Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
167 results about "Tert-Butyl formate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tert-Butyl formate, also known as formic acid tert-butylester and TBF, is a chemical compound with molecular formula C₅H₁₀O₂. TBF is one of the possible daughter products of methyl tert-butyl ether biodegradation.
Synthetic method of anti-tumor medicine
ActiveCN104817541AHigh yieldMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationCarbamateMethyl-1H-indole
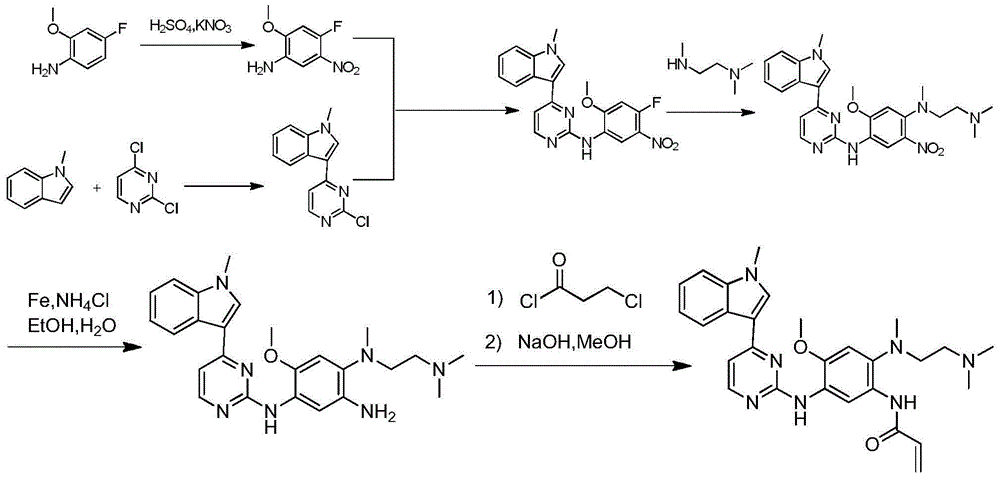
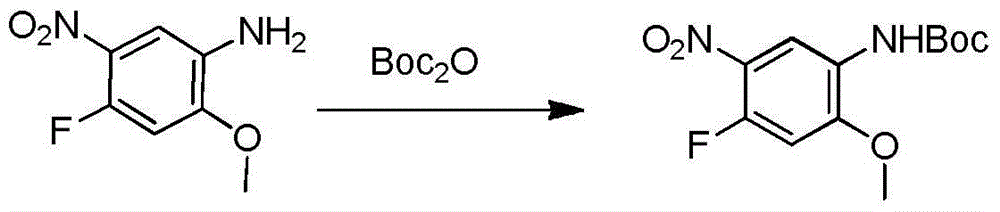
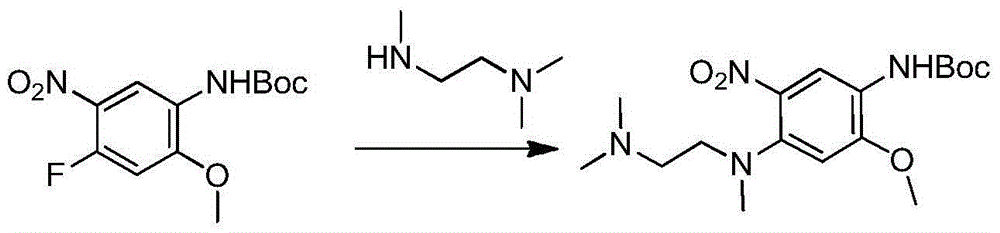
The invention relates to a synthetic method of an anti-tumor medicine, namely N-[2-[[2-(dimethylamino) ethyl] methyl amino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3-yl)-2-pyrimidyl] amino] phenyl]-2-acrylamide (AZD9291) and a key intermediate of the anti-tumor medicine. The synthetic method comprises the following steps: performing Boc acid anhydride protection on 4-fluoro-2-methoxy-5-nitroaniline to obtain 4-fluoro-2-methoxy-5-nitroanilino tert-butyl formate, then reacting with N,N,N'-trimethylethylenediamine to obtain 4-(N,N,N'-trimethylethylenediamino)-2-methoxy-5-nitroanilino tert-butyl formate, then reducing to obtain 2-(N,N,N'-trimethylethylenediamino)-4-methoxy-5-tert-butyl carbamate phenylamine, then completely reacting with acryloyl chloride and directly removing a Boc protecting group to obtain 2-methoxy-4-N,N,N'-trimethylethylenediamino-5-acrylamido phenylamine, and finally reacting with 3-(2-chloropyrimidine-4-yl)-1-methylindole to obtain AZD9291. A process disclosed by the invention is simple in step, relatively high in yield, mild in reaction condition and easy for realization of industrial production.
