Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Methyl-1H-indole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
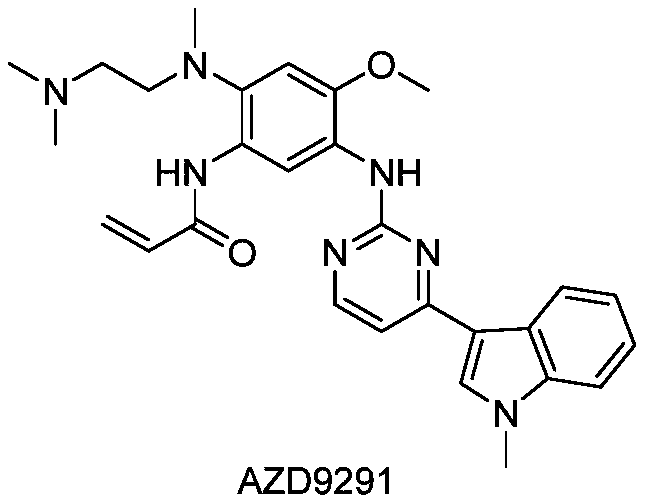
Synthetic method of anti-tumor medicine
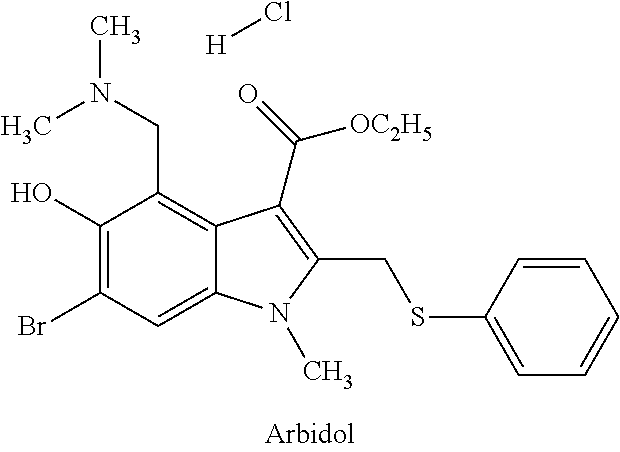
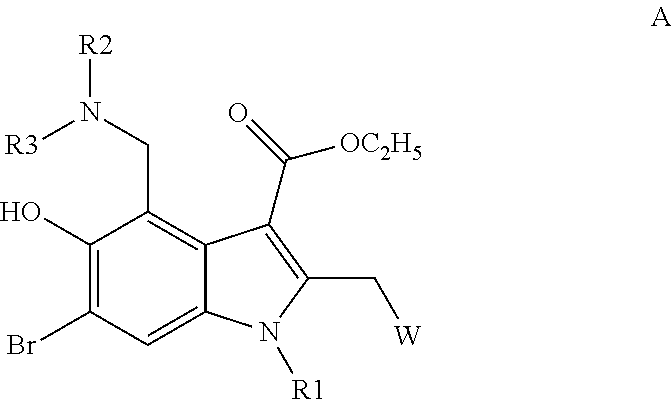
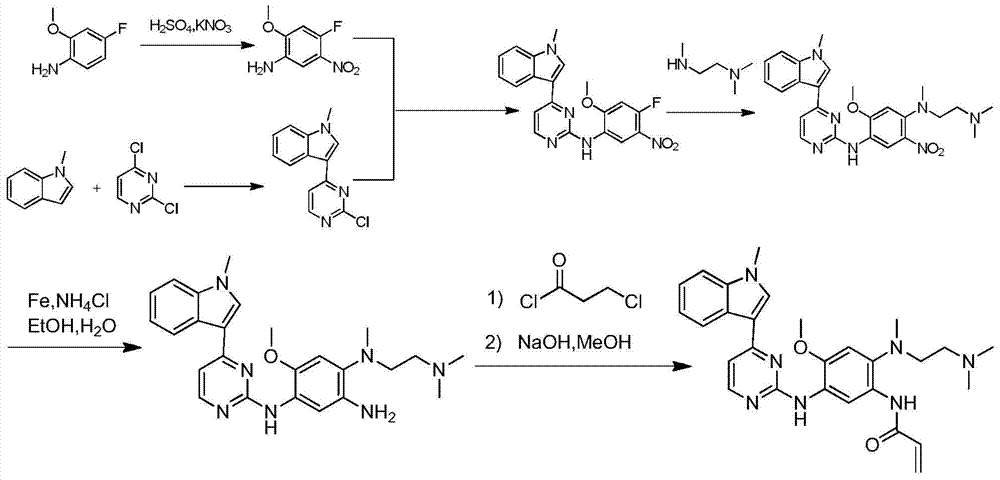
ActiveCN104817541AHigh yieldMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationCarbamateMethyl-1H-indole
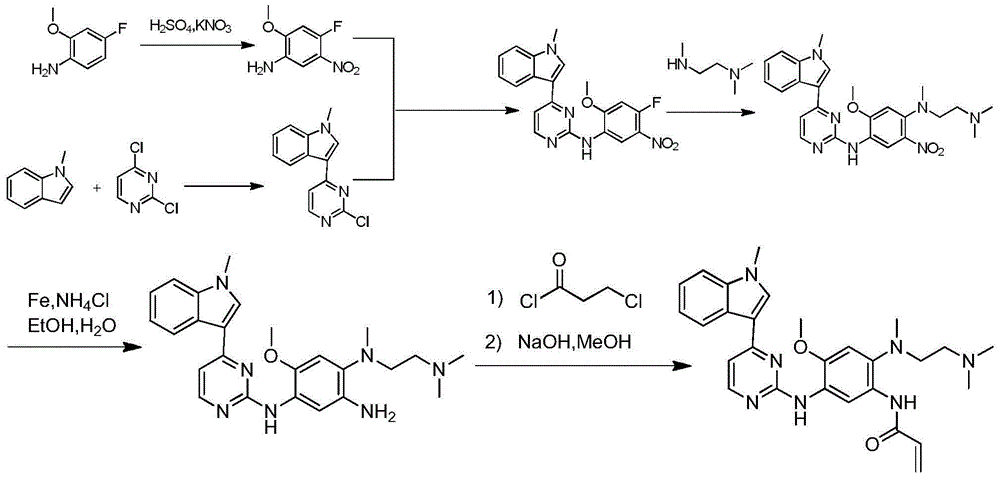
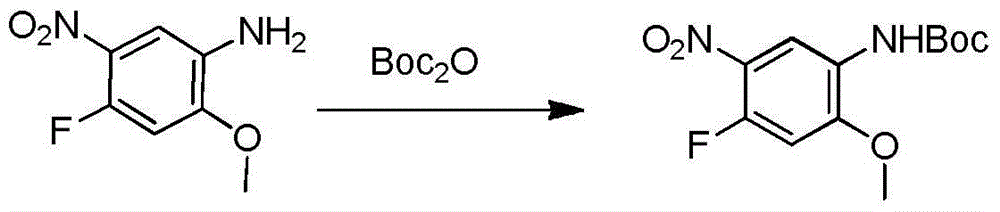
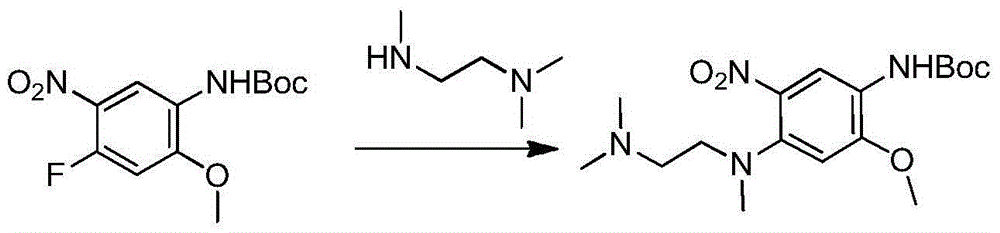
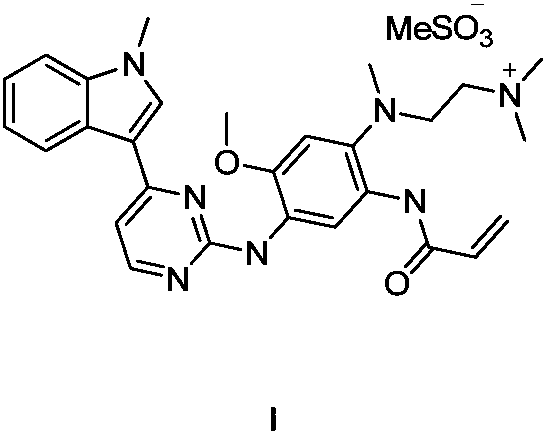
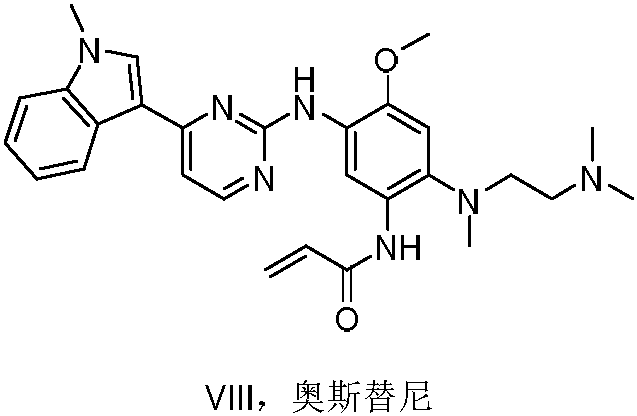
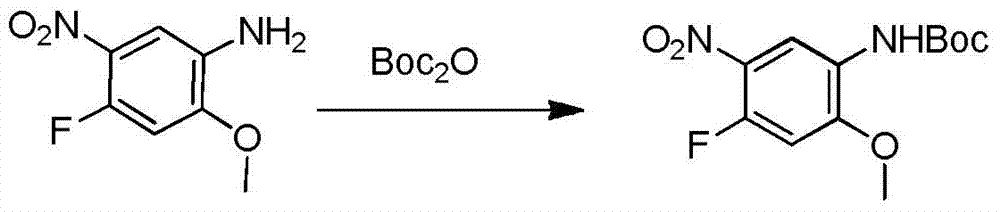
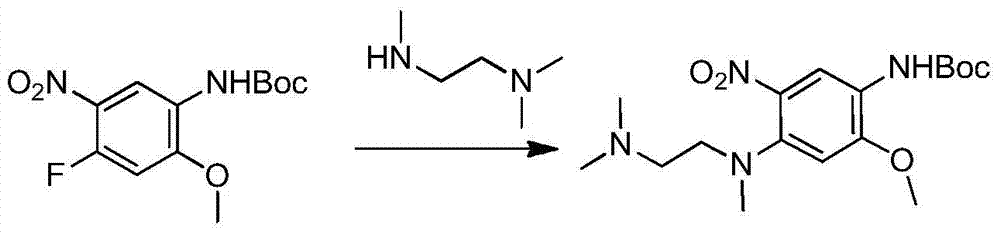
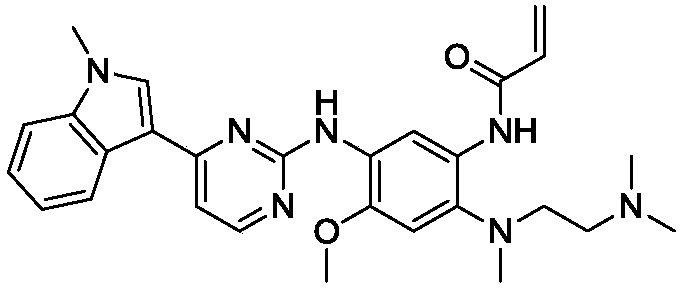
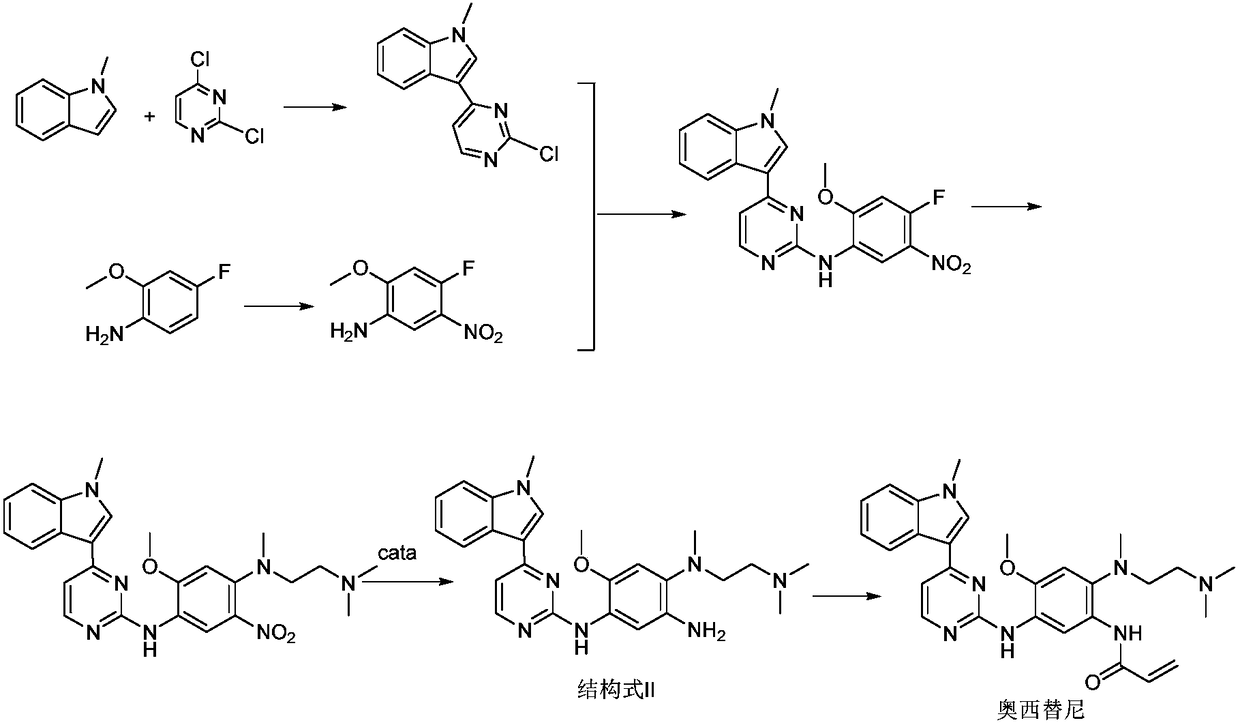
The invention relates to a synthetic method of an anti-tumor medicine, namely N-[2-[[2-(dimethylamino) ethyl] methyl amino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3-yl)-2-pyrimidyl] amino] phenyl]-2-acrylamide (AZD9291) and a key intermediate of the anti-tumor medicine. The synthetic method comprises the following steps: performing Boc acid anhydride protection on 4-fluoro-2-methoxy-5-nitroaniline to obtain 4-fluoro-2-methoxy-5-nitroanilino tert-butyl formate, then reacting with N,N,N'-trimethylethylenediamine to obtain 4-(N,N,N'-trimethylethylenediamino)-2-methoxy-5-nitroanilino tert-butyl formate, then reducing to obtain 2-(N,N,N'-trimethylethylenediamino)-4-methoxy-5-tert-butyl carbamate phenylamine, then completely reacting with acryloyl chloride and directly removing a Boc protecting group to obtain 2-methoxy-4-N,N,N'-trimethylethylenediamino-5-acrylamido phenylamine, and finally reacting with 3-(2-chloropyrimidine-4-yl)-1-methylindole to obtain AZD9291. A process disclosed by the invention is simple in step, relatively high in yield, mild in reaction condition and easy for realization of industrial production.
