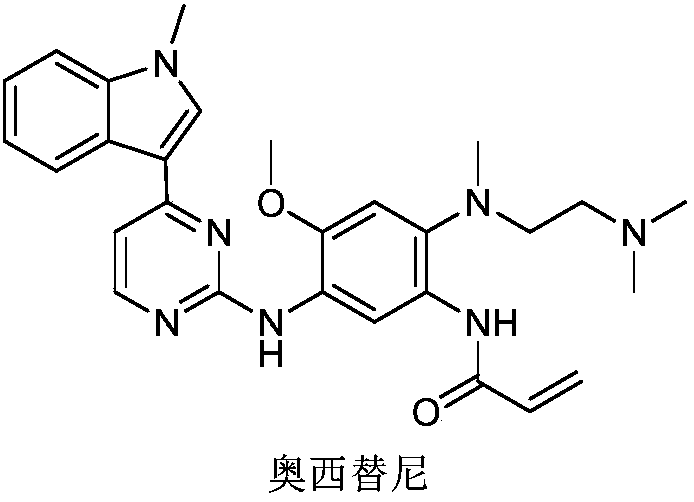
Preparation of biomass derived palladium catalyst and application to synthesis of anti-tumor drug osimertinib
An anti-tumor drug, osimertinib technology, applied in the preparation of biomass-derived palladium catalyst, the application field of anti-tumor drug osimertinib synthesis, can solve the difficulty of increasing processing, metal palladium residue, easy blackening of products, etc. problem, to achieve the effect of long service life, easy reaction and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of Biomass-Derived Palladium Catalysts
[0042] In a four-neck flask, add palladium acetate and ethanol solvent, and slowly add chitosan, wherein the molar ratio of palladium acetate to chitosan is 1:1.5; the system is heated to 70°C and reacted for 20 hours. Cool to room temperature, remove ethanol by filtration, rinse the filter cake with ethanol three times, and dry the solid in a vacuum oven at 60°C for 12 hours. The catalyst (Pd(OAc) 2 @Chitosan).
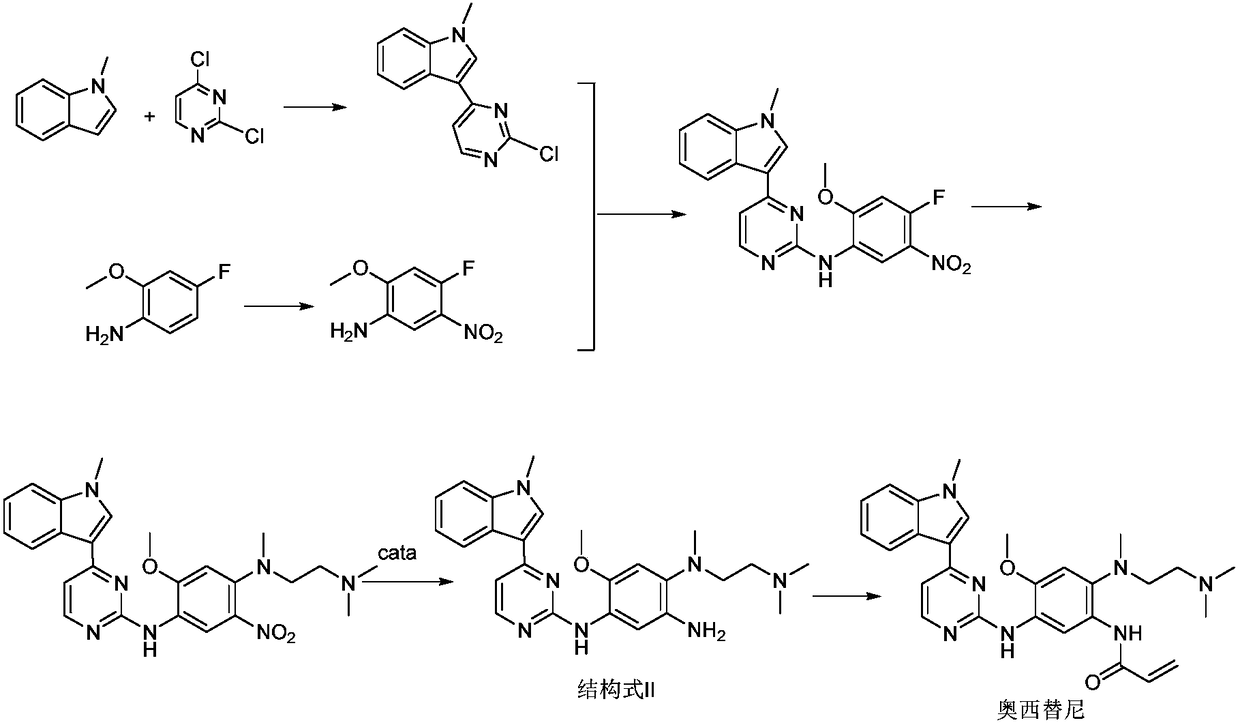
[0043] N-(2-Dimethylamino-ethyl)-2-methoxy-N-methyl-N-[4-(1-methyl-lH-indol-3-yl-pyrimidin-2-yl The preparation method of ]-5-amino-benzene-1,4-diamine, the synthetic route is as follows:
[0044]
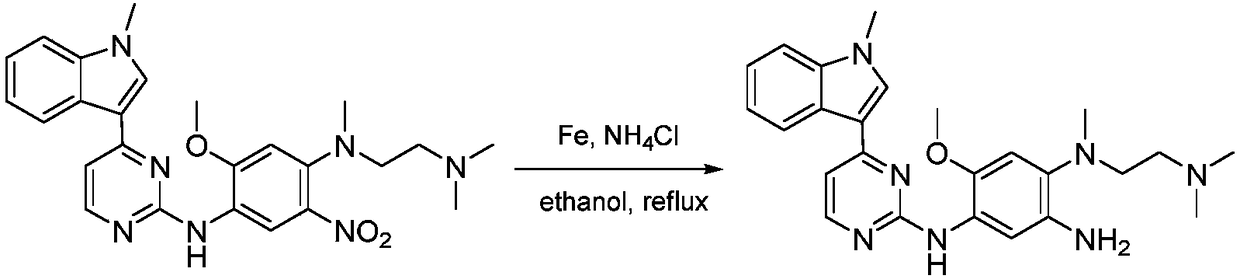