Owner:苏州东南药业股份有限公司
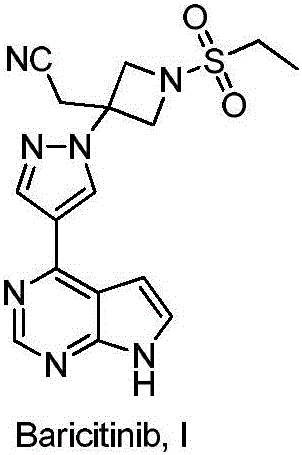
Method for preparing baricitinib
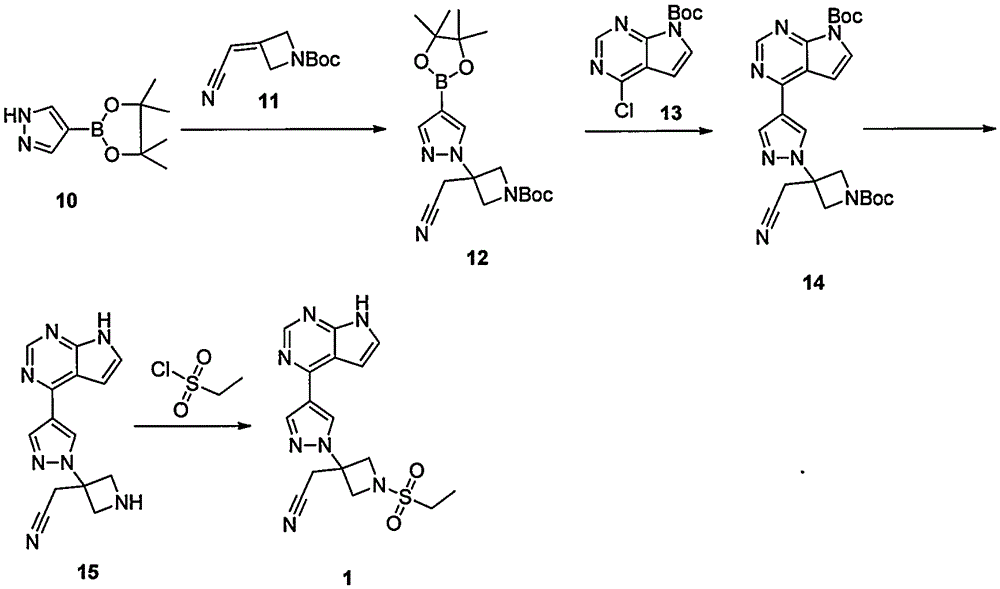
ActiveCN105294699ARaw materials are easy to getSimple processOrganic chemistryCyclobutaneCyanomethylidyne
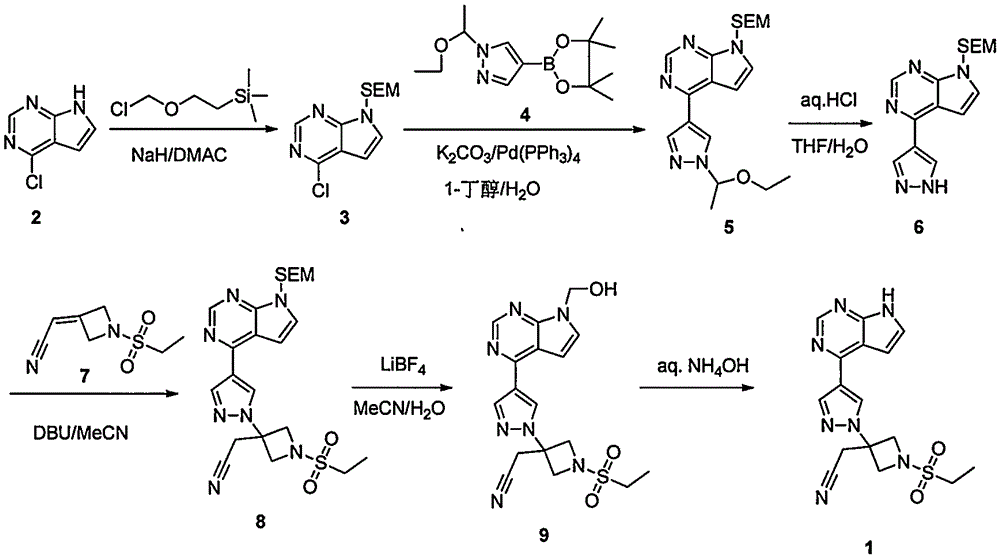
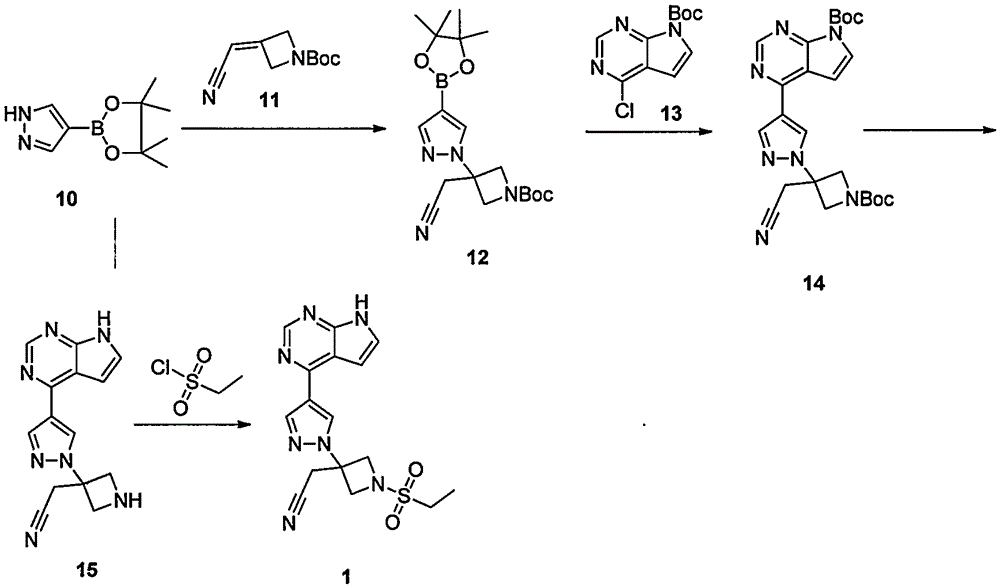
The invention provides a method for preparing baricitinib. The method comprises the following steps: by taking 4-pyrazol boric acid pinacol ester (10) as an initial raw material, performing Michael addition reaction on the initial raw material and 3-(icyanomethylene) azacyclo-cyclobutane-1-tert-butyl formate (11) so as to prepare an intermediate 12, and performing catalytic coupling reaction on 12 and 13, thereby preparing an intermediate 14; removing two-molecule tert-butyl formate of the intermediate 14, thereby preparing an intermediate 15; performing sulfamide reaction on the intermediate 15 and ethanesulfonyl chloride in an organic solvent, thereby obtaining a final product baricitinib (1). The method for preparing baricitinib has the advantages that the raw materials are easy to obtain, the process is simple, the operation is convenient, the reaction yield is high when being compared with that of document records, the atom utilization rate is high, industrial production can be easily achieved, and the like. The reaction general formula is as shown in the specification.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