Owner:苏州东南药业股份有限公司
Therapeutic agent for bile duct cancer
PendingUS20180015079A1Effective treatmentOrganic active ingredientsDigestive systemMethyl-1H-indoleMethyl group
The present invention provides a therapeutic agent for bile duct cancer comprising 5-((2-(4-(1-(2-hydroxyethyl)piperidin-4-yl)benzamide)pyridin-4-yl)oxy)-6-(2-methoxyethoxy)-N-methyl-1H-indole-1-carboxamide or a pharmacologically acceptable salt thereof.
Owner:NAT CANCER CENT +1
Therapeutic agent for breast cancer
PendingUS20180303817A1Reduce tumor volumeOrganic active ingredientsAntineoplastic agentsMethyl-1H-indoleMethyl group
The present application discloses a therapeutic agent for breast cancer, comprising 5-((2-(4-(1-(2-hydroxyethyl)piperidin-4-yl)benzamide)pyridin-4-yl)oxy)-6-(2-methoxyethoxy)-N-methyl-1H-indole-1-carboxamide or a pharmacologically acceptable salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
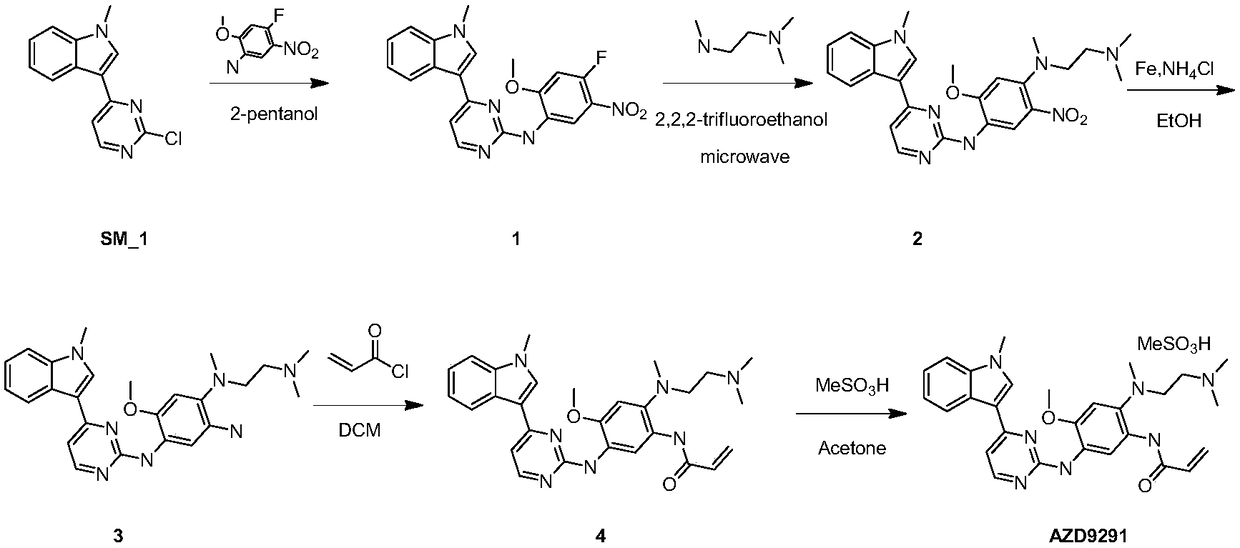
Osimertinib preparation method
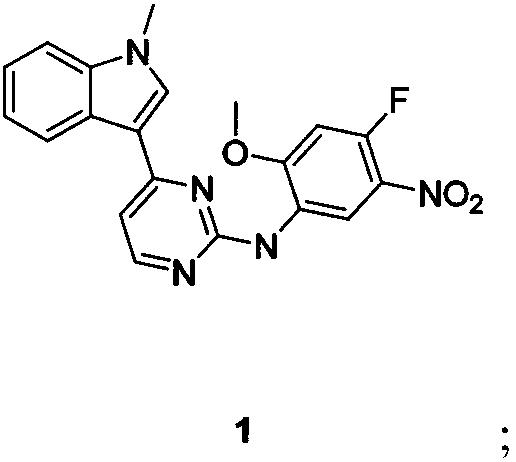
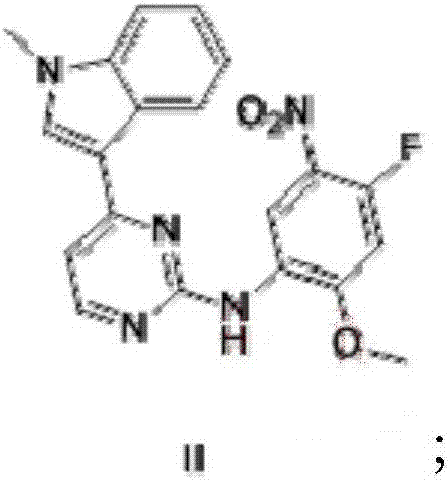
The present invention relates to an osimertinib preparation method, which comprises: (1) carrying out a nucleophilic substitution reaction on 3-(2-chloro-4-pyrimidinyl)-1-methyl-1H-indole and 4-fluoro-2-methoxy-5-nitroaniline to prepare a compound 1; (2) carrying out a substitution reaction on the compound 1 and N,N,N-trimethylethylenediamine in the presence of an organic alkali to prepare a compound 2; (3) reducing the compound 2 in the presence of a reducing agent to prepare a compound 3; (4) carrying out condensation on the compound 3 and 3-chloropropionyl chloride in the presence of an alkali to obtain a compound 4; (5) carrying out a heat elimination reaction on the compound 4 through heating in the presence of an alkali to prepare a compound 5; and (6) carrying out salification on the compound 5 and methanesulfonic acid under a heating condition to obtain osimertinib. According to the present invention, the osimertinib synthesis process has characteristics of stability, controllability, no high-toxicity solvent is used, energy saving and environmental protection.
Owner:上海赛诺克医药科技有限公司
Preparation method of pyridylaminopyrimidine derivative and intermediate thereof
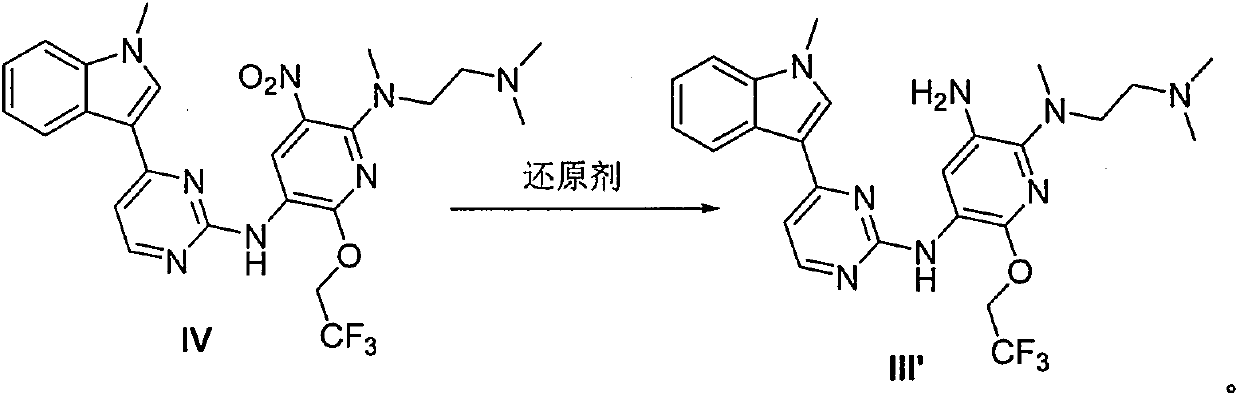
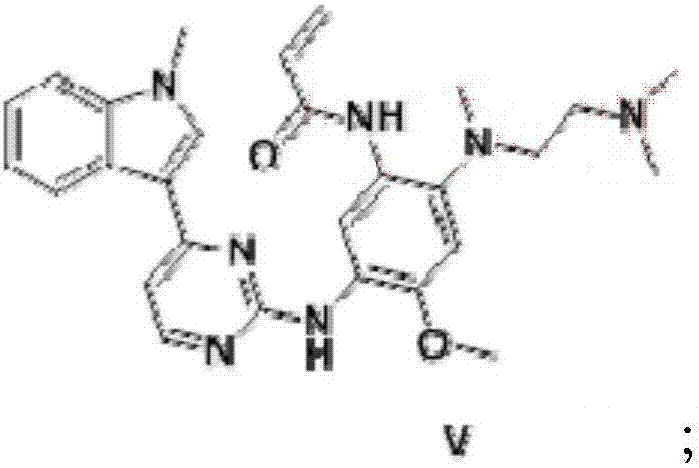
The invention provides a preparation method of a compound 2-[2-(dimethylaminoethyl) methylamino]-3-acrylamido-5-[4-(1-methyl-1H-indole-3-yl) pyrimidine-2-amino]-6-(2, 2, 2-trifluoroethoxy) pyridine asshown in a formula I, a used intermediate and a preparation method of a related intermediate. According to the method, 3-(2-chloropyrimidine-4-yl)-1-methyl-1H-indole and a compound shown as a formulaVII are subjected to a condensation reaction, a substitution reaction, a reduction reaction, an acylation reaction and an elimination reaction, and a compound shown as a formula I is obtained. The preparation method disclosed by the invention is environment-friendly, low in cost, mild in condition, simple to operate, high in yield, high in final product purity and suitable for industrial production.
Owner:SHANGHAI ALLIST PHARM CO LTD +1
Synthesis method of Osimertinib
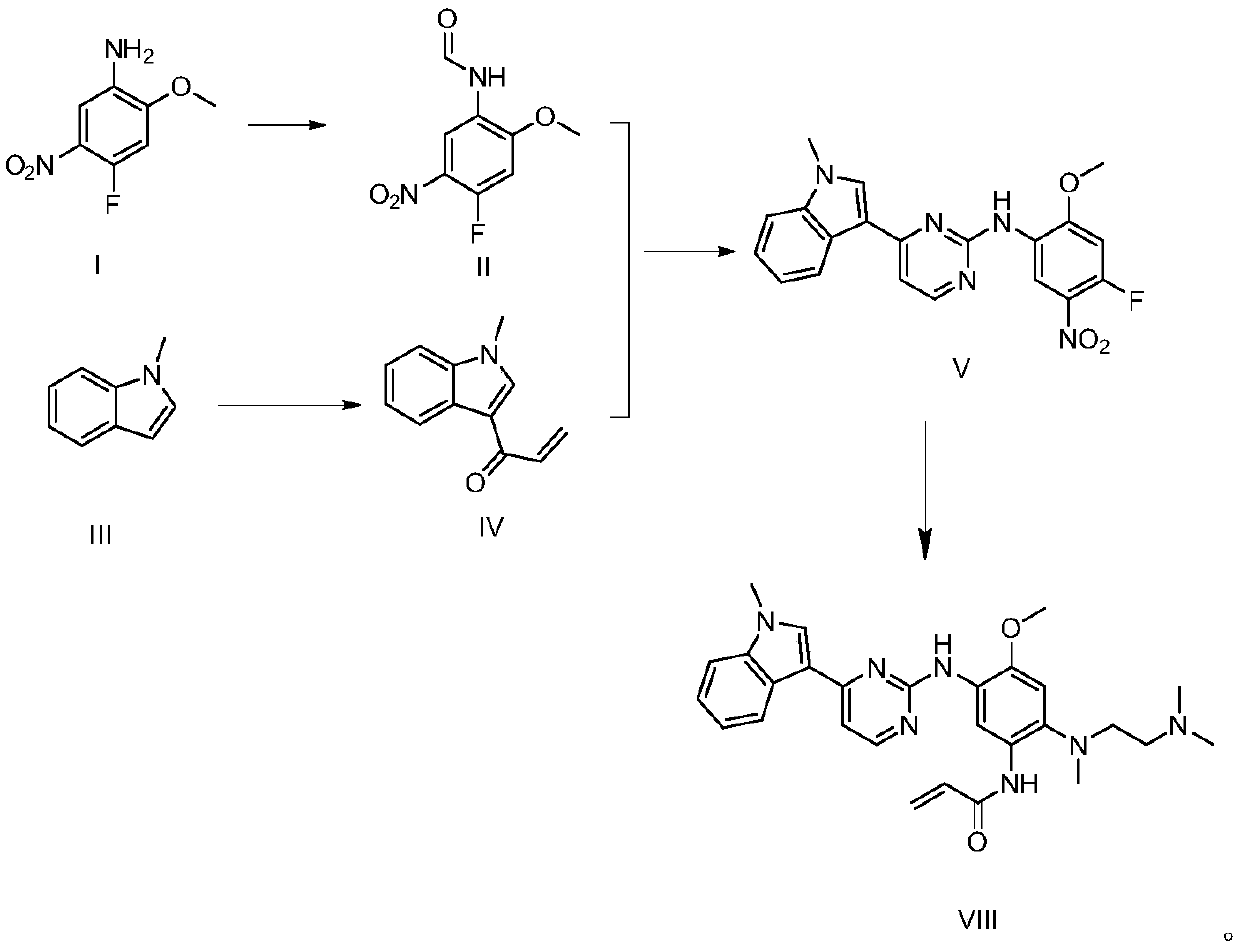
The invention discloses a synthesis method of Osimertinib. The method comprises the following steps that (1) 4-fluoro-2-methoxyl-5-nitroaniline is subjected to formylation to obtain N-(4-fluoro-2-methoxyl-5-nitrophenyl) methanamide; (2) N-methylindole is subjected to acylation reaction to prepare 3-(1-methyl-1H-indole-3-yl)prop-2-en-1-one; (3) products generated in the former two-step reactions are subjected to one-step cyclization to obtain 2-(4-fluoro-2- methoxyl-5- nitroanilino)-4-(1-methyl-1H-indole-3-yl) pyrimidine; (4) the product generated in the step (3) is sequentially subjected to nucleophilic substitution reaction, reduction reaction and condensation amidation reaction to obtain the Osimertinib. The method provided by the invention has the advantages that the raw materials can be easily obtained; the steps are simple; the yield is high; the reaction conditions are mild; the synthesis method is suitable for industrial production.
