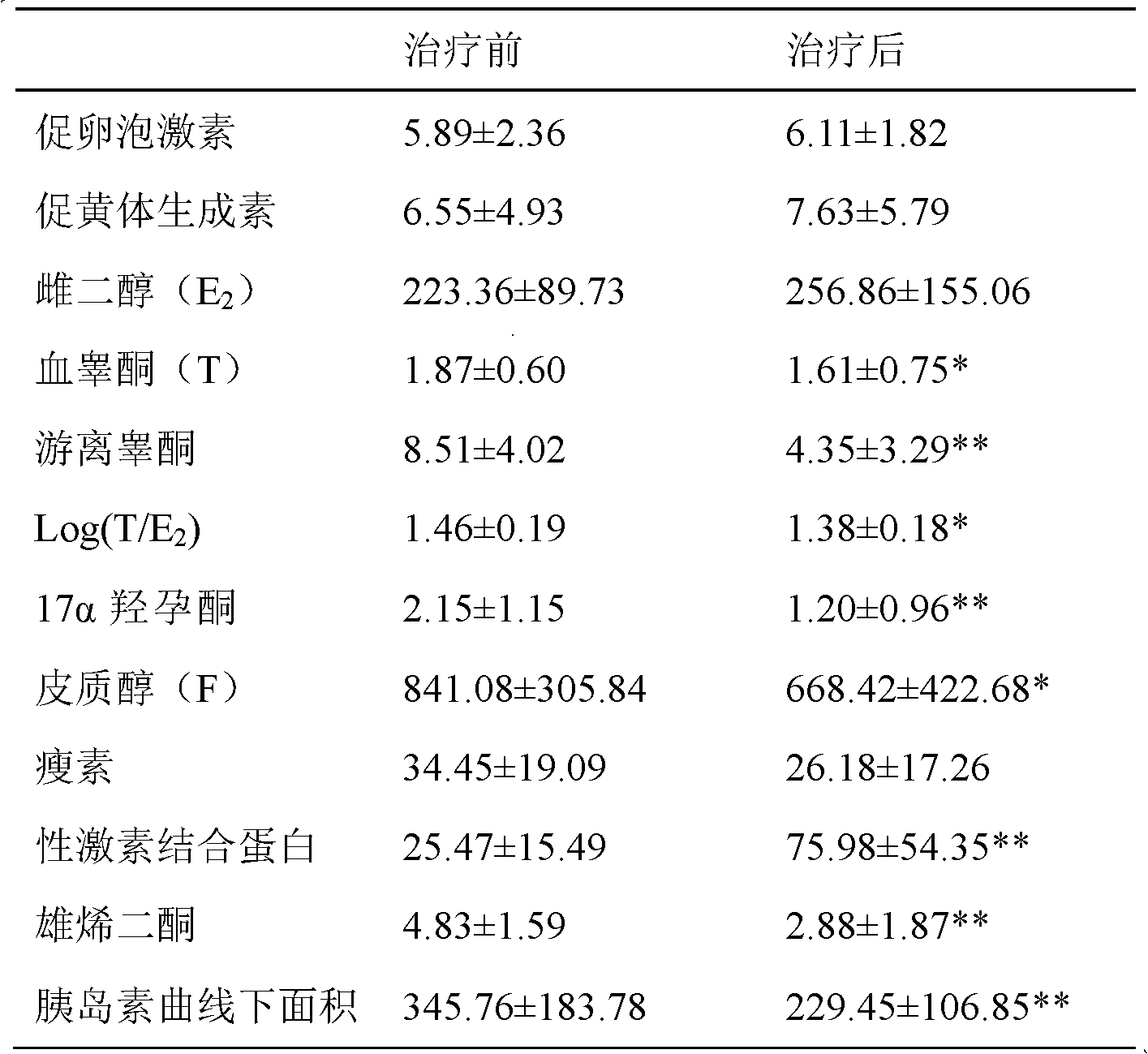
The invention provides a pharmaceutical composition for treating polycystic ovary syndrome (PCOS). The pharmaceutical composition consists of a traditional Chinese medicine composition and chemical drugs, wherein the traditional Chinese medicine composition comprises the medicinal materials such as anemarrhena, fritillary bulb, tortoise plastron, fleece-flower root, lilyturf root, hippocampus andthe like; and the chemical drugs comprise one or more compound molecules of dexamethasone, spirolactone, ethinyloestradiol, estradiol valerate, conjugated estrogen and pharmaceutically acceptable salt. The pharmaceutical composition has the advantages of reducing the level of androgens and androgen receptors of patients suffering from PCOS, improving the level of sex hormone-binding globulin (SHBG), lowering the level of free testosterone (F-TESTO), further raising the level of in-vivo estrogens, improving growth and development of follicles and increasing ovulation rate and pregnancy rate ofthe patients.
Owner:上海泰坤堂中医医院有限公司
Formulations of conjugated estrogens and bazedoxifene
The present invention relates to solid dosage formulations containing conjugated estrogens and bazedoxifene, or a salt thereof. In some embodiments, the compositions include a core comprising conjugated estrogens, and at least one coating that comprises bazedoxifene, or a salt thereof.
Owner:WYETH LLC
Vaginal cream compositions, kits thereof and methods of using thereof
The present invention is directed to pharmaceutical vaginal cream compositions comprising a conjugated estrogen and a stabilizer. The present invention is also directed to a method of treating a menopausal condition in a female in need thereof, said method comprising vaginally administering a pharmaceutical vaginal cream composition comprising a conjugated estrogen twice per week for at least 2 weeks.