Magnetic wedge and production method thereof
ActiveCN101295894AHigh strengthReduce iron consumptionWindingsManufacturing dynamo-electric machinesGlass fiberAdhesive cement
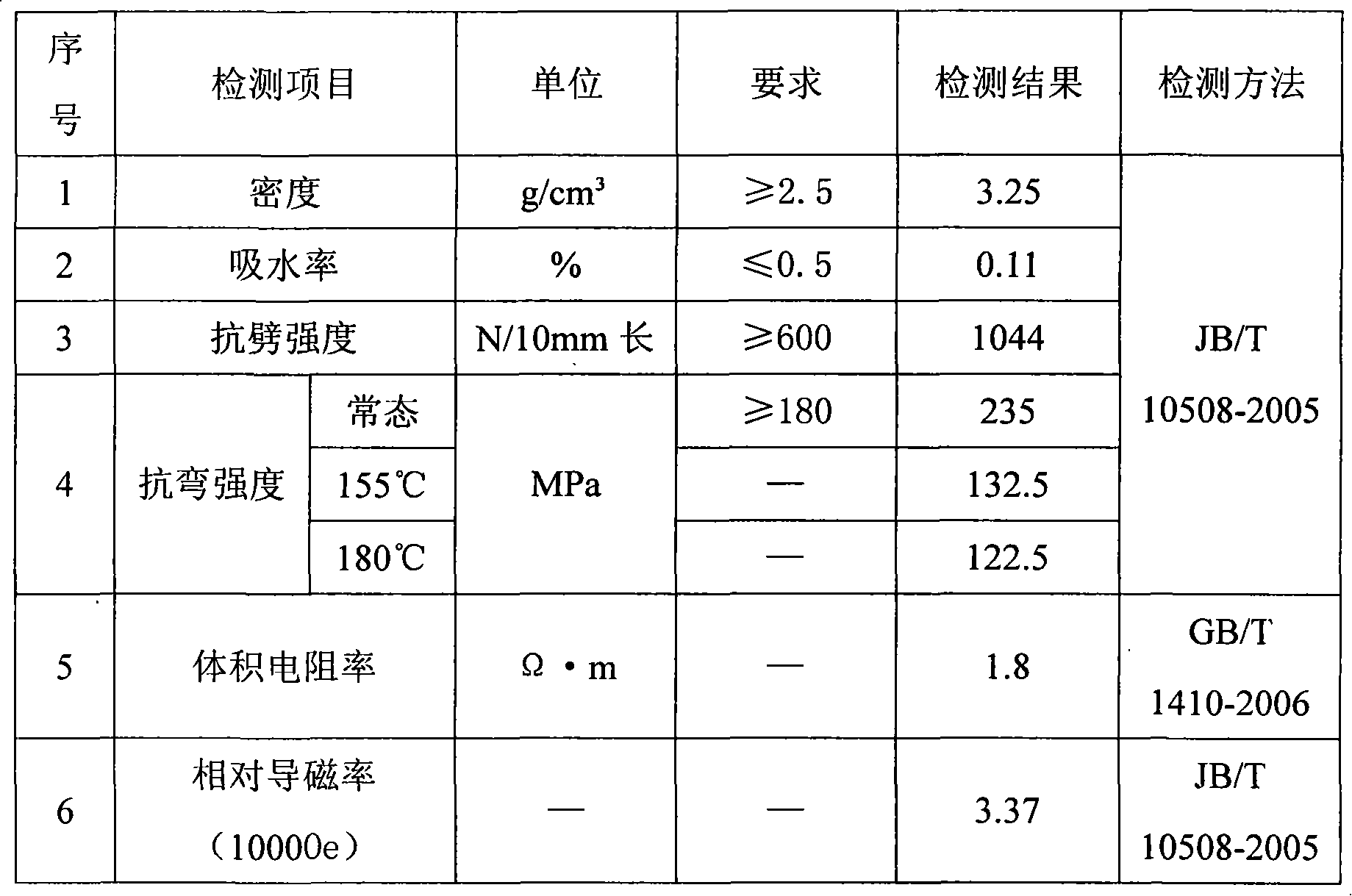
The invention discloses a magnetic slot wedge and a production method thereof. The magnetic slot wedge comprises 18 percent to 20 percent of 943 unsaturated polyester resins, 0.5 percent to 1 percent of an internal releasing agent, 0.2 percent to 0.5 percent of tert-butyl peroxy benzoate, 0.1 percent to 0.3 percent of benzoyl peroxide, 54 percent to 57 percent of magneto-increasing material and 24 percent to 25 percent of alkali free glass fiber. The production method comprises the steps that: 1. the unsaturated polyester resins, the internal releasing agent and a peroxide evocating agent are prepared into an adhesive according to proportion; 2. a mould is heated to be 150 DEGC -200 DEG C and extraction speed is 0.8-1.40r / min; 3. the alkali free glass fiber soaking the adhesive is treated with pultrusion by the mould; 4. cooling and cutting are carried out; 5. the cut slot wedge is treated with head grinding; 6. the surface of the slot wedge is treated with paint treatment to become bright and clean with no burr; and 7. airing, detecting and packaging are carried out. The magnetic slot wedge has high magnetoconductivity, high cleavage strength, great bending strength and simple production method.