Owner:安庆奇创药业有限公司
Method for preparing Sumatriptan Succinate
This invention discloses a method for preparing sumatriptan succinate. The method comprises: (1) reacting 4-chlorobutyraldehyde and sodium pyrosulfite aqueous solution, filtering and drying to obtain sodium 4-chlorobutane-1,1-disulfonate; (2) heating and reacting 4-hydrazino-N-methyl phenylmethansulfonamide and 4-chlorobutane-1,1-disulfonate in the presence of inorganic acid or organic acid catalyst, neutralizing with an alkali solution, and extracting with an organic solvent to obtain 3-(2-chloroethyl)-N-methyl-1H-indole-5-methansulfonamide; (3) adding phase transfer catalyst into 3-(2-chloroethyl)-N-methyl-1H-indole-5-methansulfonamide, reacting with dimethylamine, extracting, decolorizing, refining to obtain sumatriptan product, dissolving in a solvent under heating, adding succinic acid, reacting, and precipitating the crystal to obtain white sumatriptan succinate. The method has such advantages as short process, easy operation, high yield, low cost and stable product quality.
Owner:ZHEJIANG SUPOR PHARM CO LTD
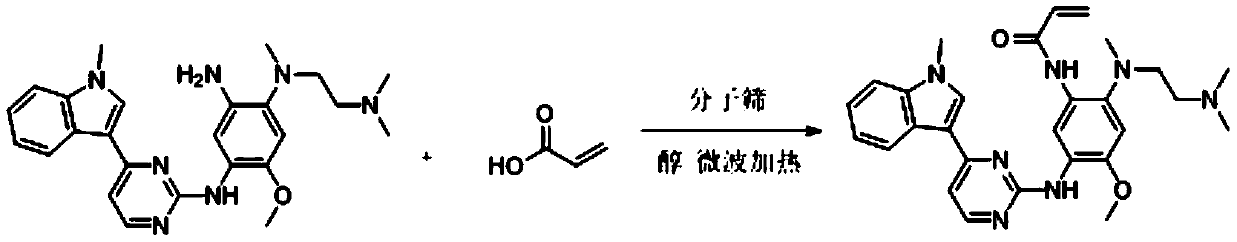
Method for synthesizing osimertinib by molecular sieve catalysis
The invention relates to the field of organic synthesis, in particular to a novel method for synthesizing osimertinib through molecular sieve catalysis. N-1-[2-(dimethylamino) ethyl]-5-methoxy-N1-methyl-N4-[4-(1-methyl-1H-indole-3-yl-2-pyrimidinyl]-1,2,4-triaminobenzene and acrylic acid as raw materials, acrylic acid is added into an alcohol solvent, reaction is carried out through molecular sievecatalysis and microwave heating, and after the reaction is completed, post-treatment is carried out to obtain the osimertinib. According to the method, the reaction conditions are mild, industrial production is facilitated, the raw materials are environmentally friendly in use, used highly toxic raw materials are decreased, the yield is high, and the reaction efficiency is high.
Owner:滨州市鸿源工程有限公司
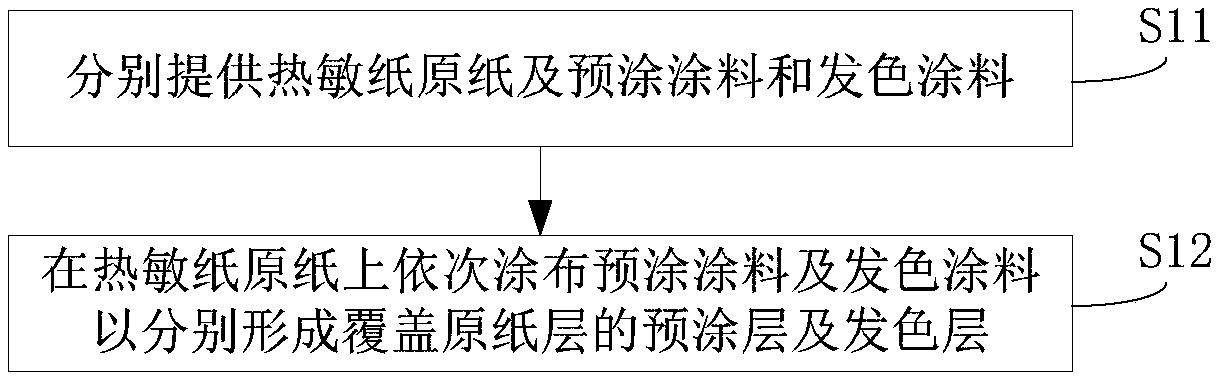
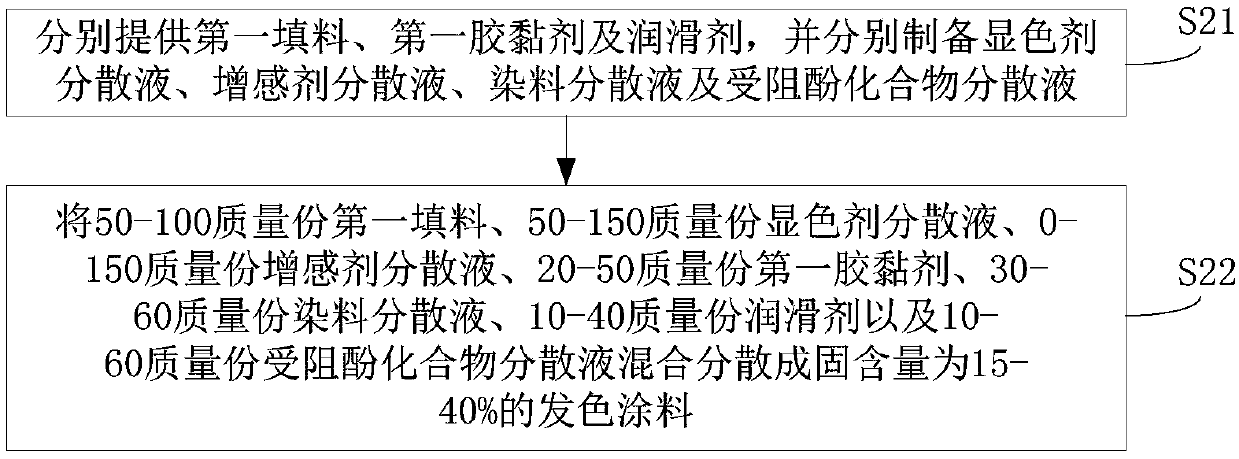
Thermosensitive paper and preparation method thereof
The invention discloses thermosensitive paper and a preparation method thereof. The thermosensitive paper comprises a base paper layer, a precoating layer and a chromogenic layer, wherein the precoating layer and the chromogenic layer are successively formed on the base paper layer; the chromogenic layer is formed by coating with a chromogenic coating, and a chromogenic coating comprises a dye, wherein the dye is at least one of crystal violet lactone, 10-benzoyl-3,7-bis(dimethylamino)phenothiazine, and 7-[4-(diethylamino)-2-(hexyloxy)phenyl]-7-(1-ethyl-2-methyl-1H-indole-3-yl)furano[3,4-B]pyridine-5(7H)-one. Through the method, the amount of a dye and a color developer in the thermosensitive paper can be substantially reduced, and thus adhesion of the dye and the color developer, on a printing head, in the thermosensitive paper in the printing process is reduced, and the printing head is protected to a certain extent.
Owner:GOLD HUASHENG PAPER SUZHOU IND PARK
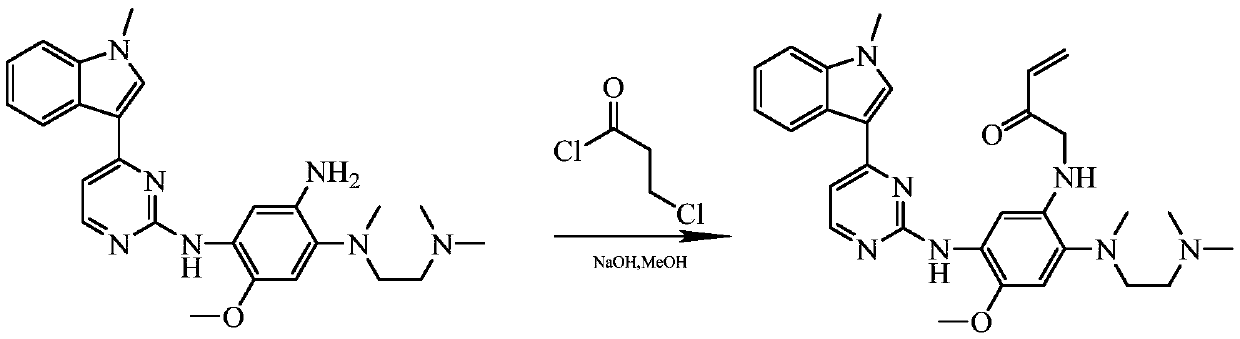
Method for preparing osimertinib mesylate
InactiveCN107188888AImprove protectionLow costSulfonic acids salts preparationEthylenediamineMethyl-1H-indole
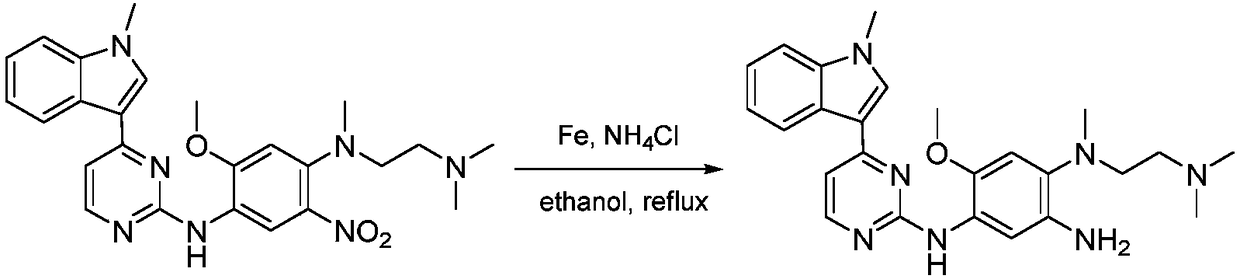
The invention discloses a method for preparing Osimertinib mesylate. The method comprises the steps that after 3-(2-chlorine-4-pyrimidinly)-1-methyl-1H-indole is subjected to substitution reaction with 4-fluorine-2-methoxy-5-nitroaniline, 3-(2-chlorine-4-pyrimidinly)-1-methyl-1H-indole is subjected to substitution reaction with N,N,N'-trimethyl-ethylenediamine, nitro is restored after catalytic hydrogenation, then 3-(2-chlorine-4-pyrimidinly)-1-methyl-1H-indole is subjected to coupling reaction with 3-chloropropionyl-chloride, osimertinib is obtained after elimination with sodium hydroxide, and osimertinib reacts with salt mesylate in acetone and water to obtain osimertinib mesylate, wherein a catalyst for restoring nitro by the catalytic hydrogenation is raney nickel, palladium carbon or palladium-carbon hydroxide. The method for preparing osimertinib mesylate is low in cost, high in yield, little in environmental pollution, simple and easy in production process operation and suitable for industrialized production.