[0045] With N-(2-dimethylamino-ethyl-2-methoxy-N-methyl-N-[4-(1-methyl-1H-indol-3-yl-pyrimidin-2-yl ]-5-nitro-benzene-1,4-diamine as raw material, dissolve the above raw material with ethanol, add the catalyst (Pd(OAc) 2 @Chitosan), the molar ratio of the catalyst to the raw material is 0.025:1, the tempe...
Embodiment 2
[0047] Under the catalytic conditions of Example 1, the catalyst was recycled 5 times, and the catalytic effect did not significantly decrease, as shown in Table 1:
[0048] Table 1
[0049] serial number
Embodiment 3
[0051] In four-necked flasks, different palladium salts (a: Pd(OAc) 2 , b: PdCl 2 , c: PdBr 2 , d: Pd(NO 3 ) 2 ), methanol as solvent, chitosan was added slowly, the system was heated up to 60°C, and reacted for 20 hours. Cool to room temperature, remove methanol by filtration, rinse the filter cake with methanol three times, and dry the solid in a vacuum oven at 60°C for 12 hours. The catalyst was obtained by carbonizing at 600°C for 2 hours under the protection of argon, and then elemental analysis was carried out on the catalyst, and it was found that the loading capacity of palladium acetate was the largest, with 9.8%, and palladium chloride, palladium bromide, and palladium nitrate were 9.6%, respectively. 9.5%, 8.5%, and then verify the reaction of the four catalysts, consistent with the experimental results, the greater the metal loading, the better the catalyst activity, respectively 97, 96, 96, 93, so the preferred catalyst is palladium acetate . Afterwards, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com