Owner:TEVA WOMENS HEALTH
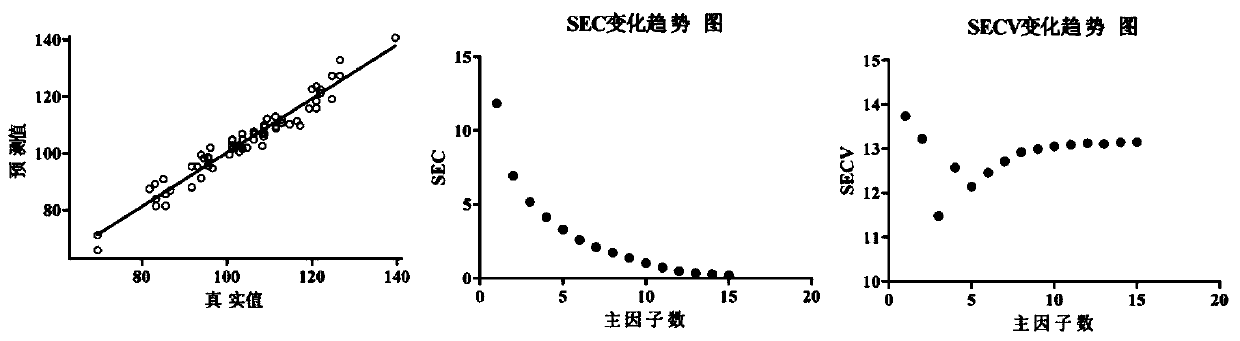
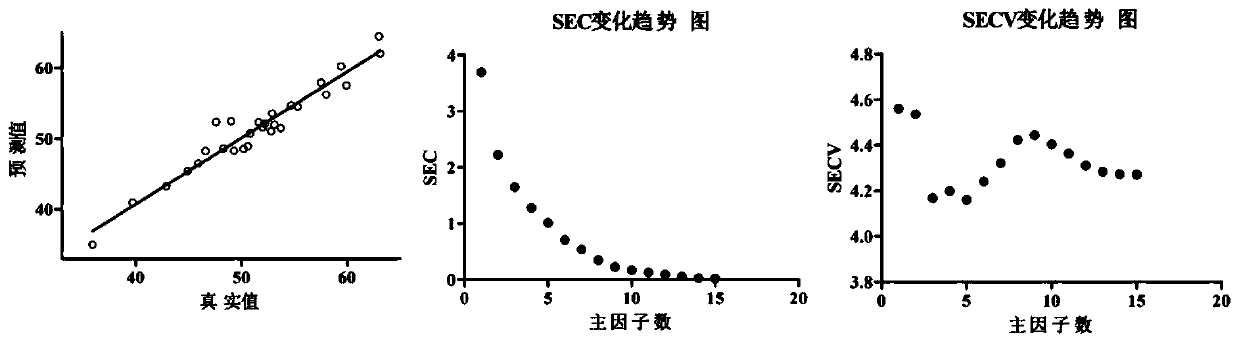
Method for rapidly detecting content of conjugated estrogens in pregnant mare urine by utilizing near infrared spectroscopy
PendingCN111579523AEasy to operateSimple and fast operationComponent separationMaterial analysis by optical meansSpectral bandsPharmaceutical drug
The invention relates to the technical field of chemical detection, and provides a method for rapidly detecting the content of conjugated estrogens in pregnant mare urine by utilizing near infrared spectroscopy. Aiming at different conjugated estrogen components, a quantitative analysis and correction model of each conjugated estrogen is established by adopting a partial least square method through preprocessing and selecting characteristic spectral bands. Compared with an HPLC (high performance liquid chromatography) method, the near infrared spectroscopy provided by the invention is simpler,more convenient and quicker to operate, is suitable for content determination of large-batch samples, can meet the requirements on large-scale sample information acquisition in industrial production,and has important significance on the drug production process and stability controllability of a terminal product. Results of the embodiment show that the method provided by the invention is relatively high in detection accuracy, relative deviation between a predicted value obtained by adopting the method provided by the invention and a real value is less than 15%, and accuracy is relatively high.
Owner:XINJIANG NUZILINE BIO PHARMA CO LTD +1