Owner:LUOXIN PHARM SHANGHAI CO LTD +1
Industrial production method for bazedoxifene acetate
ActiveCN107793344AQuality improvementHigh yieldCarboxylic acid salt preparationAcetic acidBenzaldehyde
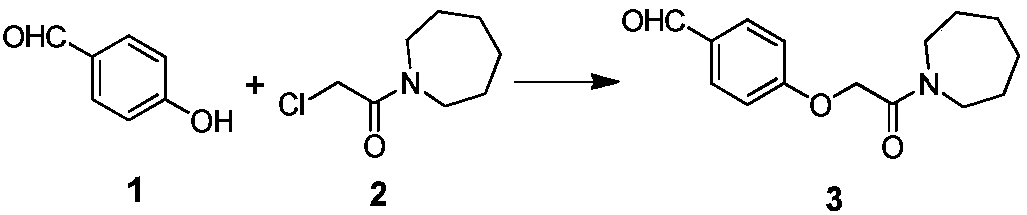
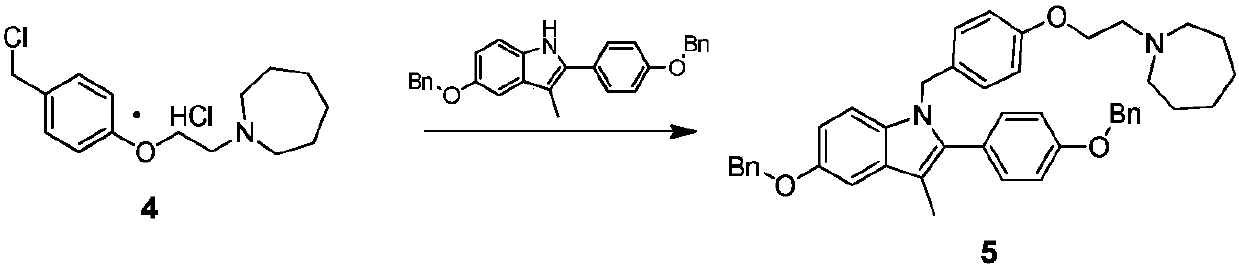
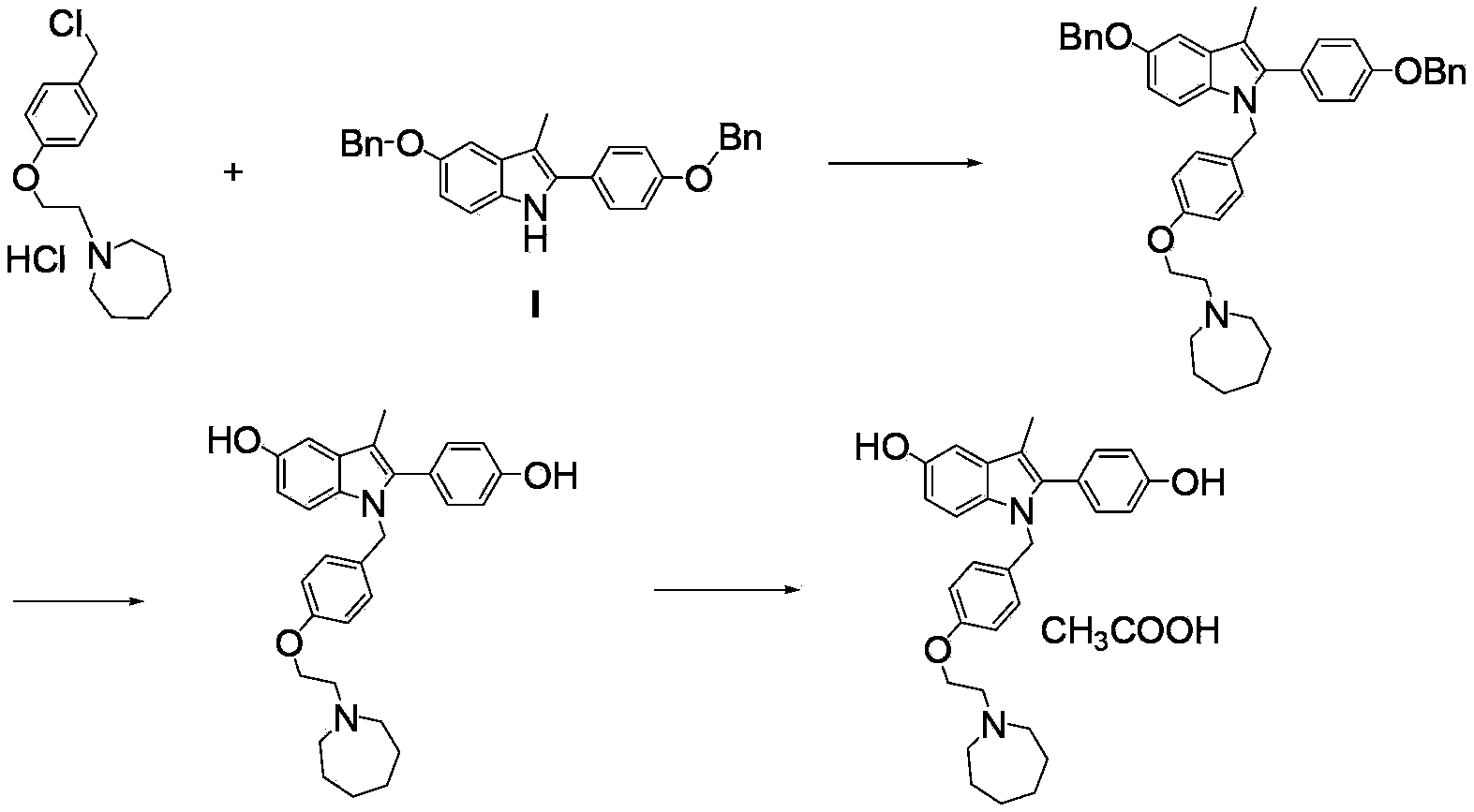
The invention discloses an industrial production method for bazedoxifene acetate. The production method comprises the following steps: taking p-hydroxy benzaldehyde as a starting material, substituting with chloracetyl-hexamethyleneimine, reducing with borohydride and chlorinating with a chlorinating agent to obtain a compound 4; reacting the compound 4 with 5-(benzyloxy)-2-(4-(benzyloxy)phenyl)-3-methyl-1H-indole to obtain a compound 5; and carrying out debenzylation to obtain a compound 6, and salifying with acetic acid to obtain a target compound which is the bazedoxifene acetate. The defective workmanship of preparation in the prior art is solved, the used reagent is low in cost and easy to obtain, environmental pollution is small, safety is high, an operation process is simple, and thus, the bazedoxifene acetate is suitable for being produced industrially.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Intermediates and processes for the preparation of 4- (acetylamino) ) -3- [ (4-chloro-phenyl) thio] -2-methyl-1h-indole-1-acetic acid
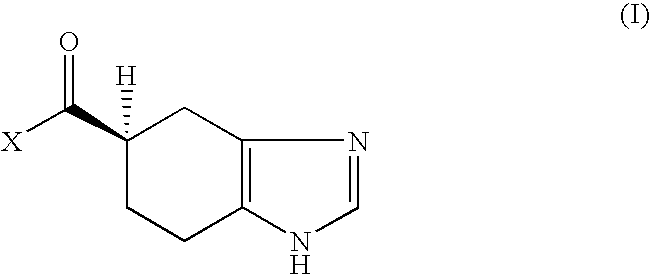
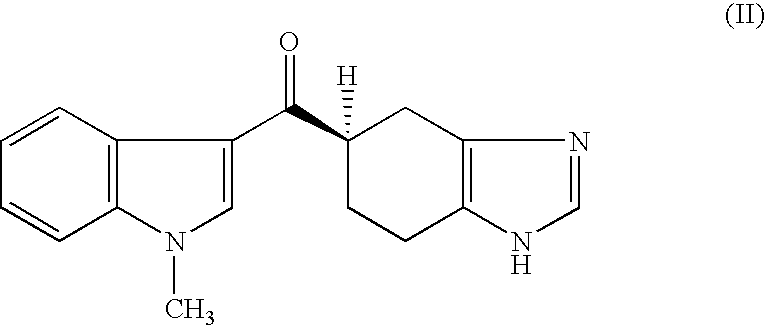
The invention relates to compounds of formula (X), and salts thereof, and their use as intermediates in improved manufacturing processes for the synthesis of pharmaceutical compound of formula (I): X is =0, =N-0H or =N-OC(O)Me; Y is hydrogen, PhS- or p-chlorophenylsulfanyl; Z is hydrogen or -CH2COOR1 wherein R1 is selected from hydrogen, optionally substituted hydrocarbyl and optionally substituted heterocyclyl.
Owner:ASTRAZENECA AB
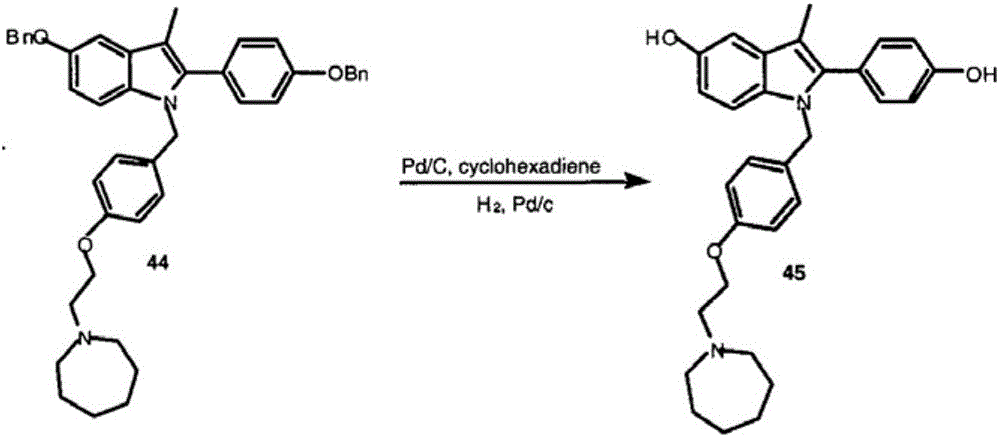
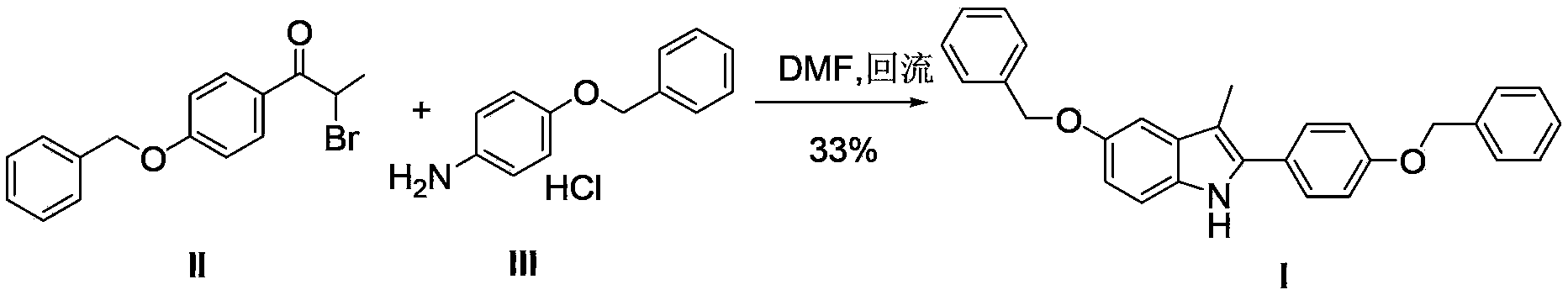
Process for the preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
The present invention is related to a process for the preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-Indole (formula-1, a useful intermediate for the synthesis of bazedoxifene) using 4-benzyloxy propiophenone and 4-benzyloxy phenyl hydrazine hydrochloride.
Owner:DIVI S LAB LTD
Efficient preparation method of bazedoxifene
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
A kind of synthetic method of antitumor drug
ActiveCN104817541BHigh yieldMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationCarbamateMethyl-1H-indole
The invention relates to a synthetic method of an anti-tumor medicine, namely N-[2-[[2-(dimethylamino) ethyl] methyl amino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3-yl)-2-pyrimidyl] amino] phenyl]-2-acrylamide (AZD9291) and a key intermediate of the anti-tumor medicine. The synthetic method comprises the following steps: performing Boc acid anhydride protection on 4-fluoro-2-methoxy-5-nitroaniline to obtain 4-fluoro-2-methoxy-5-nitroanilino tert-butyl formate, then reacting with N,N,N'-trimethylethylenediamine to obtain 4-(N,N,N'-trimethylethylenediamino)-2-methoxy-5-nitroanilino tert-butyl formate, then reducing to obtain 2-(N,N,N'-trimethylethylenediamino)-4-methoxy-5-tert-butyl carbamate phenylamine, then completely reacting with acryloyl chloride and directly removing a Boc protecting group to obtain 2-methoxy-4-N,N,N'-trimethylethylenediamino-5-acrylamido phenylamine, and finally reacting with 3-(2-chloropyrimidine-4-yl)-1-methylindole to obtain AZD9291. A process disclosed by the invention is simple in step, relatively high in yield, mild in reaction condition and easy for realization of industrial production.
Owner:苏州东南药业股份有限公司
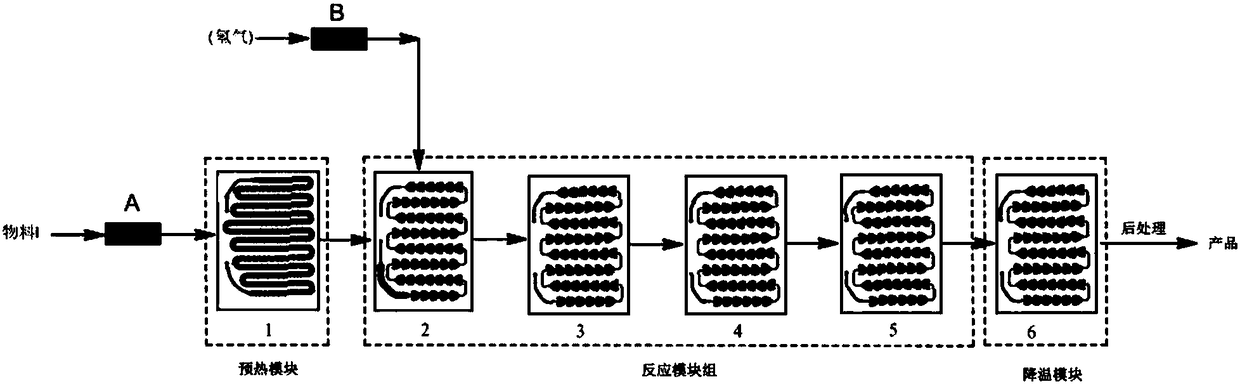
Method for synthesizing osimertinib intermediate through micro-channel reactor
InactiveCN108484579AQuick responseSlow reaction speedOrganic chemistryOrganic synthesisMethyl-1H-indole
The invention discloses a method for synthesizing an osimertinib intermediate through a micro-channel reactor and belongs to the technical field of anticancer drug synthesis in organic synthesis. Aiming at the problems in the traditional high-pressure catalytic hydrogenation reactor synthesis process that the yield is low, the purity is low, violent explosion easily occurs to cause danger, and thecatalyst recycling and reusing frequency is low, the method for synthesizing the osimertinib intermediate through the micro-channel reactor is provided. The osimertinib intermediate is N-1-[2-(dimethylamino)ethyl]-5-methoxy-N1-methyl-N4-[4-(1-methyl-1H-indole-3-yl)-2-pyrimidyl]-1,2,4-triaminobenzene. The method comprises the following synthesis steps: adding N-(2-(dimethylamino-ethyl)-2-methoxy-N-(4-(1-methyl-1-indole-3-yl)-pyrimidyl-2-yl)-5-nitro-phenyl-1,4-diamine into a mixture of an organic solvent and concentrated hydrochloric acid in a microchannel reactor, adding an activated carbon supported noble metal catalyst, and preheating to form a material I; performing reaction on hydrogen and the preheated material I, thereby obtaining the osimertinib intermediate. The method is applicable to synthesis of anticancer drugs.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
Compression coated tablets
A pharmaceutical composition for oral administration comprising a compression coated solid dosage form of a bitter or unpleasant tasting pharmaceutically active agent. Included are compression coated oral dosage forms of 3-[2-(dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulphonamide or a pharmaceutically acceptable salt or solvate thereof as active ingredient. The compression coated solid dosage forms are of use in the treatment of conditions associated with cephalic pain, in particular migraine.