Owner:浙江博菲电气股份有限公司
Tert-butyl carbamate derivative and preparation method and application thereof
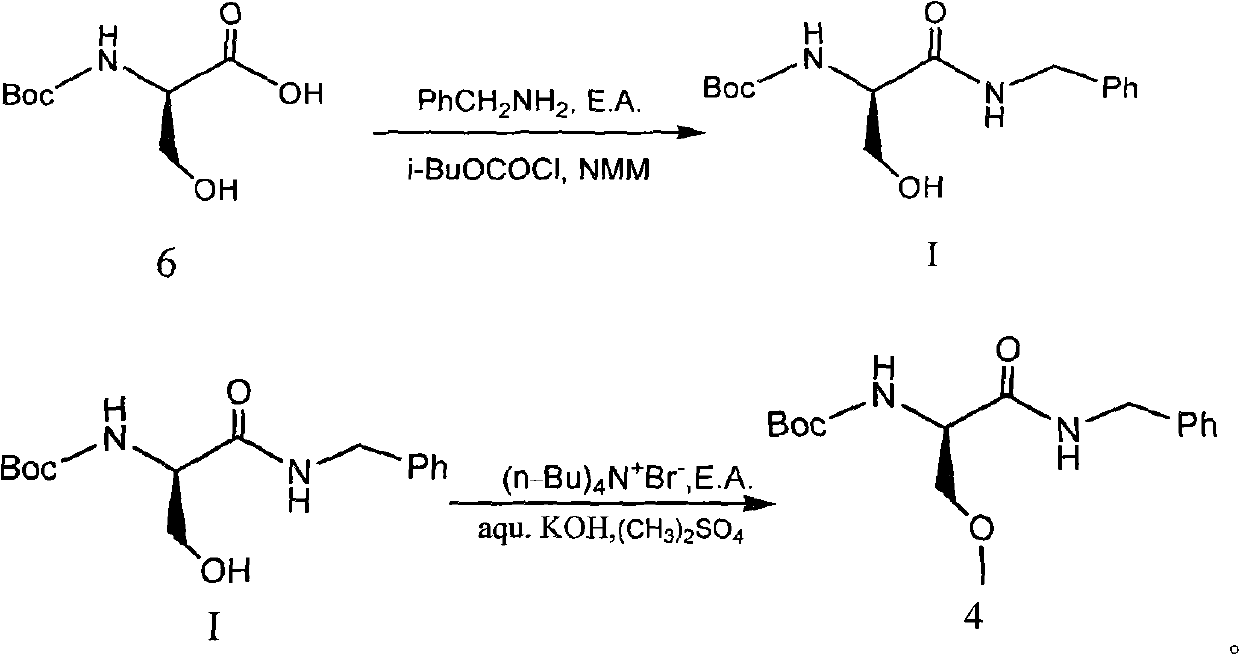
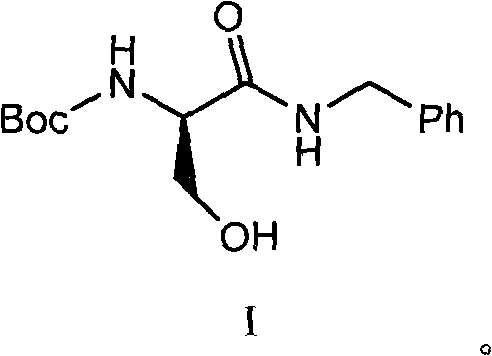
ActiveCN102020589ASolve the problem that it cannot be used to synthesize the drug lacosamideFix bugsCarbamic acid derivatives preparationOrganic compound preparationAlkyl transferChemical reaction
The invention relates to a synthetic intermediate of lacosamide and a preparation method and application thereof. In the method, a compound shown as a formula I is prepared by condensing a compound 6; the compound shown as the formula I is a novel intermediate compound for synthesizing the lacosamide; (R)-2-(amino-Boc)-N-benzyl-3-methoxyl propionamide (compound 4) can be conveniently prepared from the compound shown as the formula I through alkylation reaction; and the (R)-2-(amino-Boc)-N-benzyl-3-methoxyl propionamide is another important intermediate for synthesizing the lacosamide. The related chemical reaction formulas are shown in the specifications.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
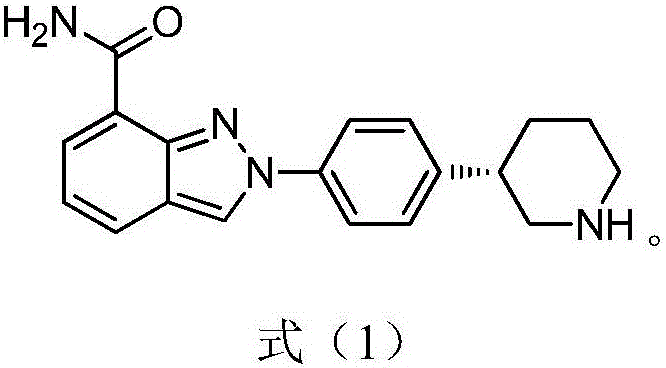
Preparation method of niraparib intermediate of (3S)-3-(4-aminophenyl) piperidine-1-tert-butyl formate
The invention belongs to the field of medicine synthesis, and relates to a preparation method of a niraparib intermediate of (3S)-3-(4-aminophenyl) piperidine-1-tert-butyl formate. The method is characterized in that a D-phenylglycine derivative is used as a resolving agent; 3-(4-aminophenyl) piperidine-1-tert-butyl formate racemic bodies are used as raw materials; splitting is performed in a solvent; separation is performed to obtain a split salifying product; acid is added into the split salifying product; hydrolysis is performed; the resolving agent is extracted and separated; the pH is regulated to 8 to 10; distillation is performed to obtain the (3S)-3-(4-aminophenyl) piperidine-1-tert-butyl formate. The method has the advantages that the resolving efficiency is high; the product optical purity is high; the resolving agent can be easily recycled and reused; the application cost is low; the preparation method is suitable for large-scale production; the technical support is provided for further preparation of high-purity niraparib.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
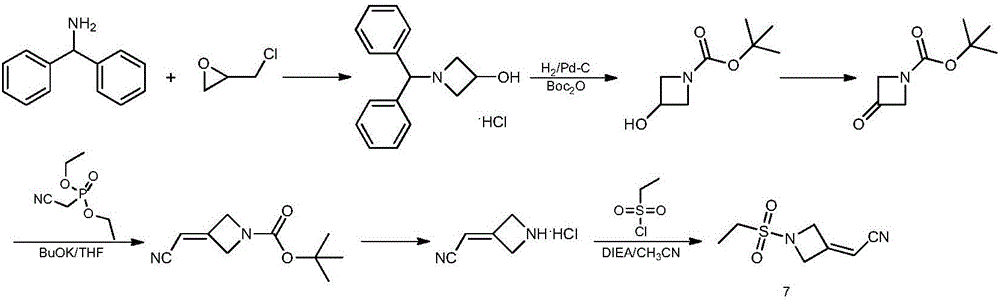
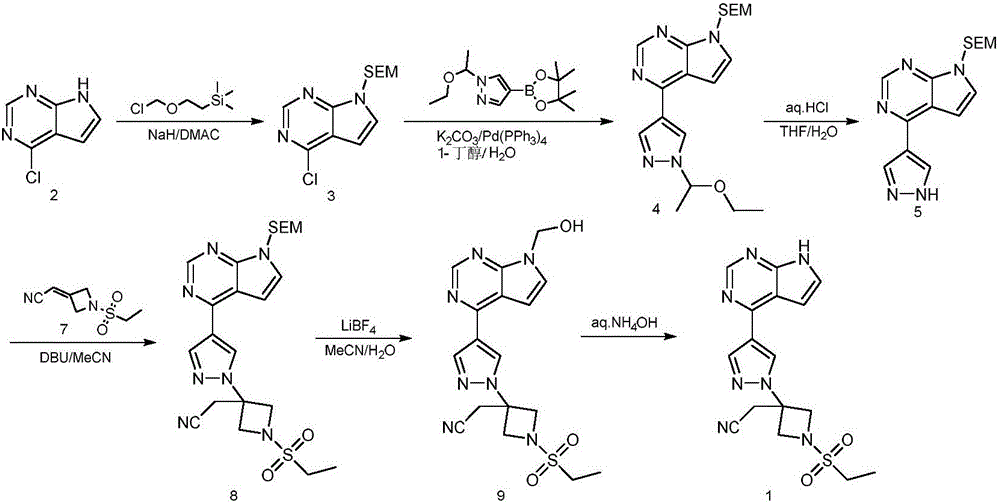
Preparation method of baricitinib
ActiveCN107176955ARaw materials are easy to getSimple processOrganic chemistrySulfonyl chlorideBoronic acid