Method For Obtaining Conjugated Estrogen Mixtures From Pregnant Mare Urine and Use of a Macroporus Resin in the Method
ActiveUS20090131698A1Reduce adsorptionLow costOrganic active ingredientsBiocideDivinylbenzenePre treatment
The present invention relates to a method for obtaining a natural mixture of conjugated estrogens from urine of pragnant mares (PMU) and use of a macroporous adsorption resin in the method. The method for obtaining a natural mixture of conjugated estrogen from PMU includes the steps of pretreating raw PMU; adsorbing the natural mixture of conjugated estrogens contained in PMU with a macroporous adsorption resin; washing the macroporous adsorption resin laden with the mixture of conjugated estrogens with an alkaline aqueous solution; and eluting the washed adsorption resin with an eluting agent to obtain the mixture of conjugated estrogens. The macroporous adsorption resin is a styrene-divinylbenzene semipolar macroporous adsorption resin with ester group structure. The method according to the invention solves the problems of low adsorptive capacity and high cost existed in the conventional methods, and is suitable for large-scale production.
Owner:XINJIANG NUZILINE BIO PHARMA CO LTD
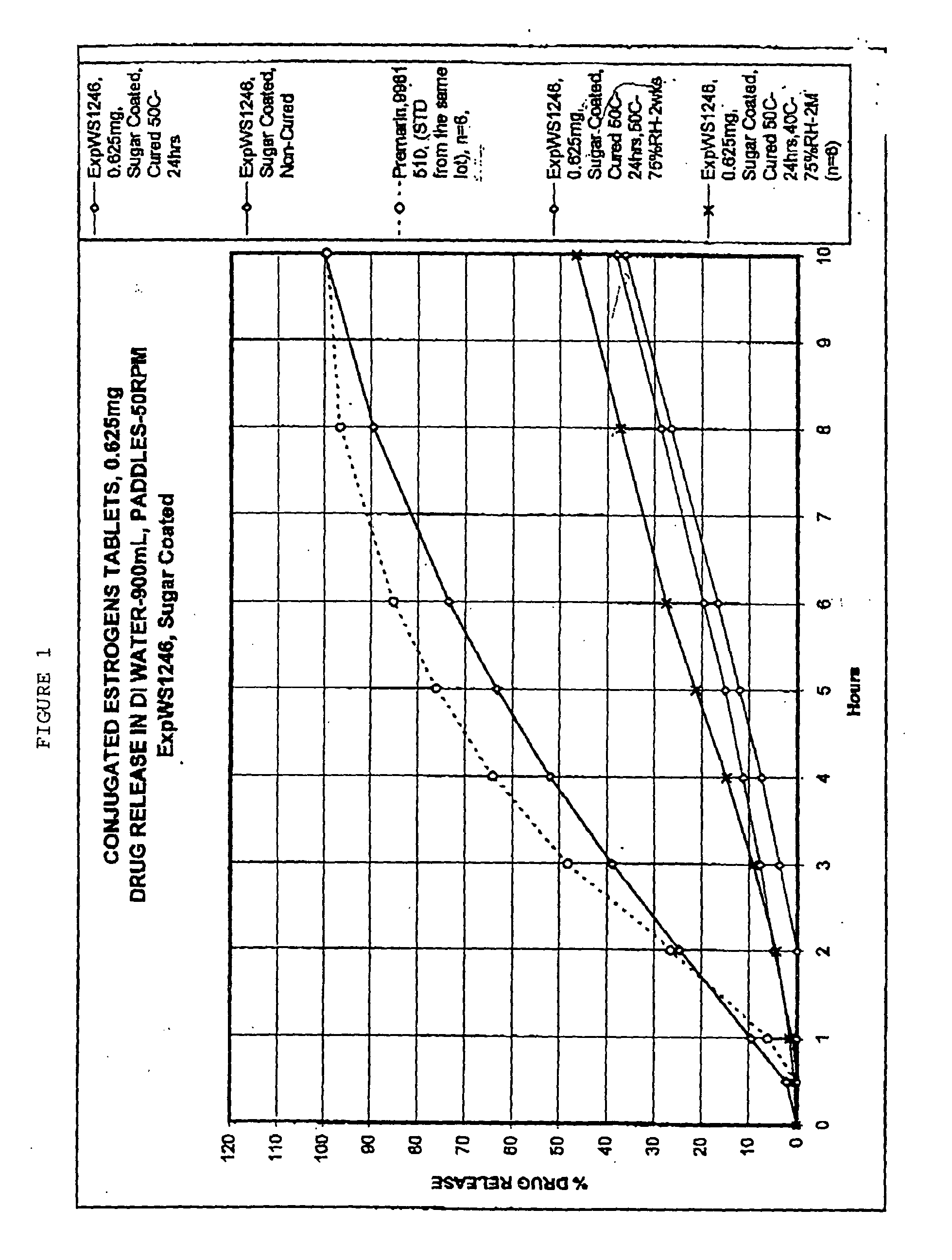
Sugar coatings and methods therefor
InactiveUS20080107780A1Convenient coatingImprove barrier propertiesDough treatmentConfectioneryCoated tabletsSugar Coated Tablet