Owner:GUO MINTONG +3
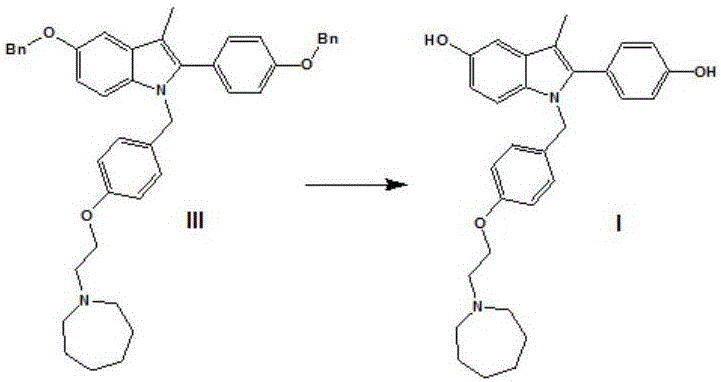
The preparation method of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1h-indole
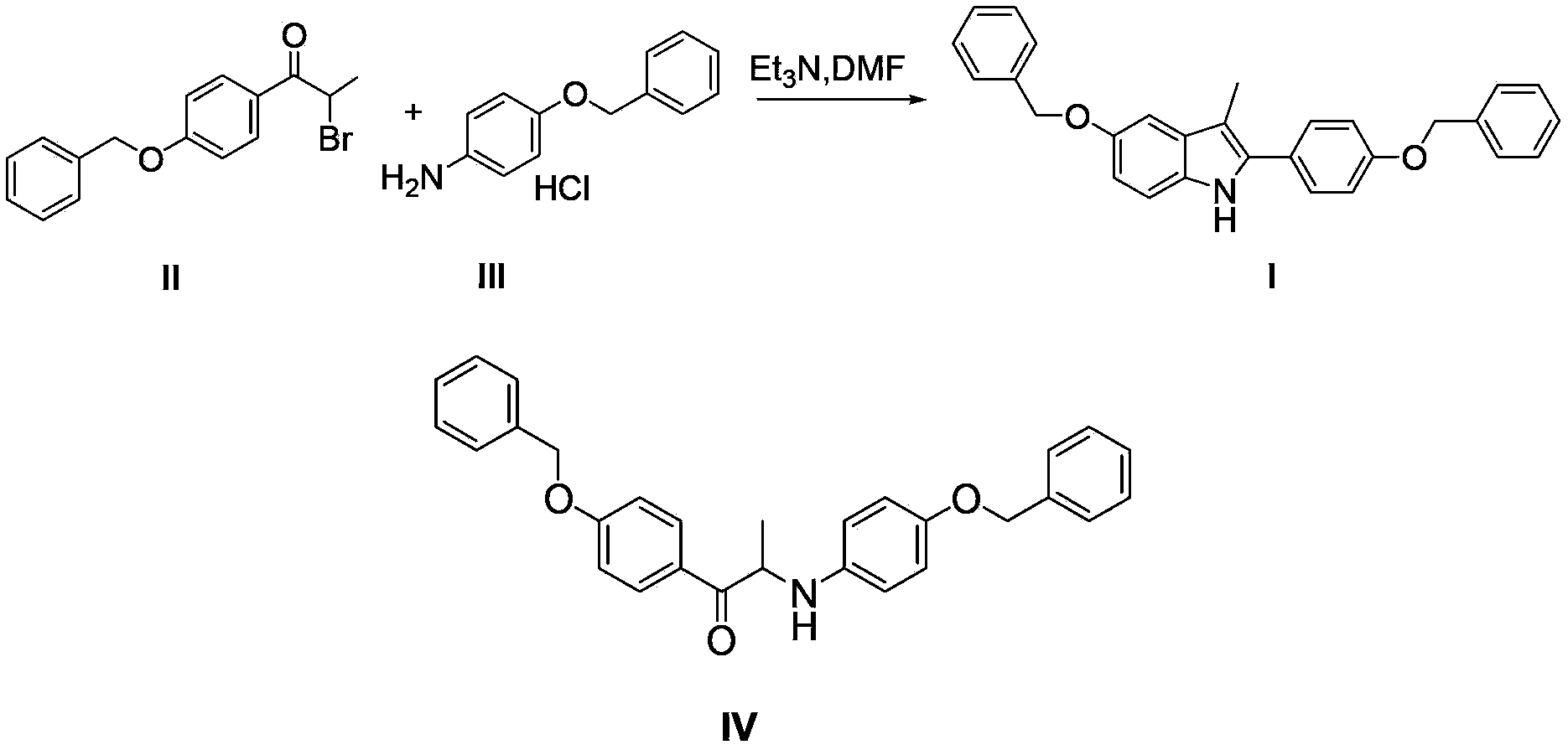
The invention discloses a preparation method for 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole as shown in the specification. The preparation method comprises the following steps: (1) subjecting a compound II and a compound III to a condensation reaction in an alcohol solvent under the action of alkali; and (2) mixing a reaction solution obtained in the step (1) with protonic acid and carrying out Bischler-Mohlau indole cyclization. Compared with the prior art, the invention has the following advantages: the condensation reaction in the first step and Bischler-Mohlau indole cyclization at the second step are integrated into a one-pot reaction, so operation is simple and convenient; a reaction product I is directly precipitated from the reaction solvent, so reaction post-treatment is substantially simplified, and high purity and high reaction yield are realized; and the method is applicable to enlarged production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
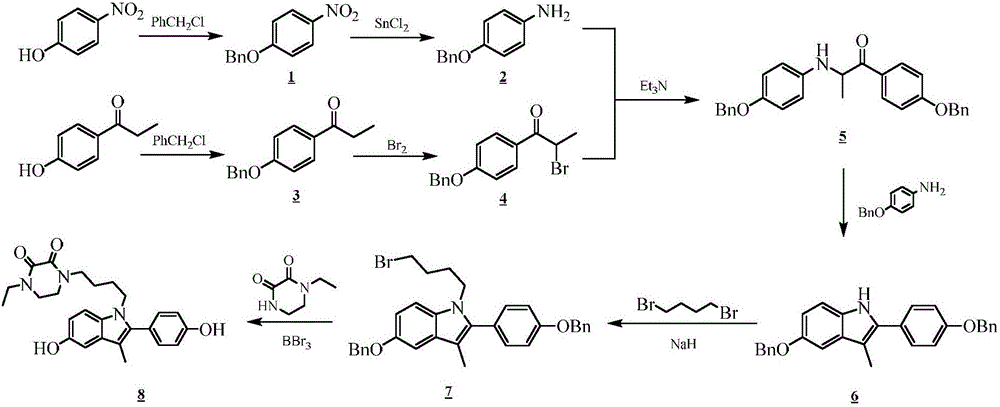
Piperazinone compounds and application thereof
ActiveCN105801564AEasy to prepareHigh yieldOrganic active ingredientsOrganic chemistryHormone Receptor ModulatorsHectic fever
The invention belongs to the technical field of medicine and relates to 1-ethyl-4-[4-[5-hydroxy-2-(4-hydroxyphenyl)-3-methyl-1H-indole-1-yl]butyl]piperazine-2,3-dione as well as a medical application, a stereoisomer and pharmaceutically acceptable salt thereof. The structural formula of 1-ethyl-4-[4-[5-hydroxy-2-(4-hydroxyphenyl)-3-methyl-1H-indole-1-yl] butyl]piperazine-2,3-dione is represented in the specification; 1-ethyl-4-[4-[5-hydroxy-2-(4-hydroxyphenyl)-3-methyl-1H-indole-1-yl]butyl]piperazine-2,3-dione and pharmaceutically acceptable acid addition salt of the compound can be combined with existing drugs or can be independently used as an estrogen receptor modulator for treating or preventing various estrogen function related diseases such as bone loss, fracture, osteoporosis, hectic fever, LDL cholesterol level rise, cardiovascular disease, cognitive impairment, brain degeneration disease and anxiety, as well as depression, sexual dysfunction, hypertension, retinal degeneration and cancer caused by estrogen deficiency, especially osteoporosis.
Owner:SHENYANG PHARMA UNIVERSITY
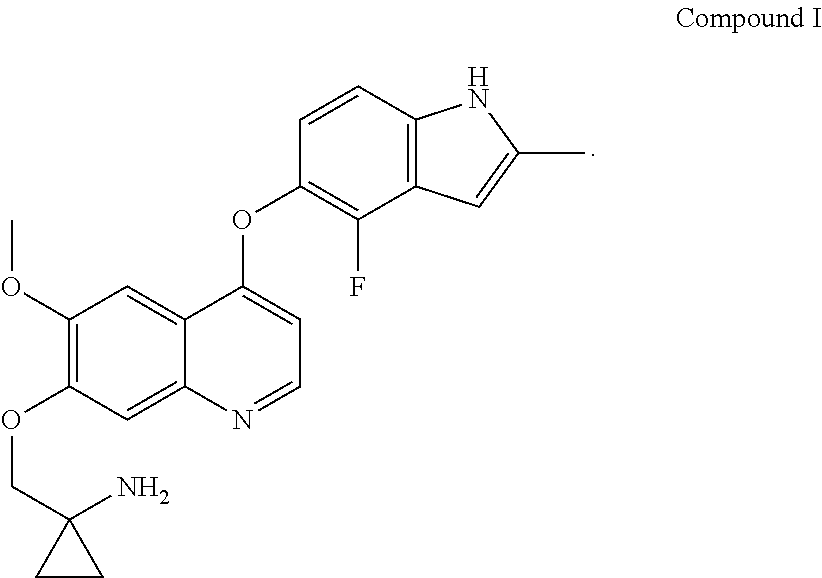
Use of quinoline derivatives for treating oesophageal cancer and treatment method, pharmaceutical composition and kit thereof
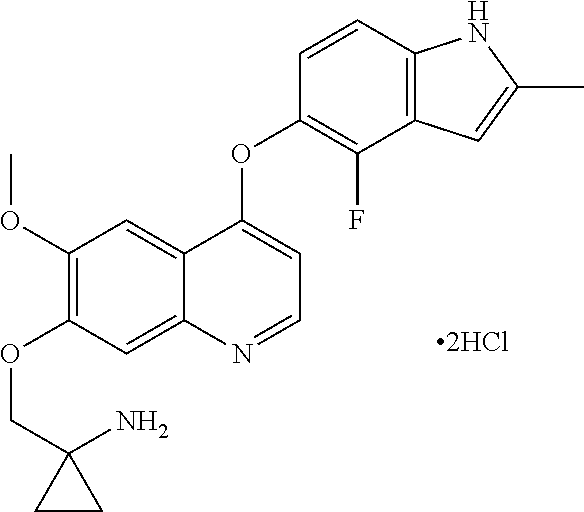
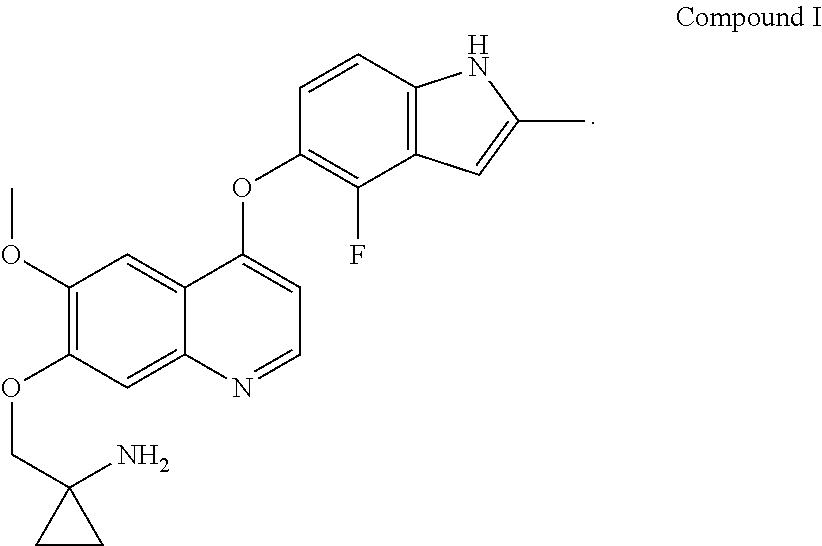
Provided in the present invention are a use of quinoline derivatives for treating oesophageal cancer and a treatment method, a pharmaceutical composition and a kit thereof. The 1-[[[4-(4-fluoro-2-methyl-1H-indole-5-yl)oxy-6-methoxyquinoline-7-yl]oxy]methyl]cyclopropylamine provided by the present invention can effectively treat oesophageal cancer, and reduce the sum of the diameters of patient's target lesions.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Quinoline for treating cholangiocarcinoma
The invention provides quinoline for treating cholangiocarcinoma, and an application thereof to preparation of a medical composition for treating tumors, and particularly relates to an application ofquinoline derivative 1-[[[4-(4-fluoro-2-methyl-1H-indole -5-)oxy-6-methoxyquinolin-7-yl]oxy]methyl cyclopropylamine to treatment cholangiocarcinoma: as shown in the description.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
A novel process for preparation of indole derivatives
Owner:POTLURI RAMESH BABU
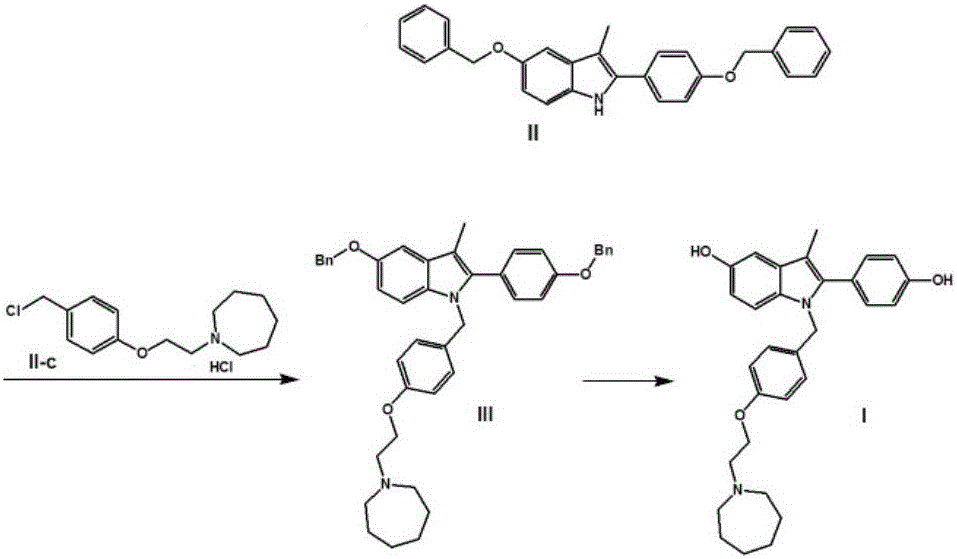
Preparation method for 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
The invention discloses a preparation method for 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole as shown in the specification. The preparation method comprises the following steps: (1) subjecting a compound II and a compound III to a condensation reaction in an alcohol solvent under the action of alkali; and (2) mixing a reaction solution obtained in the step (1) with protonic acid and carrying out Bischler-Mohlau indole cyclization. Compared with the prior art, the invention has the following advantages: the condensation reaction in the first step and Bischler-Mohlau indole cyclization at the second step are integrated into a one-pot reaction, so operation is simple and convenient; a reaction product I is directly precipitated from the reaction solvent, so reaction post-treatment is substantially simplified, and high purity and high reaction yield are realized; and the method is applicable to enlarged production.
Owner:SHANGHAI INST OF PHARMA IND +2
Preparation of biomass derived palladium catalyst and application to synthesis of anti-tumor drug osimertinib
ActiveCN108187666AWide variety of sourcesLow priceOrganic chemistryCatalyst activation/preparationDispersitySolvent
The invention relates to a preparation method of a biomass derived palladium catalyst and application to synthesis of an anti-tumor drug osimertinib. The preparation method comprises the following process: enabling chitosan and palladium salt to react in the presence of a solvent to form a complex; then cooling to room temperature; drying a solid in vacuum to remove a solvent; under the protectionof argon gas, carbonizing at high temperature to obtain the catalyst; dissolving a raw material N-(2-dimethylamino-ethyl-2-methoxy-N-methyl-N-[4-(1-methyl-1H-indole-3-yl-pyrimidine-2-yl)-5-nitryl-benzene-1,4-diamine and adding the catalyst; stirring at temperature of 0 to 120 DEG C for 5 to 12h; dropwise adding hydrazine hydrate; after completely reacting, cooling to room temperature and filtering; spinning and drying the solvent to obtain a faint yellow solid. The preparation method provided by the invention has the advantages of wide raw material source, relatively low price, moderate reaction conditions and high yield; the catalyst has high activity, high stability, high dispersity of active components and long service life; the catalyst can be repeatedly used for a plurality of timesand the preparation method also has the advantages of short flow, easiness for controlling reaction and simple equipment requirements; complicated post-treatment is avoided and environment pollution is avoided.