The invention discloses a preparation method of baricitinib. The method comprises the following steps: performing a substitution reaction on 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (II) serving as a raw material and benzene sulfonyl chloride in the presence of an alkali to obtain an intermediate III; then, performing a Suzuki coupling reaction on the intermediate III and 4-pyrazole-4-boronic acid pinacol ester in the presence of a palladium catalytic system and an alkali to obtain an intermediate V; then performing a Michael addition reaction on the intermediate V and 3-(cyanomethylene)azetidine-1-tert-butyl formate in the presence of a catalyst to obtain an intermediate VII; then removing Boc protection from the intermediate VII under the action of hydrochloric acid to obtain an intermediate VIII; then performing a sulfoamidate reaction on the intermediate VIII and ethyl sulfonyl chloride in an organic solvent in the presence of an alkali to obtain an intermediate IX; lastly, removing benzenesulfonyl protection from the intermediate IX under the action of tetramethylammonium fluoride or tetrabutylammonium fluoride or a trihydrate of the tetramethylammonium fluoride or the tetrabutylammonium fluoride to obtain baricitinib (I). Compared with the prior art, the method has the advantages of adoption of readily-available raw materials, low cost, high product yield and easiness for industrial production.
Owner:NANJING YOKO PHARMA +2
Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine
The invention belongs to the technical field of chemical synthesis, and particularly relates to a preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine, which comprises the followingsteps: 1) synthesizing an intermediate I, namely 1-Boc-1-aminopyrrole, by taking tert-Butyl carbazate and 2, 5-dimethoxytetrahydrofuran as raw materials; 2) enabling the intermediate I to react withan isocyanate methanesulfonate to synthesize an intermediate II, namely 1-Boc-1-amino-(9ci)-1H-pyrrole-2-carbonitrile; 3) performing deamination protection on the intermediate II under an acidic condition to obtain an intermediate III, namely 1-amino-(9ci)-1H-pyrrole-2-carbonitrile hydrochloride; 4) performing a ring closing reaction on the intermediate III and formamidine acetate to obtain an intermediate IV, namely 4-aminopyrrolo [2, 1-f] [1, 2, 4] triazine; and 5) reacting the intermediate IV with a bromination reagent to obtain a final product, namely 7-bromopyrrolo [2, 1-f] [1, 2, 4] thiazine-4-amine. The preparation method disclosed by the invention has the advantages of cheap and easily available raw materials, few reaction steps, high yield, mild reaction conditions and capabilityof realizing large-scale production.