The invention provides sugar-containing compositions suitable for use in coating solid preparations such as tablets, pills, granules and grains. Methods of using such coatings are provided, as are solid dosage forms coated with the compositions. In some embodiments, the methods provide sugar coated tablets comprising conjugated estrogens, and a progestin, for example medroxyprogesterone acetate.
Owner:WYETH LLC
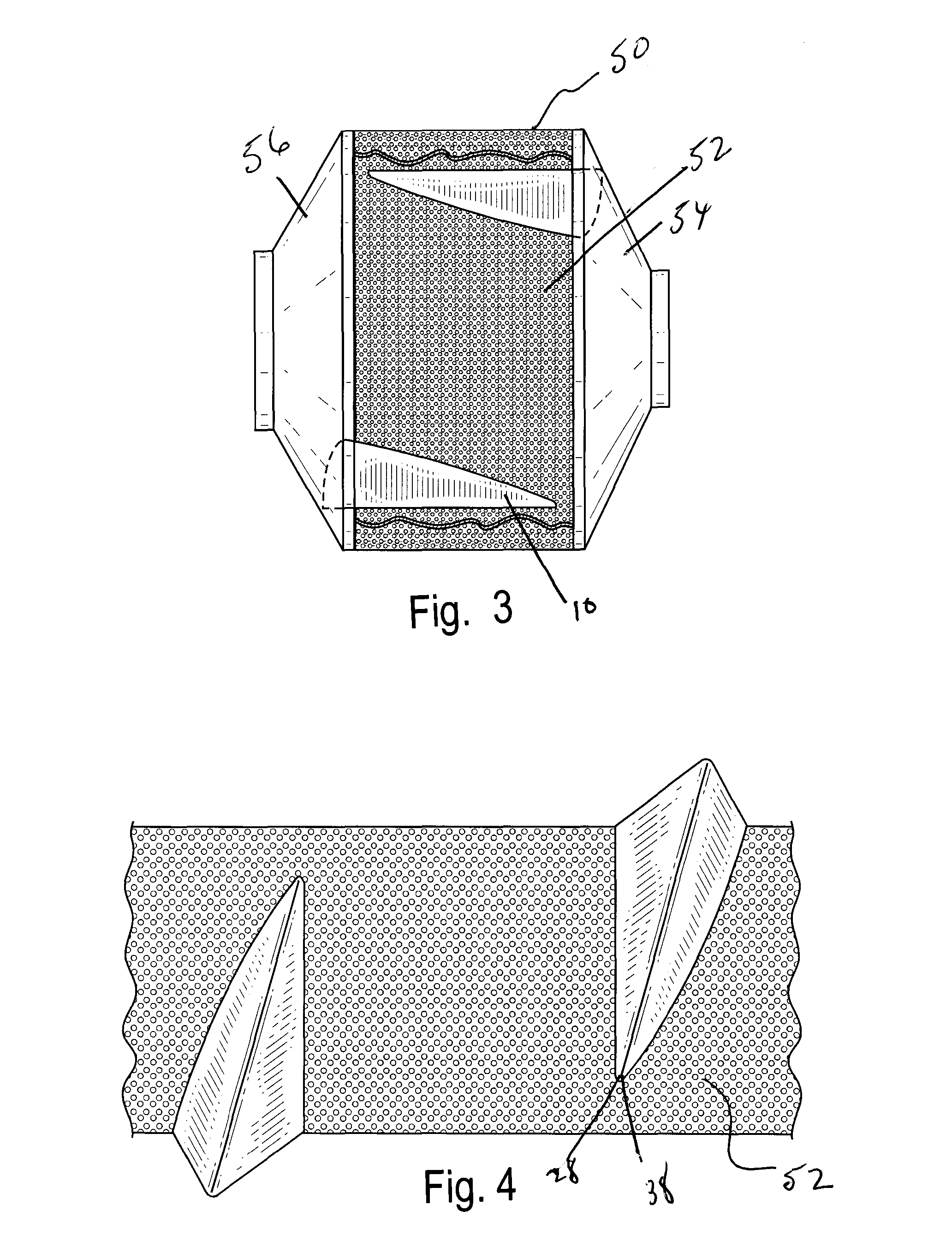
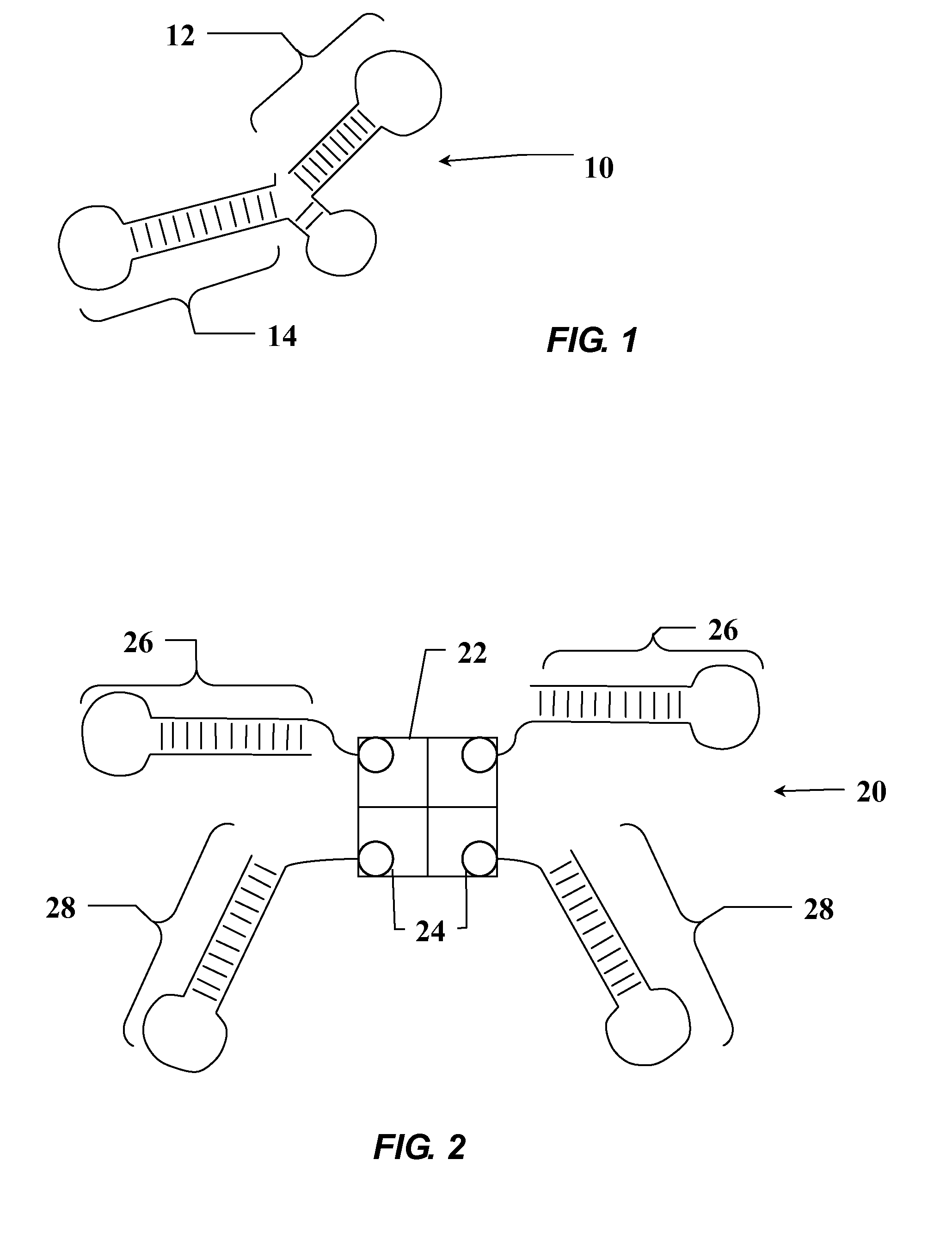
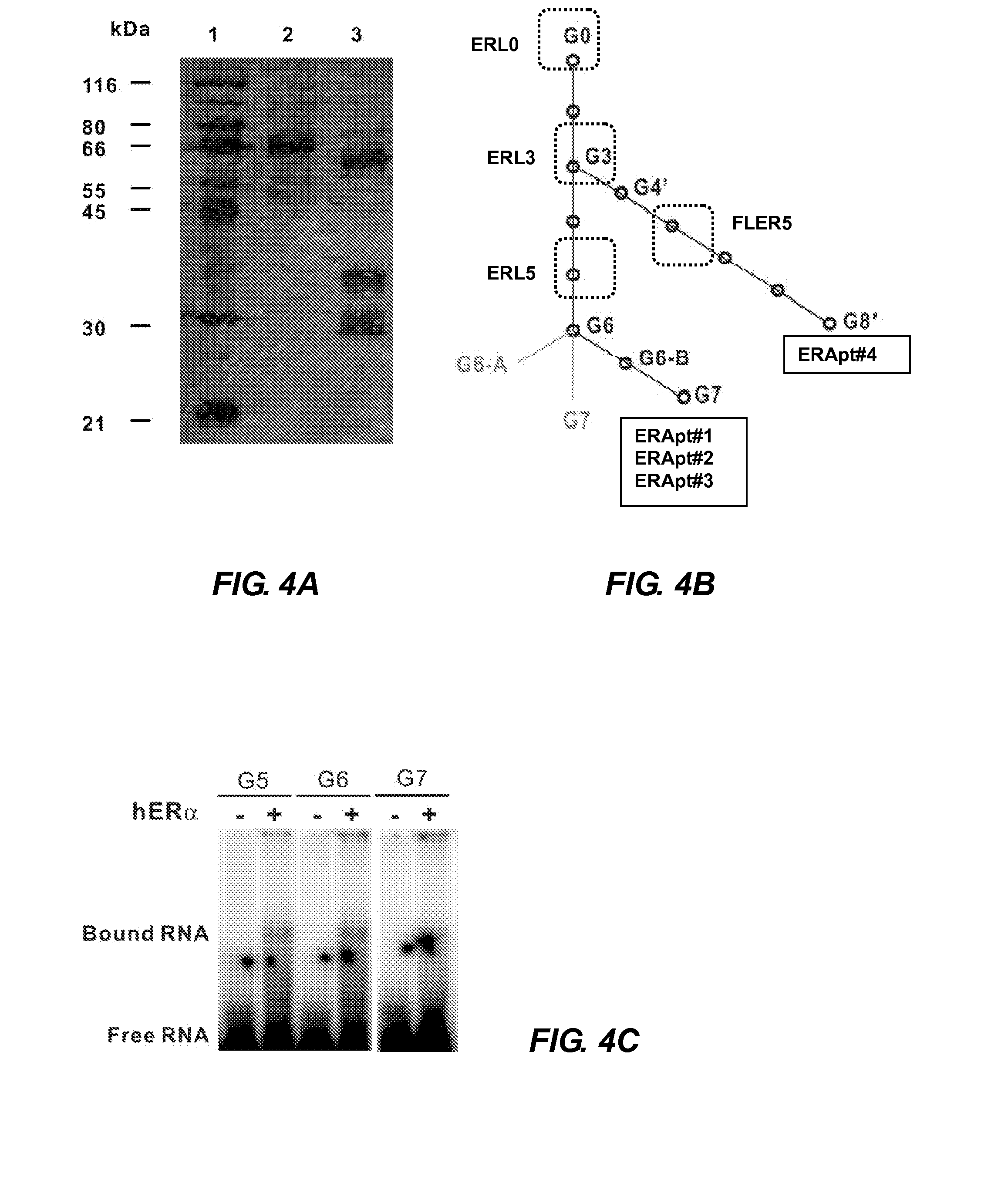
Aptamer modulators of estrogen receptors
The present invention relates to a nucleic acid aptamer molecule that includes a domain that binds to an estrogen receptor, molecular complexes that include the nucleic acid aptamer molecule and an estrogen receptor, and constructed DMA molecules and expression systems, as well as host cells, that the contain an RNA aptamer molecule of the invention. Use of these aptamers and encoding constructs to inhibiting estrogen receptor activity in a cell and to treat estrogen receptor-positive cancers is also described.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Method for obtaining estrogens from mare's urine
InactiveUS20050014738A1Easy to useIncrease contentOrganic active ingredientsBiocideMedicineSolid phase extraction
A method for obtaining an extract from a natural mixture of conjugated estrogens from the urine of pregnant mares (PMU) by solid-phase extraction on semipolar adsorption resins in which the resulting extract meets the pharmaceutical specifications for conjugated estrogens and the content of free estrogens is minimized even if old pregnant mares' urine and / or pregnant mares' urine which has been stored or transported at elevated temperatures is used.