Owner:HANGZHOU LUPU BIOTECH CO LTD
Novel Process for Producing Ramosetron or Its Salt
InactiveUS20090069570A1High optical purityHigh yieldOrganic active ingredientsBiocideHalogenMedicine
[Problems] To provide a novel process for producing ramosetron or its salt that is useful as a pharmaceutical, especially as a therapeutic and / or preventive agent for digestive symptoms caused by administration of an anti-malignant tumor agent, diarrheal-type irritable bowel syndrome, diarrheal symptoms of irritable bowel syndrome, etc.[Means for Resolution] Ramosetron or its salt can be produced by reacting a compound of the formula (I):[wherein X is a halogen] or a salt thereof with 1-methyl-1H-indole in the presence of a Lewis acid selected from the group consisting of a lower alkylaluminum dihalide, a di-lower alkylaluminum halide, a tri-lower alkylaluminum and a lower alkylaluminum sesquihalide.
Owner:ASTELLAS PHARMA INC
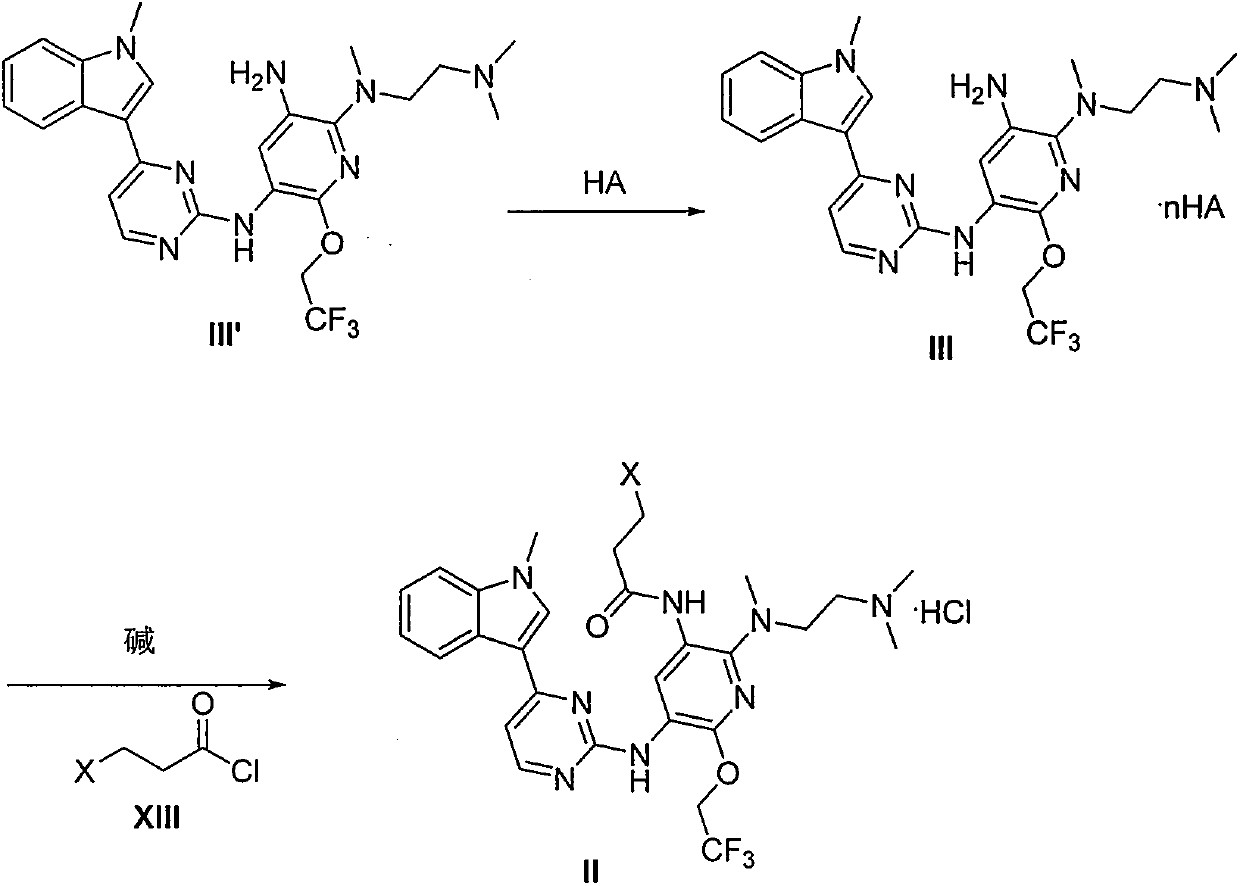
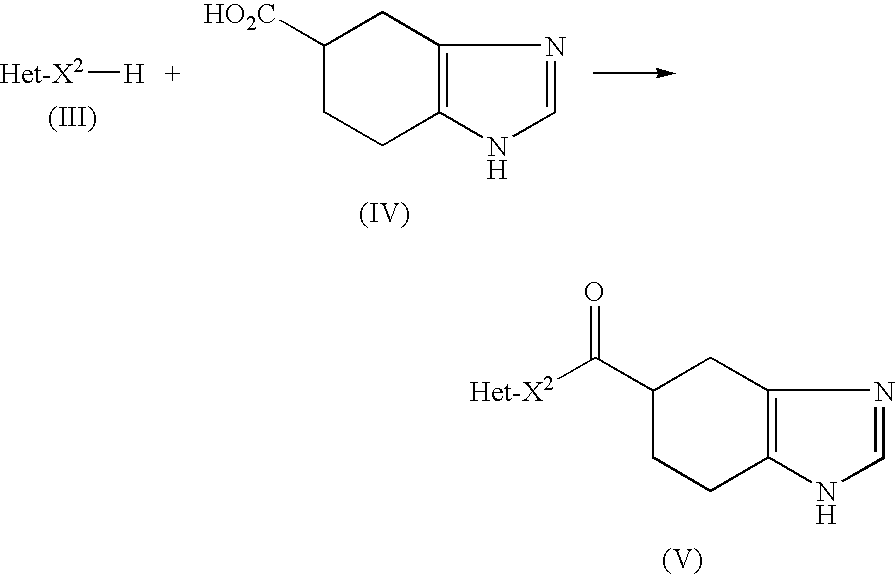
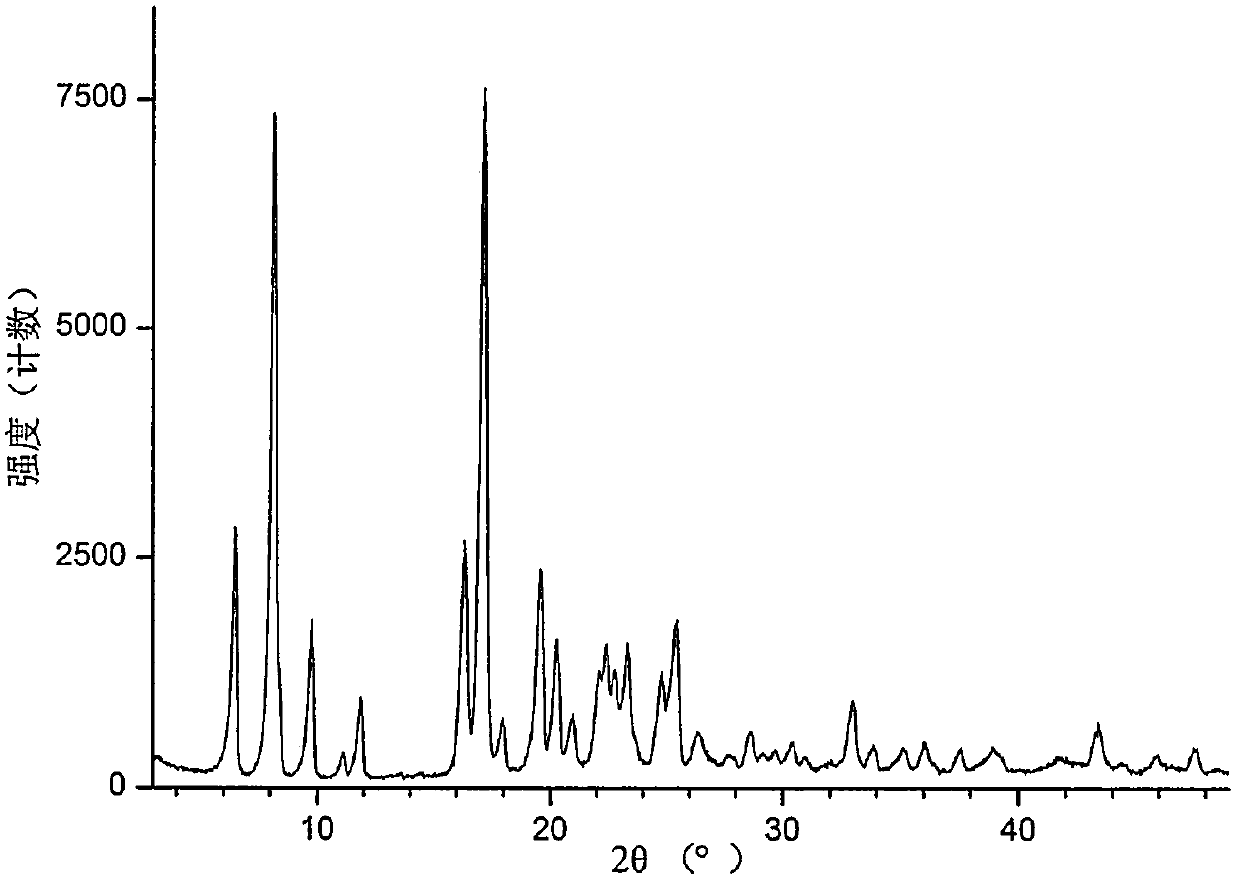
Crystal form of pyridylaminopyrimidine derivative and preparation method thereof
InactiveCN110606841AImprove stabilityGood repeatabilityOrganic active ingredientsOrganic chemistry methodsMethyl-1H-indolePyridine
The invention discloses a crystal form of N-{2-{[2-(dimethylamino)ethyl](methyl)amido}-6-(2,2,2-trifluoroethoxy)-5-{[4-(1-methyl-1H-indole-3-yl)pyrimidine-2-yl]amido}pyridine-3-yl}acrylamide as well as a preparation method and an application thereof, wherein the X-ray powder diffraction pattern of the crystal form has characteristic peaks when diffraction angles 2[theta] are 6.5 + / -0.2 degrees, 8.2 + / -0.2 degrees, 9.8 + / -0.2 degrees, 11.9 + / -0.2 degrees, 16.4 + / -0.2 degrees, 17.2 + / -0.2 degrees, 19.6 + / -0.2 degrees and 20.3 + / -0.2 degrees. The crystal form is good in stability, simple in preparation method, good in repeatability and suitable for industrial production.