Owner:CHENGDU AF BIOCHEM
Preparation method of alogliptin benzoate
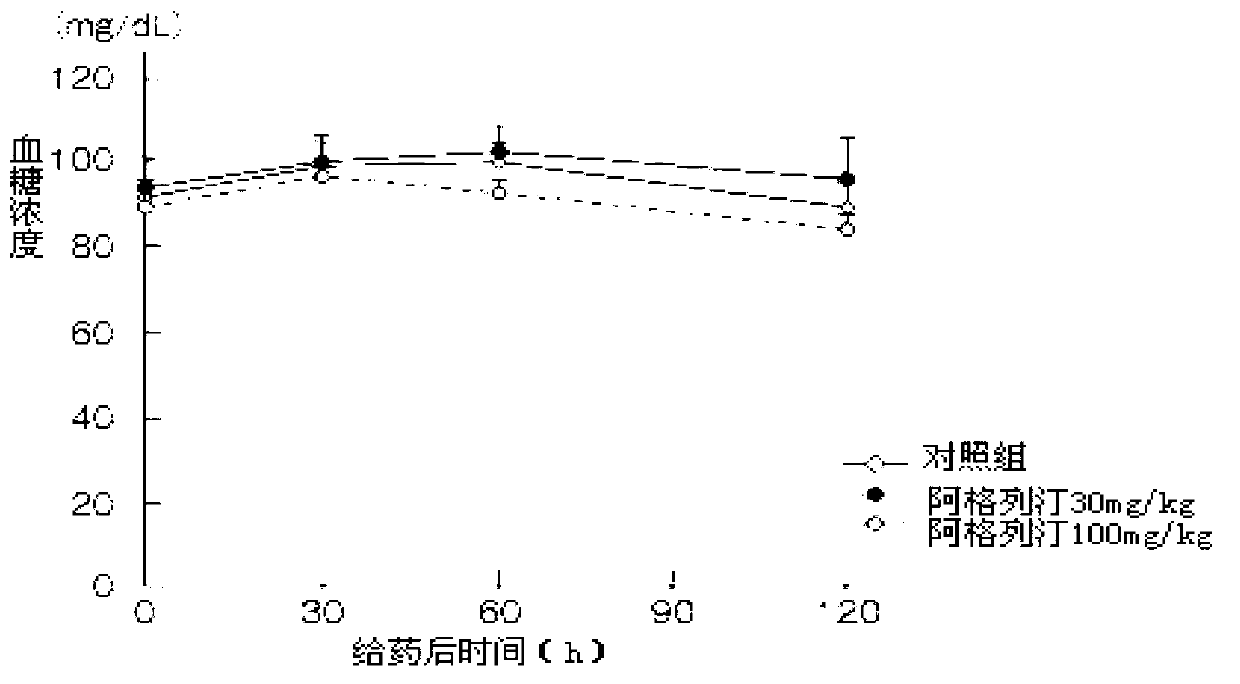
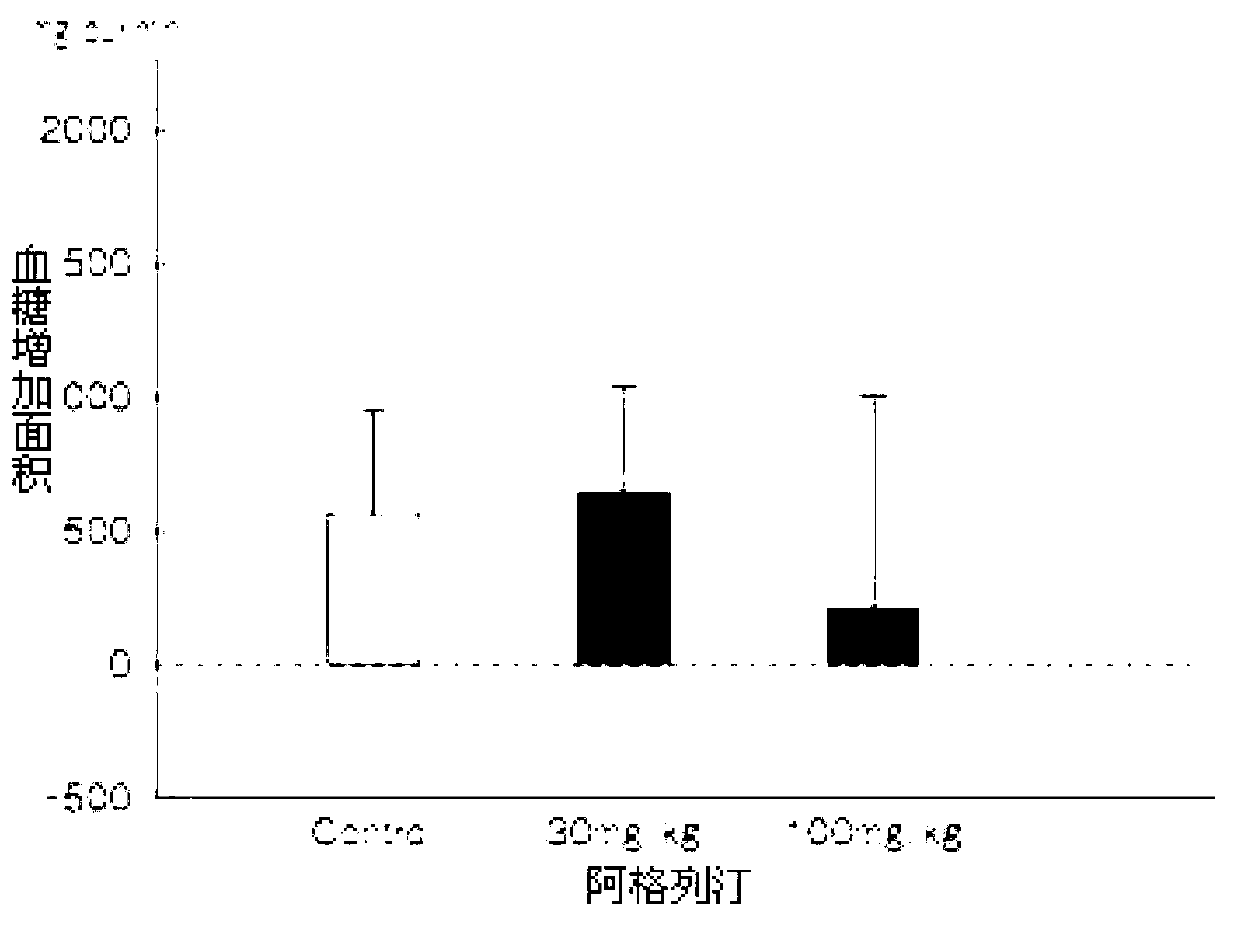
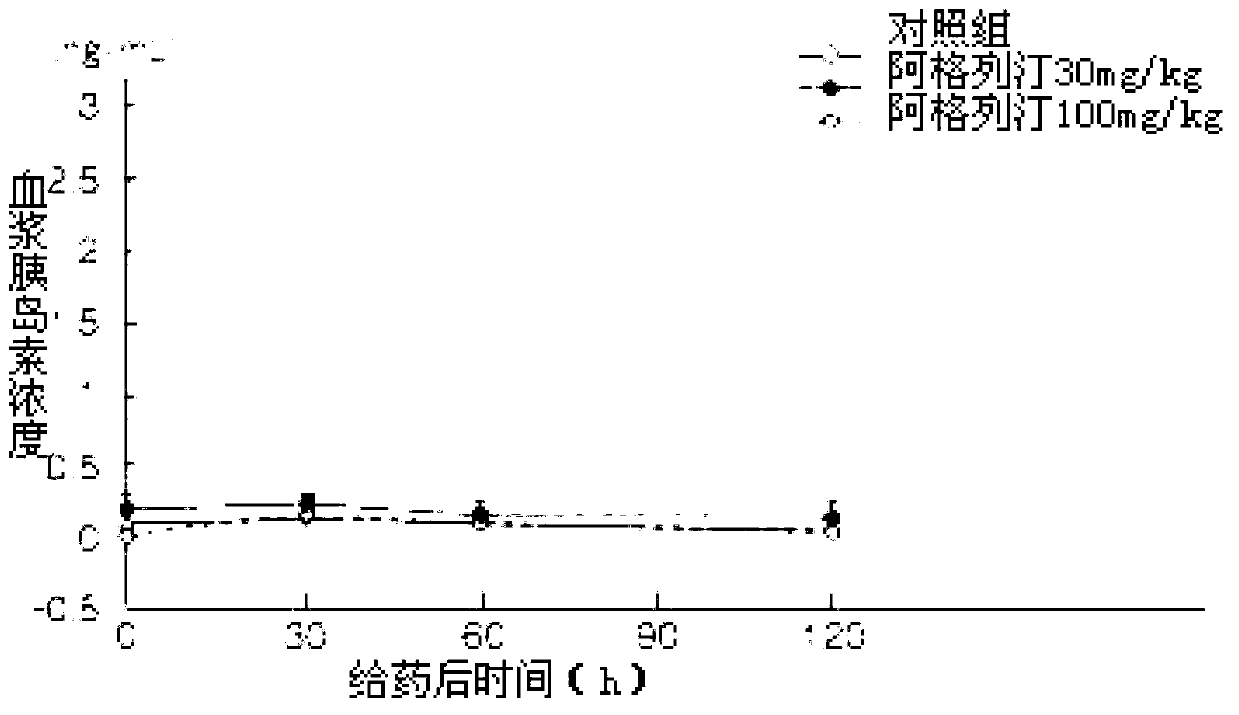
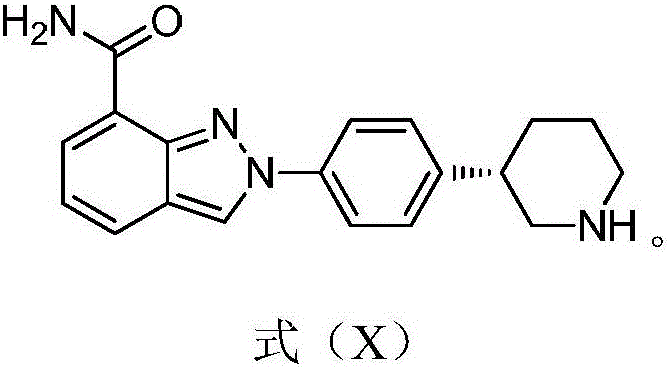
ActiveCN103193762ACarboxylic acid salt preparationBenzoic acidTert-Butyloxycarbonyl protecting group
The invention relates to the field of preparation of medicaments and specifically relates to a preparation method of alogliptin benzoate. The preparation method comprises the following steps of: taking 6-chlorouracil as a starting raw material, reacting with o-cyanobenzyl bromide to obtain 2-((6-chloro-2, 4-dioxo-3, 4-dihydro-2H-pyrimidin-1-yl) methyl) benzonitrile, further performing methylation with iodomethane to obtain 2-((6-chloro-3-methyl-2, 4-dioxo-3, 4-dihydro-2H-pyrimidin-1-yl) methyl) benzonitrile, then reacting with (3R)-3-tert-butoxycarbonylamino-piperidine to obtain N-(3-(2-cyano-benzyl)-1-methyl-2, 6-dioxo-1, 2, 3, 6-tetrahydro-pyrimidin-4-yl) piperidine-(3R)-3-tert-butyl carbamate, performing deprotection by hydrogen chloride gas, and further forming a salt with benzoic acid to obtain the alogliptin benzoate. According to the preparation method of the alogliptin benzoate, disclosed by the invention, the raw materials which are low in cost and easy to purchase are selected, an alogliptin benzoate product is finally generated by reaction, and the overall manufacturing cost is low; and the temperature is strictly controlled in the steps, byproducts are few, the yield is high, and no toxicity is generated.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Vinyl acetate-acrylate-ethylene multipolymer emulsion and preparation method thereof
ActiveCN109517102AUniform polymerizationImprove the single-pot conversion rateMonocarboxylic acid ester polymer adhesivesHyposulfiteSodium sulfate
The invention relates to the field of emulsion polymerization and discloses a vinyl acetate-acrylate-ethylene multipolymer emulsion and a preparation method thereof. The method comprises the followingsteps: under an emulsion polymerization reaction condition, (1) in the presence of an oxidization-reduction initiating system, carrying out a first polymerization reaction on a part of mixed monomercontaining a vinyl acetate monomer and an acrylate monomer and vinyl monomer, wherein in the oxidization-reduction initiating system, the oxidizing agent is tert-butyl hydroperoxide and the reducing agent is zinc formaldehyde sulfoxylate and / or sodium formaldehyde hyposulfite; and (2) adding the vinyl monomer and residual part of the mixed monomer into the system after the first polymerization reaction for a second polymerization reaction, wherein in the second polymerization reaction, tert-butyl peroxybenzoate is added into the reaction system. The content of acrylics in the copolymer emulsion obtained by the method is obviously higher than those in common modified EVA in the prior art, and the emulsion is good in bonding strength.