Owner:ABBVIE PHARMA GMBH
Natural pregnant mare conjugated estrogen purification and refinement method and application of nonionic nonpolar fine separation resin used by same
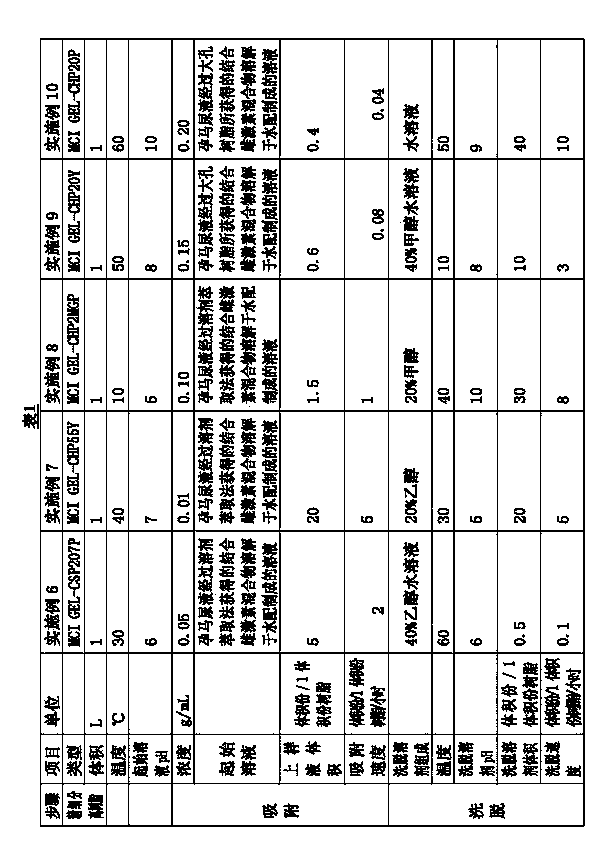
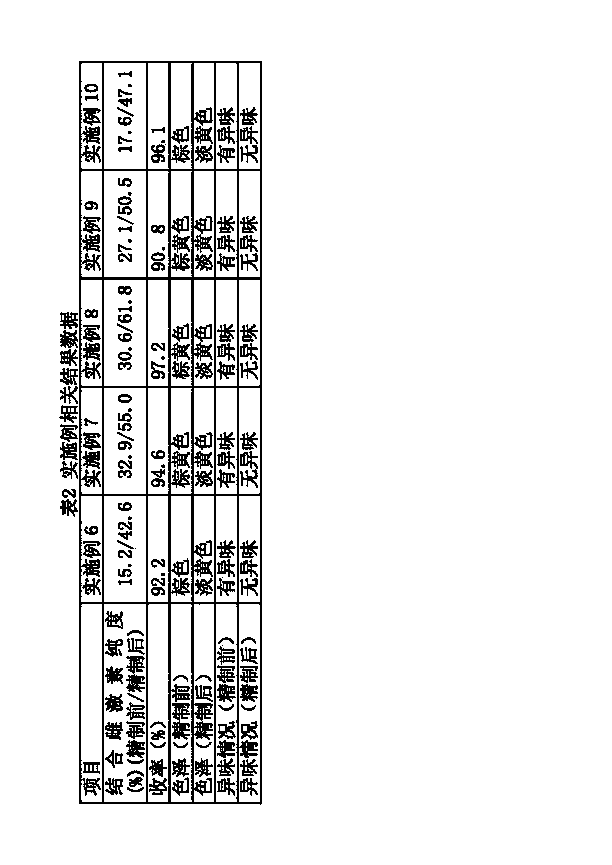
InactiveCN103720720AImprove qualityEasy to separateNervous disorderSkeletal disorderPolymeric adsorbentActive ingredient
The invention relates to a natural pregnant mare conjugated estrogen purification and refinement method and application of a nonionic nonpolar fine separation resin used by the same. The method comprises the following steps: 1. adsorbing an initial liquid containing a pregnant mare conjugated estrogen crude extract by using a nonionic nonpolar fine separation resin; and 2. eluting the nonionic nonpolar fine separation resin carrying the conjugated estrogen mixture by using an eluting solvent to obtain the purified and refined conjugated estrogen mixture liquid. Compared with the prior art, the purity of the conjugated estrogen prepared by the method is up to 42.6-61.8%, and the total conjugated estrogen yield is 90.8-97.2%; and the conjugated estrogen has no peculiar smell and obviously lighter color (fine light yellow), which is very important for preparing the high-quality conjugated estrogen active pharmaceutical ingredient and preparation products. The method has the advantages of simple technique and high refinement efficiency, and is especially suitable for large-scale production.