Owner:SHANGHAI ALLIST PHARM CO LTD
Method for synthesizing natural product (+/-)-folicanthine and intermediate product thereof
InactiveCN102329320BReduce pollutionFew reaction stepsOrganic chemistryFolicanthineMethylene Dichloride
Owner:LANZHOU UNIVERSITY
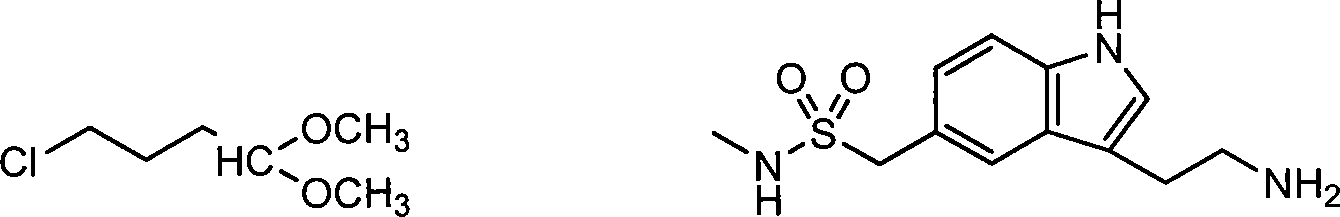
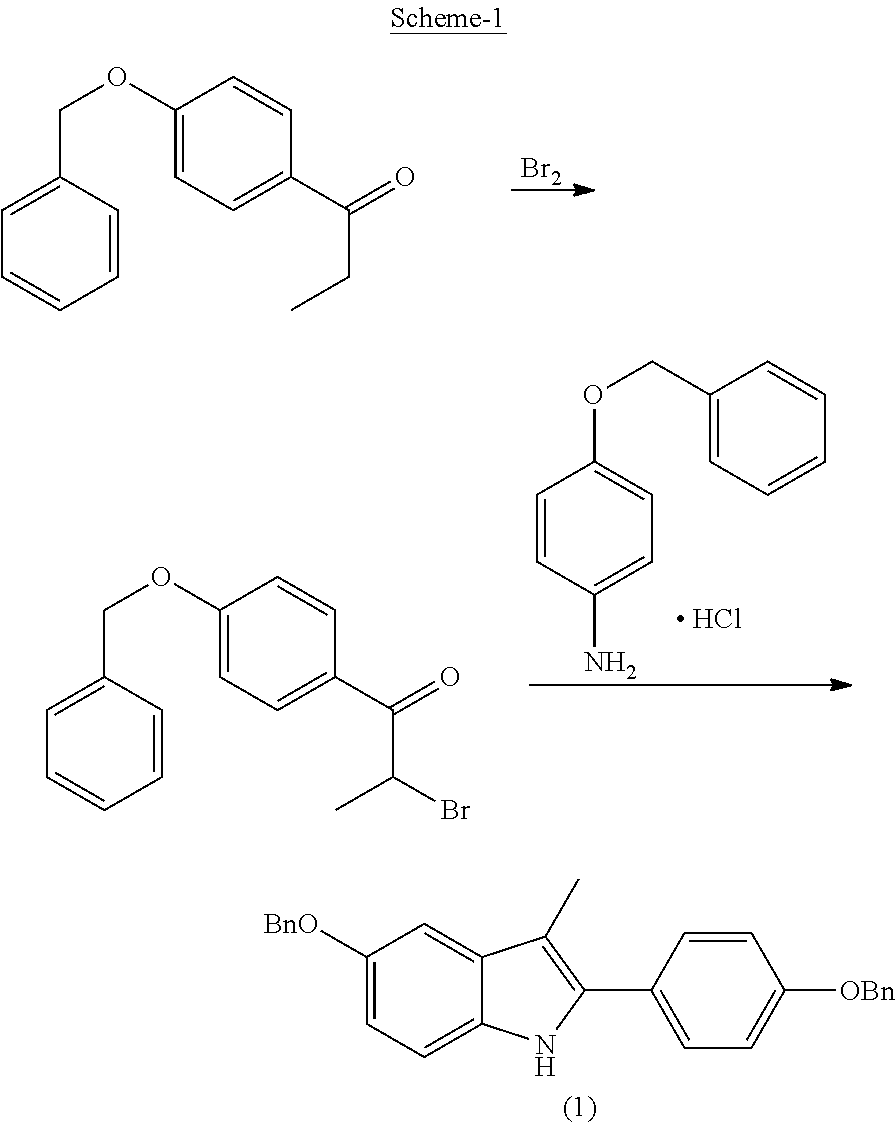
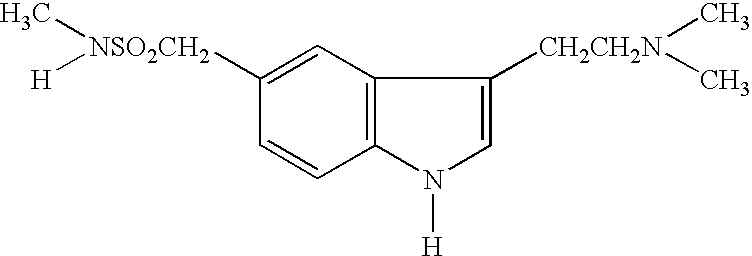
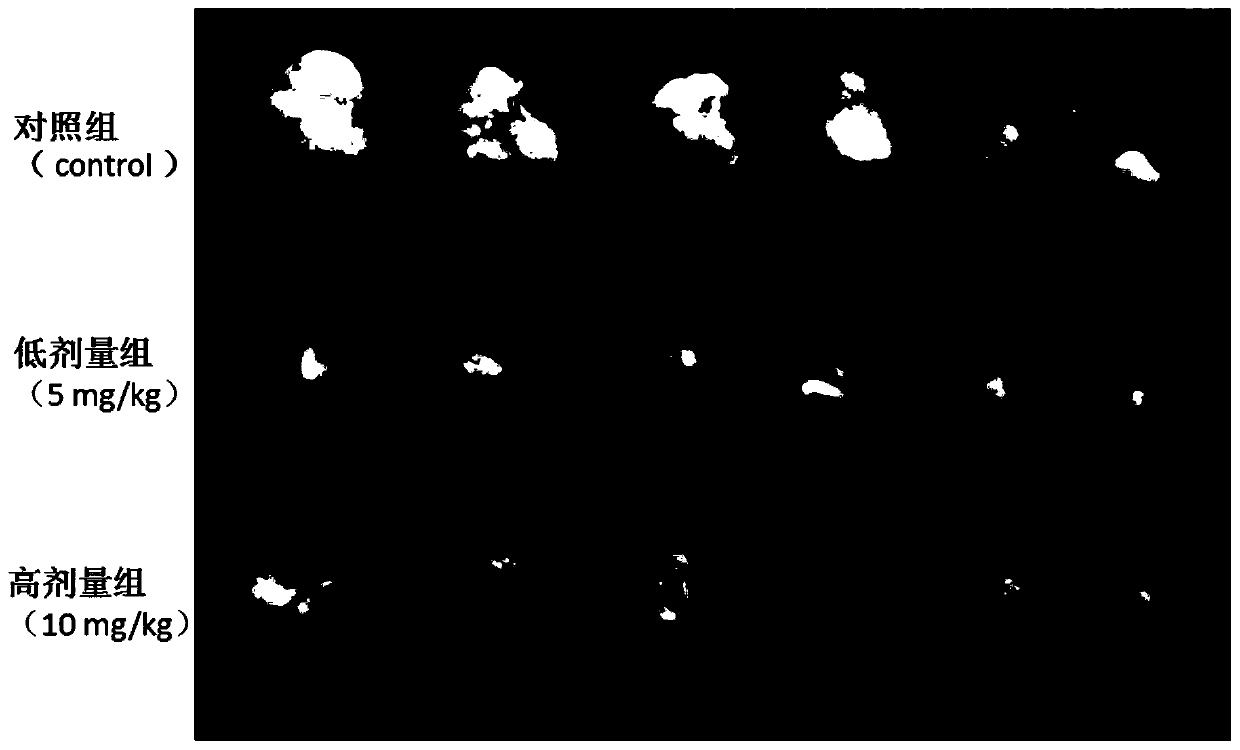
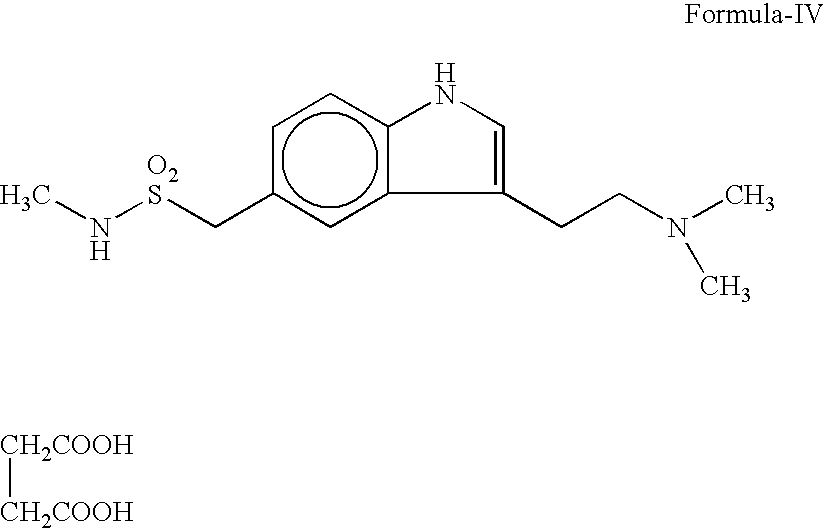
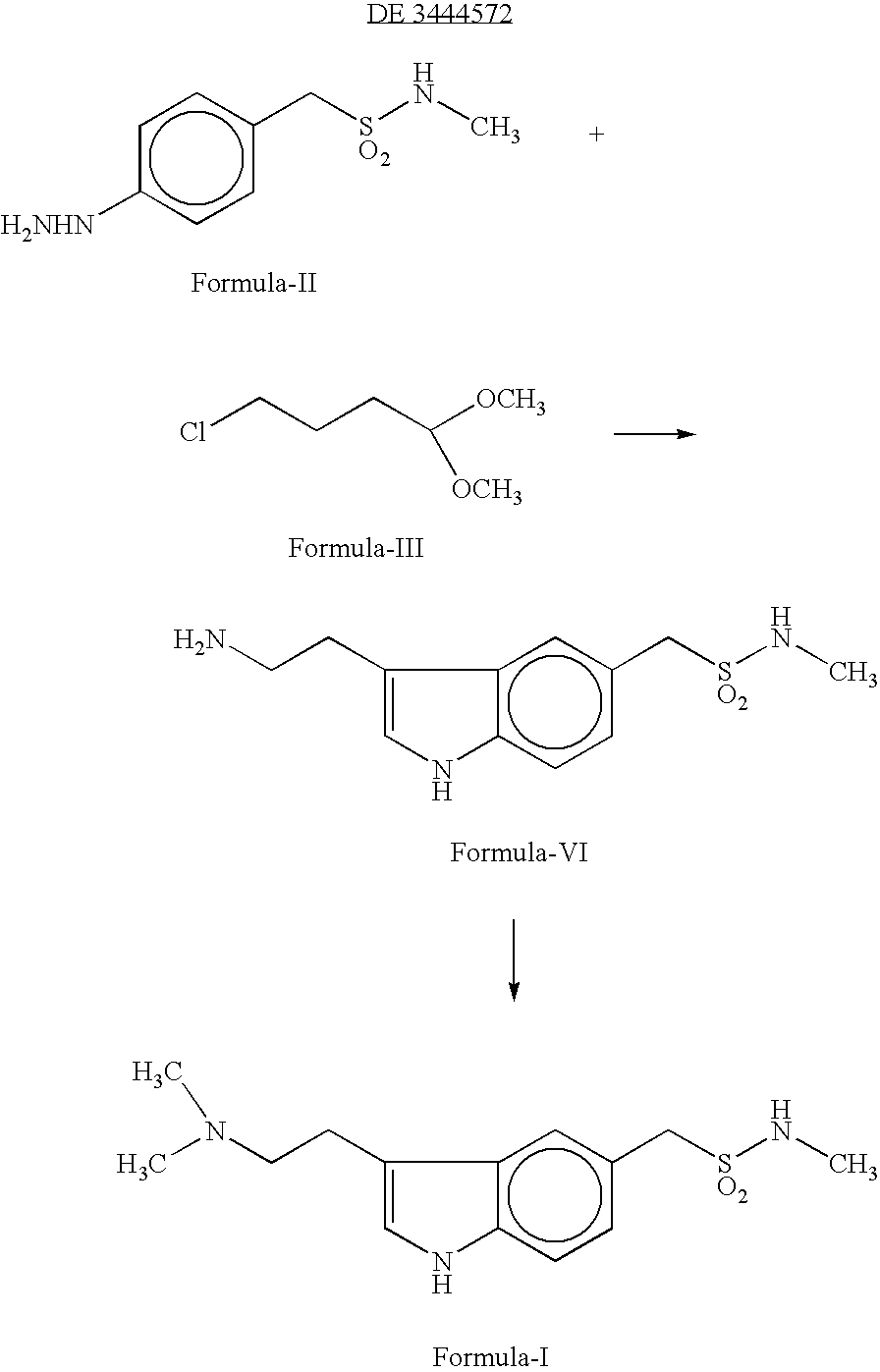
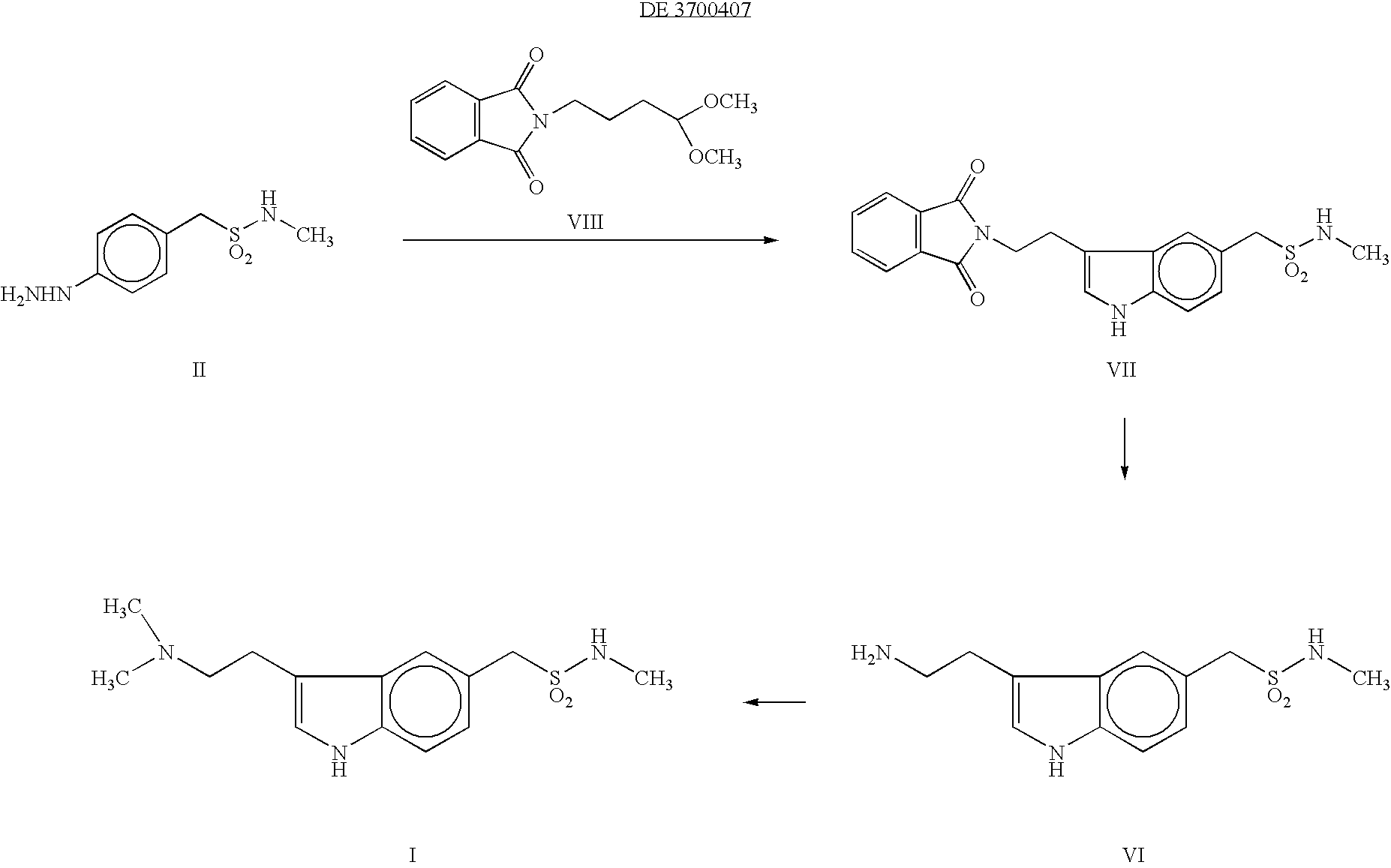
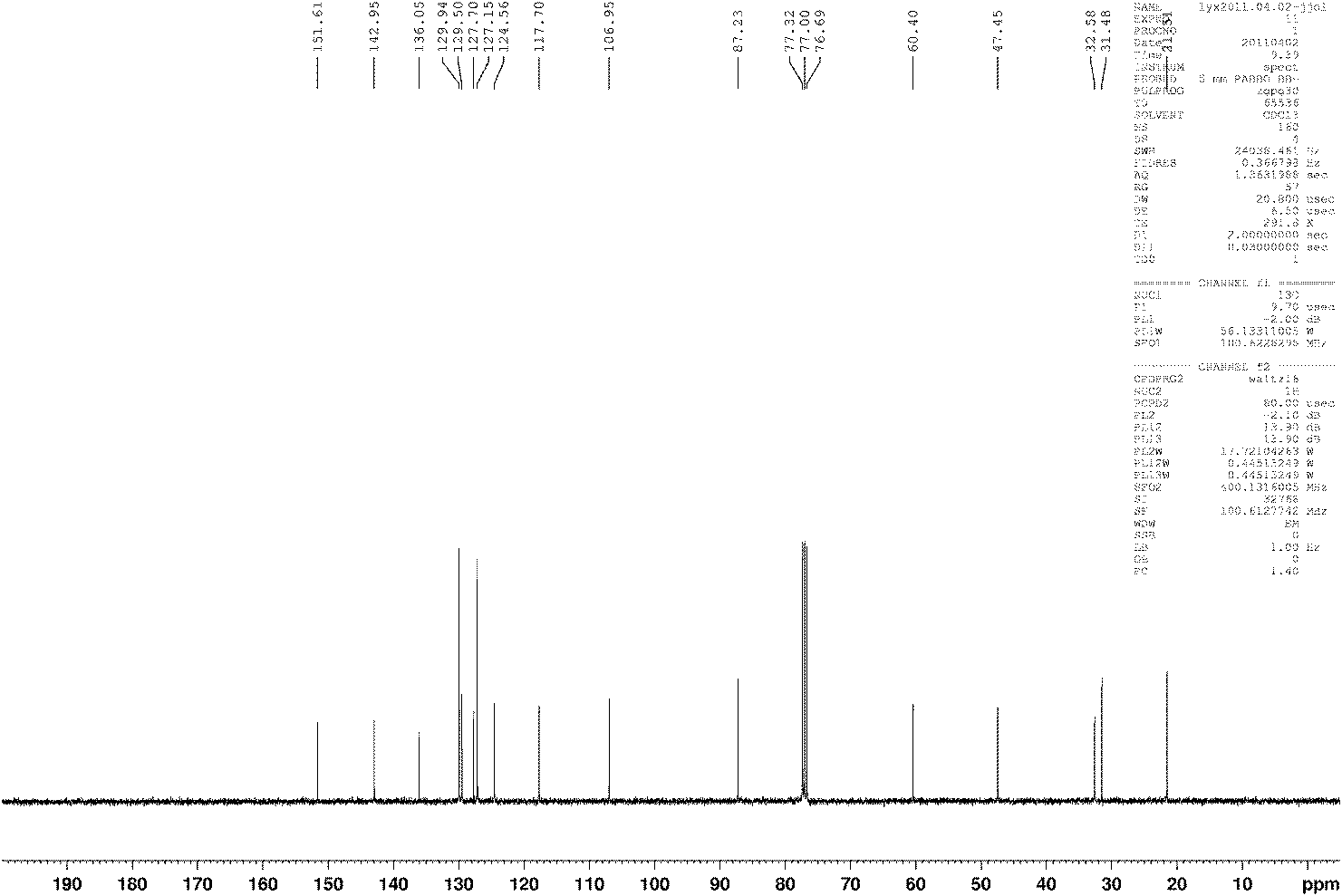
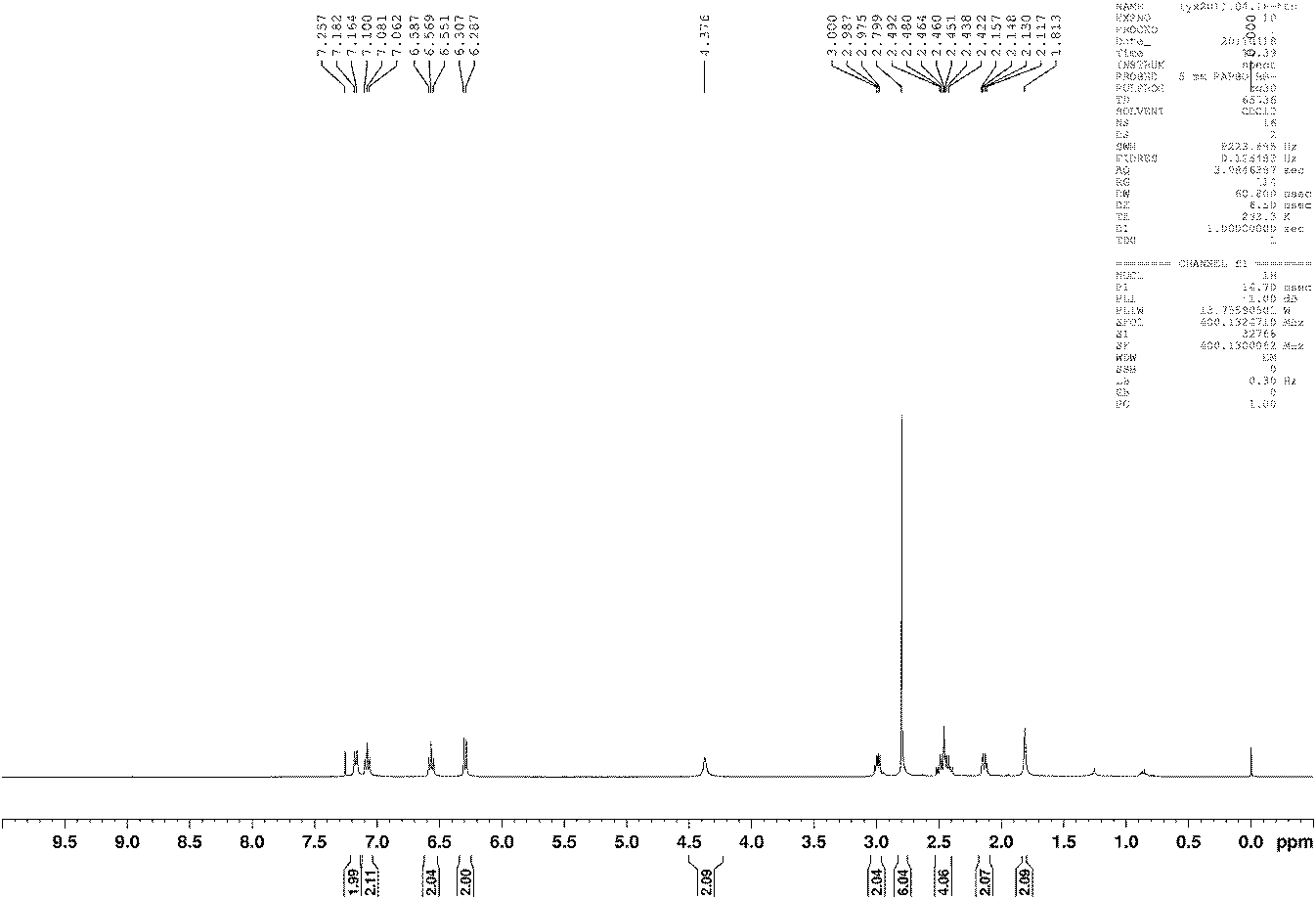
Preparation method for 3-[2-(dimethylamino)ethly]-N-methylindole-5-methyl sulfonylamine succinate
ActiveCN102432520AProcess stabilityEasy to operateOrganic chemistryMetal ions in aqueous solutionMethyl-1H-indole
The invention discloses a preparation method for 3-[2-(dimethylamino)ethly]-N-methylindole-5-methyl sulfonylamine succinate (I), which belongs to the field of preparation of medicament intermediates. The preparation method comprises the following steps of: undergoing a condensation reaction on 3-(2-aminoethyl)-N-methyl-1H-indole-5-methanesulfonamide (III) and a methanol aqueous solution in the presence of ammonium carbonate and methanol, and directly reducing an intermediate product by performing palladium / carbon catalytic hydrogen without separating to obtain 3-[2-(dimethylamino)ethly]-N-methylindole-5-methyl sulfonylamine (II); at the end of the reaction, recovering a solvent, performing post-treatment to obtain a crude product (II); and recrystallizing to obtain refined 3-[2-(dimethylamino)ethly]-N-methylindole-5-methyl sulfonylamine (II), undergoing a salt-forming reaction on the refined product and succinic acid in purified water, clarifying the reaction liquid, and adding activecarbon for decolorizing to obtain 3-[2-(dimethylamino)ethly]-N-methylindole-5-methyl sulfonylamine succinate (I). The preparation method has the characteristics of stable process and easiness for controlling.
Owner:SHANDONG XINHUA PHARMA CO LTD
Substituted 2-(5-hydroxy-2-methyl-1H-indole-3-yl)acetic acids and ethers thereof and the use of same to treat viral diseases
InactiveUS8481587B2BiocideGroup 1/11 organic compounds without C-metal linkagesMethyl-1H-indoleBULK ACTIVE INGREDIENT
The present invention relates to novel substituted 2-(5-hydroxy-2-methyl-1H-indol-3-yl)acetic acids, to novel antiviral active ingredients, pharmaceutical compositions, antiviral medicaments, methods for prophylaxis and treatment of viral diseases particularly caused by influenza viruses and infectious hepatisis C (HCV) viruses.Novel substituted 2-(5-hydroxy-2-methyl-1H-indol-3-yl)acetic acids, their esters of the general formula 1 and pharmaceutically acceptable salts and / or hydrates thereof have been disclosedwherein: R1 represents amino group substituent selected from hydrogen, optionally substituted