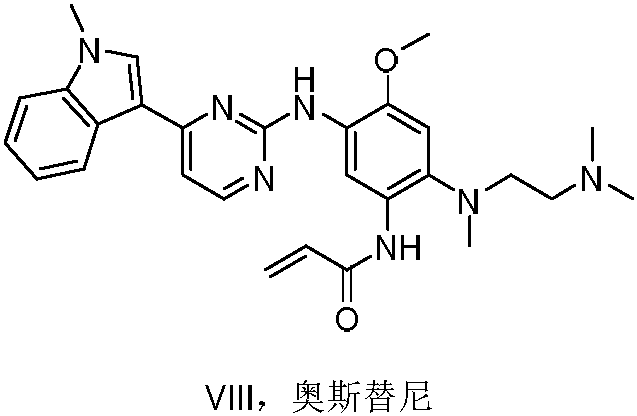
Synthesis method of Osimertinib
A synthesis method and compound technology, applied in the field of medicine and chemical industry, can solve the problems of low utilization rate of atoms, smell affecting industrialization prospects, etc., and achieve the effect of short route, high yield and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
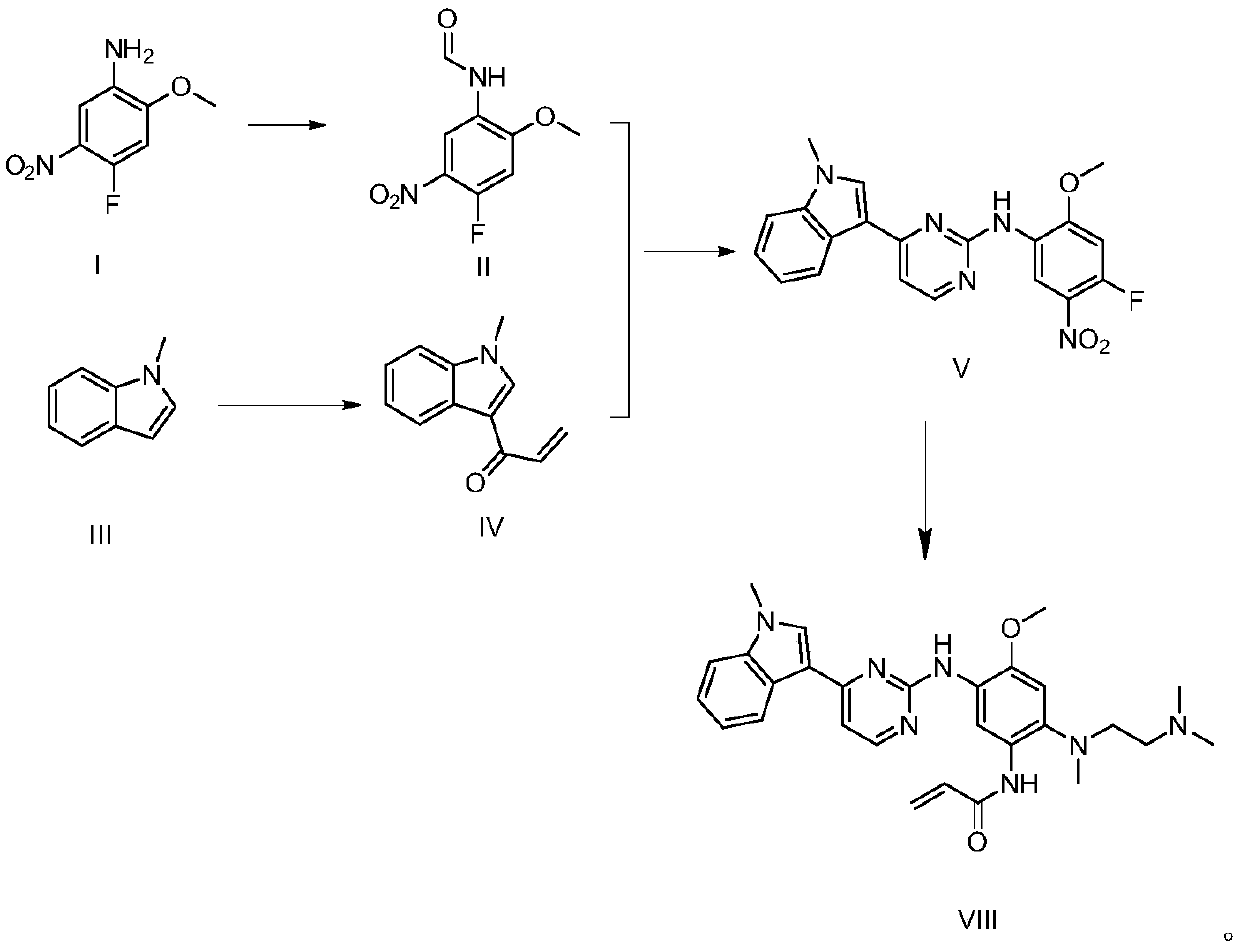
[0033] Embodiment 1, the preparation of N-(4-fluoro-2-methoxy-5-nitrophenyl) formamide (II)
[0034] Acetic anhydride (1.53 g, 15 mmol) was added into a 25 mL three-neck flask, the temperature of the solution was controlled with an ice-water bath, and magnetic stirring was performed. Formic acid (1.38g, 30mmol) was slowly added dropwise in acetic anhydride, and the temperature of the reaction solution was controlled not to exceed 35°C. Stirring was continued for 15 min at room temperature. Then, 4-fluoro-2-methoxy-5-nitroaniline (I) (1.86 g, 10 mmol) was slowly added to the above solution in batches, and the reaction temperature was controlled not to exceed 35°C. After the addition of 4-fluoro-2-methoxy-5-nitroaniline (I) was completed, stirring was continued for 48 h, 10 mL of water was added, and stirring was continued for 1 h. A yellow solid precipitated out, which was filtered and washed with water until the washed water became neutral. Dry under reduced pressure at 45°...
Embodiment 2
[0035] Embodiment 2, the preparation of 3-(1-methyl-1H-indol-3-yl) prop-2-en-1-one (IV)