Owner:XINJIANG TEFENG PHARMA +1
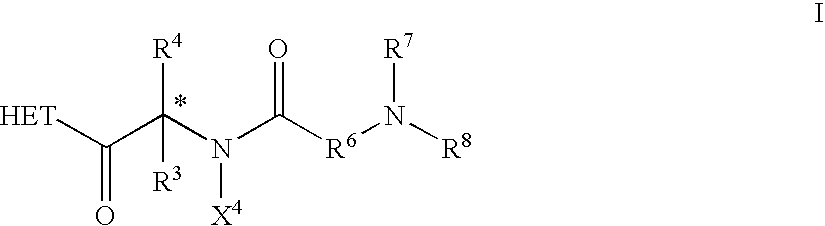
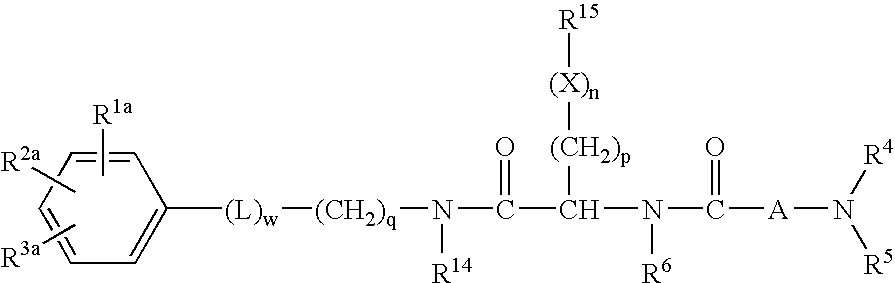
Dipeptide derivatives
InactiveUS6924280B2Reduced responseReducing cachexia and protein lossBiocideNervous disorderProgesteronesConjugated estrogen
This invention is directed to compounds of the formula and the pharmaceutically-acceptable salts thereof, where the substituents are as defined in the Specification, which are growth hormone secretogogues and which increase the level of endogenous growth hormone. The compounds of this invention are useful for the treatment and prevention of osteoporosis and / or frailty, congestive heart failure, frailty associated with aging, obesity; accelerating bone fracture repair, attenuating protein catabolic response after a major operation, reducing cachexia and protein loss due to chronic illness, accelerating wound healing, or accelerating the recovery of burn patients or patients having undergone major surgery; improving muscle strength, mobility, maintenance of skin thickness, metabolic homeostasis or renal homeostasis. The compounds of the present invention are also useful in treating osteoporosis and / or frailty when used in combination with: a bisphosphonate compound such as alendronate; estrogen, conjugated estrogens, and optionally progesterone; an estrogen agonist or antagonist; or calcitonin, and pharmaceutical compositions useful therefor. Further, the present invention is directed to pharmaceutical compositions useful for increasing the endogenous production or release of growth hormone in a human or other animal which comprises an effective amount of a compound of the present invention and a growth hormone secretagogue selected from GHRP-6, Hexarelin, GHRP-1, growth hormone releasing factor (GRF), IGF-1, IGF-2 or B-HT920. The invention is also directed to intermediates useful in the preparation of compounds of Formula I.
Owner:PFIZER INC
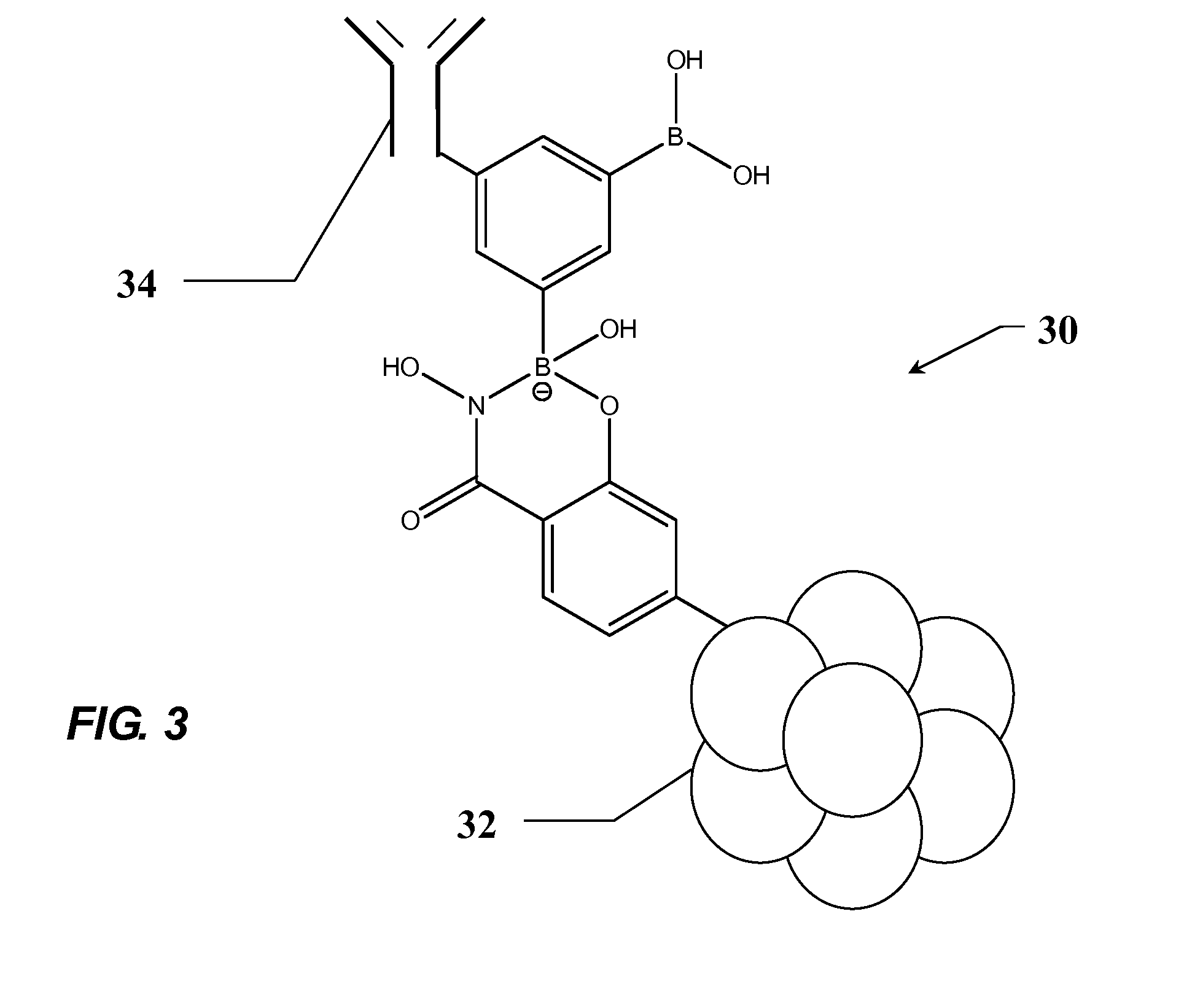
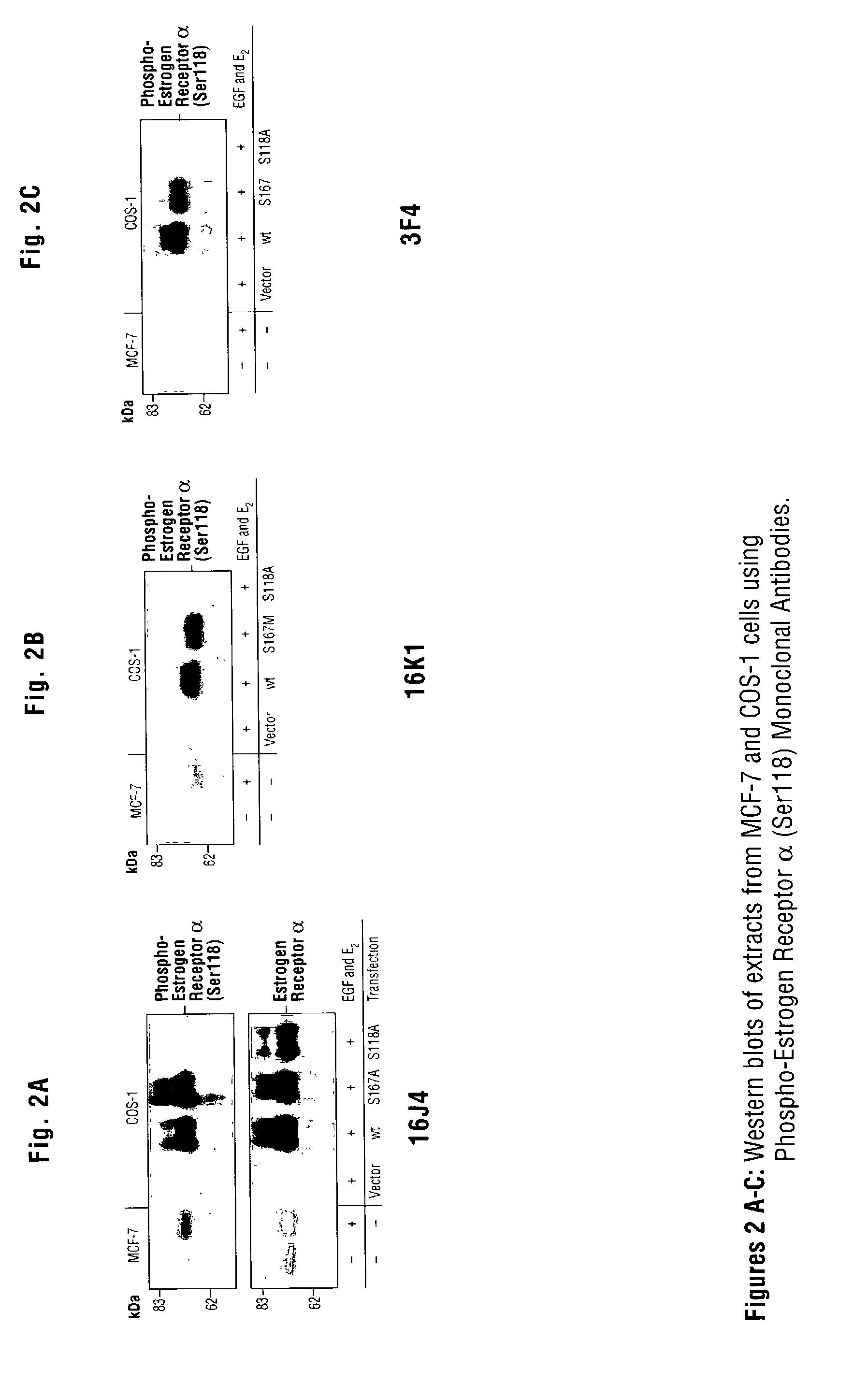
Monoclonal antibodies specific for phosphorylated estrogen receptor alpha (Ser118) and uses thereof
ActiveUS7105642B2Animal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsPhosphorylationPhosphoric acid
The invention provides monoclonal antibodies that bind the estrogen receptor α (ER α) when phosphorylated at serine 118 (Ser118) in the N-terminal domain, but do not bind to ER α when not phosphorylated at this site. Also provided are methods for determining the phosphorylation of ER α in a biological sample, profiling ER α activation in a test tissue, and identifying a compound that modulates phosphorylation of ER α in a test tissue, by using the disclosed monoclonal antibodies. The sample or test tissue may be taken from a subject suspected of having cancer, such as breast cancer. Kits comprising the phospho-ER α (Ser118) monoclonal antibodies of the invention are also provided.
Owner:CELL SIGNALING TECHNOLOGY
Compositions for conjugated estrogens and associated methods
Oral conjugated estrogen formulations are disclosed and described. In one aspect, the oral formulation may be a tablet having a core and one or more coatings thereon. In addition to conjugated estrogen ingredients, the core may include one or more organic excipients and one or more inorganic excipients. In one aspect, the organic excipients may include less than about 20% w / w of a cellulose ingredient, and less than about 50% w / w of a sugar ingredient. In another aspect, the inorganic excipients may include less than about 10% w / w of a calcium phosphate tribasic ingredient. In yet another aspect, the formulation does not crack when stored at about 40° C. and about 75% relative humidity for about 2 months.
Owner:WATSON LAB INC
Conjugated estrogen compositions, applicators, kits, and methods of making and use thereof
InactiveUS20080051377A1Increased risk of infectionInability to controlOrganic active ingredientsBiocideConjugated oestrogensPharmaceutical Substances
The present invention is directed to monophasic pharmaceutical compositions comprising a conjugated estrogen and a hydrophilic or lipophilic excipient. The present invention is also directed to kits and applicators comprising the pharmaceutical compositions. The invention is also directed to methods for treating menopausal conditions in a female comprising administration of the pharmaceutical compositions.