C1-C5 alkyl, acyl or sulfonyl; R2 and R4 independently of each other represent alkyl substituent selected from hydrogen, halogen, optionally substituted C1-C3 alkyl, optionally substituted hydroxyl, optionally substituted amino group, optionally substituted aminomethyl, substituted mercapto group; R3 represents hydrogen, optionally substituted lower C1-C5 alkyl; R5 represents cyclic system substituent selected from hydrogen, fluorine, trifluoromethyl, carboxy group, alkyloxycarbonyl, possibly substituted aryl, heterocyclyl, optionally substituted aminomethyl, cyano group; R6 represents hydroxyl group substituent selected from hydrogen, optionally substituted C1-C5 alkyl, acyl.
Owner:IVASHCHENKO ANDREY ALEXANDROVICH +6
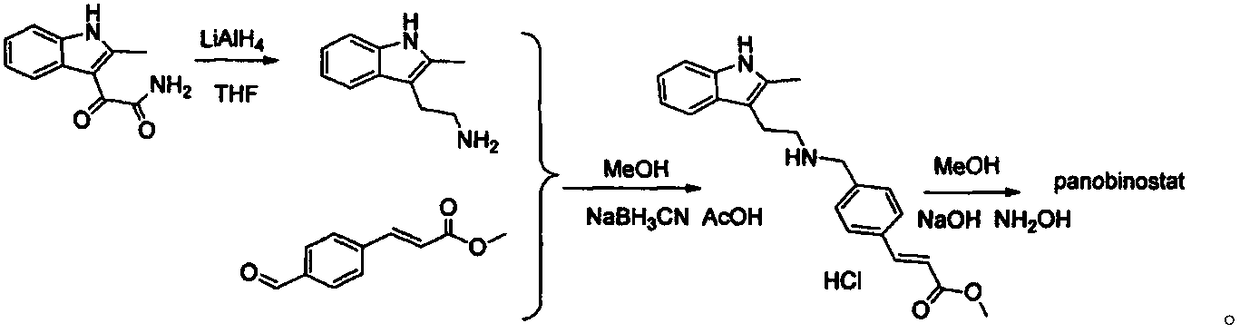
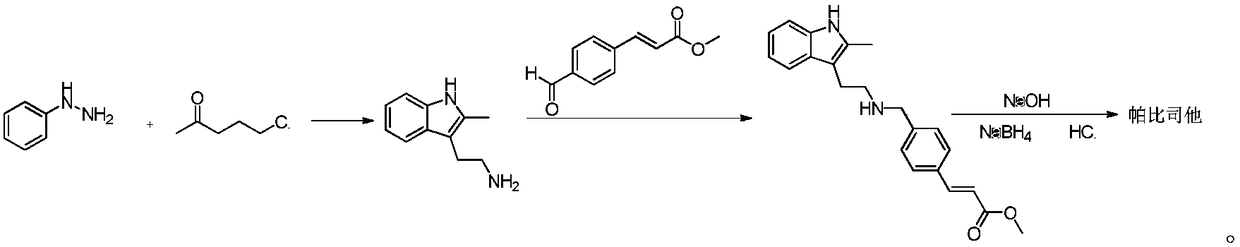
Synthesis method of panobinostat key intermediate
The invention belongs to the field of medicine synthesis, and particularly relates to a synthesis method of a panobinostat key intermediate. The key intermediate is (E)-3-[4-[[[2-(2-methyl-1H-indole-3-yl)ethyl] amino] methyl] phenyl] methyl acrylate hydrochloride. The method has the advantages that the yield is as high as 85 percent or higher; the impurities are few; the purity is 99.5 percent orhigher; meanwhile, the operation is simple and convenient.
Owner:南京众慧网络科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



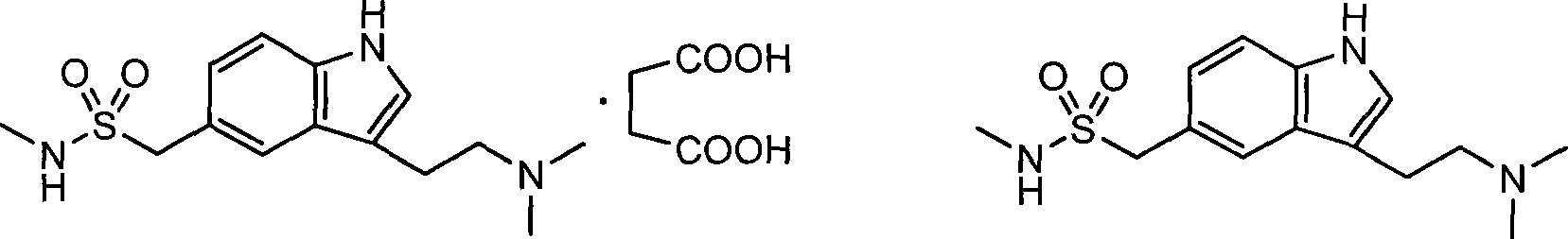



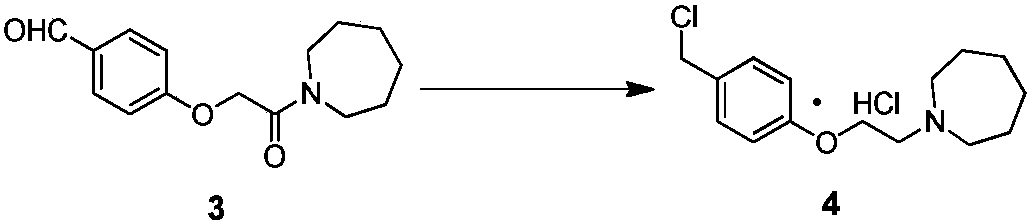
![Intermediates and processes for the preparation of 4- (acetylamino) ) -3- [ (4-chloro-phenyl) thio] -2-methyl-1h-indole-1-acetic acid Intermediates and processes for the preparation of 4- (acetylamino) ) -3- [ (4-chloro-phenyl) thio] -2-methyl-1h-indole-1-acetic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fcad2a87-607d-48ba-877a-335546ad46c5/FDA0000140919910000011.png)
![Intermediates and processes for the preparation of 4- (acetylamino) ) -3- [ (4-chloro-phenyl) thio] -2-methyl-1h-indole-1-acetic acid Intermediates and processes for the preparation of 4- (acetylamino) ) -3- [ (4-chloro-phenyl) thio] -2-methyl-1h-indole-1-acetic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fcad2a87-607d-48ba-877a-335546ad46c5/FDA0000140919910000013.png)
![Intermediates and processes for the preparation of 4- (acetylamino) ) -3- [ (4-chloro-phenyl) thio] -2-methyl-1h-indole-1-acetic acid Intermediates and processes for the preparation of 4- (acetylamino) ) -3- [ (4-chloro-phenyl) thio] -2-methyl-1h-indole-1-acetic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fcad2a87-607d-48ba-877a-335546ad46c5/FDA0000140919910000021.png)








![Preparation method for 3-[2-(dimethylamino)ethly]-N-methylindole-5-methyl sulfonylamine succinate Preparation method for 3-[2-(dimethylamino)ethly]-N-methylindole-5-methyl sulfonylamine succinate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/68a5d625-c981-4909-9938-3fb0c986ac7d/BDA0000110523370000021.PNG)