[0036] Under nitrogen protection, N-methylindole (III) (1.31 g, 10 mmol) was added into a 50 mL three-necked flask, dissolved in 20 mL of anhydrous dichloromethane, the temperature of the solution was controlled by an ice-water bath, and magnetically stirred. Aluminum trichloride (3.33 g, 25 mmol) was added to the above solution in batches, and the reaction temperature was controlled not to exceed 10°C. A dichloromethane solution of acryloyl chloride (1.80 g, 20 mmol of acryloyl chloride dissolved in 5 mL of anhydrous dichloromethane) was slowly added dropwise to the above reaction solution. After the dropwise addition, the resulting reaction solution was stirred at 0° C. for 2 h. After that, 10 mL of water was added to quench the reaction, dichloromethane was evaporated under reduced pressure, and 50 mL of ethyl acetate was added to dissolve the system, washed three times with saturated...
Embodiment 3
[0037] Example 3, Preparation of 2-(4-fluoro-2-methoxy-5-nitroanilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine (V)
[0038] Under oxygen protection, in a 250mL single-necked bottle, 3-(1-methyl-1H-indol-3-yl)prop-2-en-1-one (IV) (1.85g, 10mmol), N- (4-Fluoro-2-methoxy-5-nitrophenyl)formamide (II) (3.21g, 15mmol), ammonium iodide (3.62g, 25mmol), sodium nitrite (0.14g, 2mmol), Morpholine (0.17g, 2mmol) was dissolved in 25mL of acetic acid, heated to 80°C, and stirred for 16h. After the reaction was completed, the reaction solution was slowly added dropwise to 100 mL of saturated sodium bicarbonate ice solution, and a yellow solid was precipitated, which was dried under reduced pressure at 45°C for 12 hours to obtain 2-(4-fluoro-2-methoxy-5-nitrate Anilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine (V) 3.46g, yield 88%. 96% purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com