Owner:TEVA WOMENS HEALTH
Method for determining content of conjugated estrogens in pregnant mare urine
ActiveCN111458442AGuarantee the accuracy of content determinationImprove detection efficiencyComponent separationConjugated estrogenUrology
The invention relates to the technical field of chemical detection, and provides a method for detecting the content of conjugated estrogens in pregnant mare urine. Carbonate in the pregnant mare urinecan form neutral salt, water and carbon dioxide gas when encountering inorganic acid; the column efficiency is improved, so that the problems that 30 samples cannot be measured each time, the columnpressure is increased, the column efficiency is reduced and the like when the content of conjugated estrogens in pregnant mare urine is measured by HPLC can be solved, the measurement accuracy of thesample content is ensured, and reliable data is provided for production. The pH value is adjusted to 7.0-8.0 by using an alkaline substance because: a, the existence state of bound estrogen is relatively stable under an alkaline condition; b, the pH value of the pregnant mare urine is about 7.5-8.5, and the pH value of the pregnant mare urine is adjusted to be slightly alkaline (7.0-8.0), so thatthe pregnant mare urine is closer to the original conditions of the pregnant mare urine; and c, the authenticity and the reproducibility of a detection result have advantages when the sample is slightly alkaline.
Owner:XINJIANG NUZILINE BIO PHARMA CO LTD +1
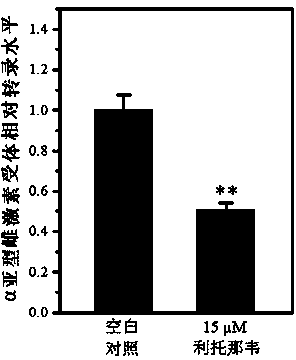
Application of ritonavir as estrogen receptor modulator
InactiveCN103816151ASignificant effectOrganic active ingredientsAntineoplastic agentsPromoter activityMetabolite
The invention discloses novel applications of ritonavir, and metabolite thereof and / or a metabolite derivative and / or a structural analogue of ritonavir as an estrogen receptor modulator. An inhibition effect of ritonavir to tumor cells is partially depended on an estrogen receptor. Ritonavir down-regulates protein expression level and gene transcription level of an alpha subtype estrogen receptor of the tumor cells; ritonavir inhibits F promoter activity of the alpha subtype estrogen receptor; interactive experiments of rotonavir and the alpha subtype estrogen receptor-ligandb inding domain demonstrate that ritonavir is a selective antagonist of the alpha subtype estrogen receptor, belongs to the estrogen receptor modulator, and can bind the estrogen receptor and modulate transcription and expression level of the estrogen receptor, thereby generating effects of inhibiting growth of the tumor cells and increasing cell mortality rate. Reposition of conventional drugs can reduce risks and reduce research cost. The drug is expected to be used for preparing tumor-treating drugs for drug resistance and chemotherapy resistance of conventional antitumor drugs.
Owner:WUHAN UNIV
Method for obtaining estrogens from mare's urine
A method for obtaining an extract from a natural mixture of conjugated estrogens from the urine of pregnant mares (PMU) by solid-phase extraction on semipolar adsorption resins in which the resulting extract meets the pharmaceutical specifications for conjugated estrogens and the content of free estrogens is minimized even if old pregnant mares' urine and / or pregnant mares' urine which has been stored or transported at elevated temperatures is used.
Owner:ABBVIE PHARMA GMBH
Method for obtaining a natural mixture of conjugated equine estrogens
InactiveUS20050032767A1Simple methodEasy to separateOrganic active ingredientsSteroids preparationConjugated Equine EstrogensNorisoprenoids
A method for obtaining an extract containing the natural mixture of conjugated equine estrogens in which a mixture of conjugated estrogens obtained by solid-phase extraction from pregnant mares' urine is depleted in phenolic urine contents and in non-conjugated lipophilic compounds selected from the group consisting of non-conjugated flavonoids, non-conjugated isoflavonoids, non-conjugated norisoprenoids, non-conjugated steroids, in particular androstane and pregnane steroids, and comparable non-conjugated compounds.
Owner:ABBVIE PHARMA GMBH
Method for obtaining a natural mixture of conjugated equine estrogens
InactiveUS7964586B2Simple methodOrganic active ingredientsBiocideConjugated Equine EstrogensNorisoprenoids
A method for obtaining an extract containing the natural mixture of conjugated equine estrogens in which a mixture of conjugated estrogens obtained by solid-phase extraction from pregnant mares' urine is depleted in phenolic urine contents and in non-conjugated lipophilic compounds selected from the group consisting of non-conjugated flavonoids, non-conjugated isoflavonoids, non-conjugated norisoprenoids, non-conjugated steroids, in particular androstane and pregnane steroids, and comparable non-conjugated compounds.
Owner:ABBVIE PHARMA GMBH
Use of conjugated estrogens in combination with trimegestone in hormone replacement therapy
This invention relates to methods and pharmaceutical compositions for providing hormone replacement therapy in perimenopausal, menopausal, and postmenopausal women through the continuous administration of combinations of conjugated estrogens and trimegestone.
Owner:WYETH LLC
Selective modulator of human estrogen receptor alpha36 subtype
InactiveCN103768064ALarge room for renovationBroad application prospectsOrganic active ingredientsSkeletal disorderDrugChemical compound
The present invention discloses a selective modulator of a human estrogen receptor alpha36 subtype, and a use of the selective modulator in preparation of drugs for prevention and treatment of diseases caused by estrogen receptor abnormality. According to the present invention, the selected compounds can be selectively bound with the estrogen receptor alpha36 subtype in theory; experiment results confirm that: the compounds can activate the ERK channel, inhibit the cancer cell cycle, and initiate apoptosis through the estrogen receptor alpha36 subtype so as to provide inhibition effects for cancer cells, wherein the cell growth inhibition activity of the compounds is superior to the cell growth inhibition activity of a variety of the currently-researched selective modulators of the estrogen receptor alpha36 subtype; and with the skeleton diversity of the compounds, the target compound has advantages of broad transformation space, synthetic route choice and the like, and the application prospects are broad.
Owner:PEKING UNIV
Molecular compound of 17 beta-estradiol and vitamin C as well as preparation method and application thereof
ActiveCN113244227AIncreased anti-osteoporosis activityOrganic active ingredientsSkeletal disorderVitamin CThrombus
The invention discloses a molecular compound of 17 beta-estradiol and vitamin C as well as a preparation method and application thereof. The molecular compound is formed by compounding 17 beta-estradiol molecules and vitamin C in a molar ratio of 0.25: 1, 0.5: 1, 0.75: 1, 1: 1, 1: 0.25, 1: 0.5 or 1: 0.75. The invention further provides a method for preparing the molecular compound of 17 beta-estradiol and vitamin C. The molecular compound of 17beta-estradiol and vitamin C provided by the invention can be distributed to bone tissues in a bone targeting manner, so that not only the anti-osteoporosis activity of 17beta-estradiol is remarkably improved, but also the side effects of hyperplasia endometrii and thrombosis caused by treating with 17 beta-estradiol and conjugated estrogens can be effectively avoided.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com