Owner:北京东方石油化工有限公司
High-strength pure liquid plugging agent plugging technology for plugging leakage layer and plugging water
PendingCN111927384AHigh strengthImprove stabilityDrilling compositionSealing/packingBenzoyl peroxideThermodynamics
The invention discloses a high-strength pure liquid plugging agent plugging technology for plugging a leakage layer and plugging water. The plugging technology includes the following steps that trailsqueezing is conducted on a to-be-plugged section, water injectivity indexes are tested, the using quantity of a plugging agent is calculated, a drillable composite bridge plug is set at the position30 meters below the to-be-plugged section, and then sand filling is carried out to the lower side of the plugged section; an oil pipe is tripped into the to-be-plugged section, a pipe column is squeezed, the plugging agent is prepared and further contains a silicone modified epoxy compound and a curing agent, the curing agent refers to tert-butyl peroxybenzoate or benzoyl peroxide peroxide, the plugging agent is squeezed into the plugged section, and a plugging agent plug is additionally arranged; and pressure shut-in waiting solidification is conducted, pressure testing is performed on the plugged plug surface, the plugging agent plug is removed in a drilling mode when pressure testing is qualified, plugging operation ending is confirmed after the pressure testing is qualified, the plugging agent with the silicone modified epoxy compound and the curing agent being mixed is used, the plugging strength can be improved and long-time plugging effectiveness is guaranteed.
Owner:大庆飞陆科技有限公司
Method for preparing (3S)-3-(4-aminophenyl)-piperidyl-1-tert-butyl formate
The invention discloses a method for preparing (3S)-3-(4-aminophenyl)-piperidyl-1-tert-butyl formate. The method is characterized by comprising the following steps: 1) performing a contact reaction on a 3-(4-aminophenyl)-piperidyl-1-tert-butyl formate racemate and (R)-(-)-binaphthyl-2,2-diyl hydrogen phosphate, cooling, crystallizing, and filtering to obtain a solid compound A; (2) hydrolyzing the solid compound A obtained in the step (1) under an acidic condition, adjusting the pH to be 8 to 10 after the hydrolysis reaction is completed, extracting with ethyl acetate, and concentrating to obtain the (3S)-3-(4-aminophenyl)-piperidyl-1-tert-butyl formate. The method provided by the invention is high in yield and high in product optical purity, and provides a new way for preparing the (3S)-3-(4-aminophenyl)-piperidyl-1-tert-butyl formate.
Owner:QINGDAO YUNTIAN BIOTECH
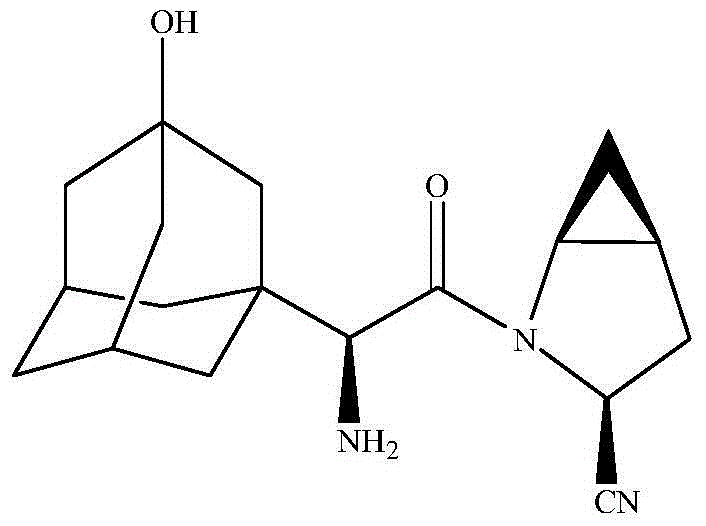
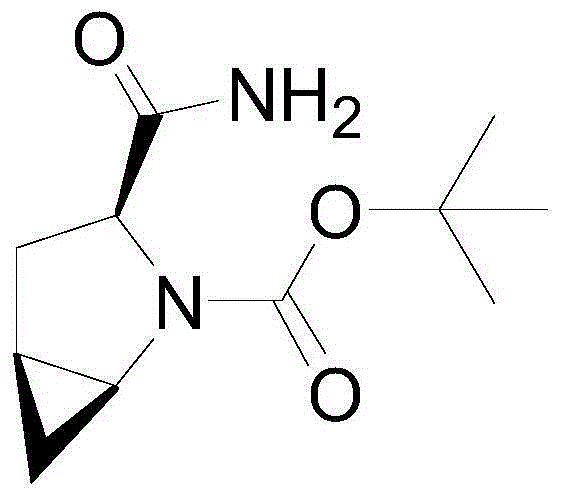
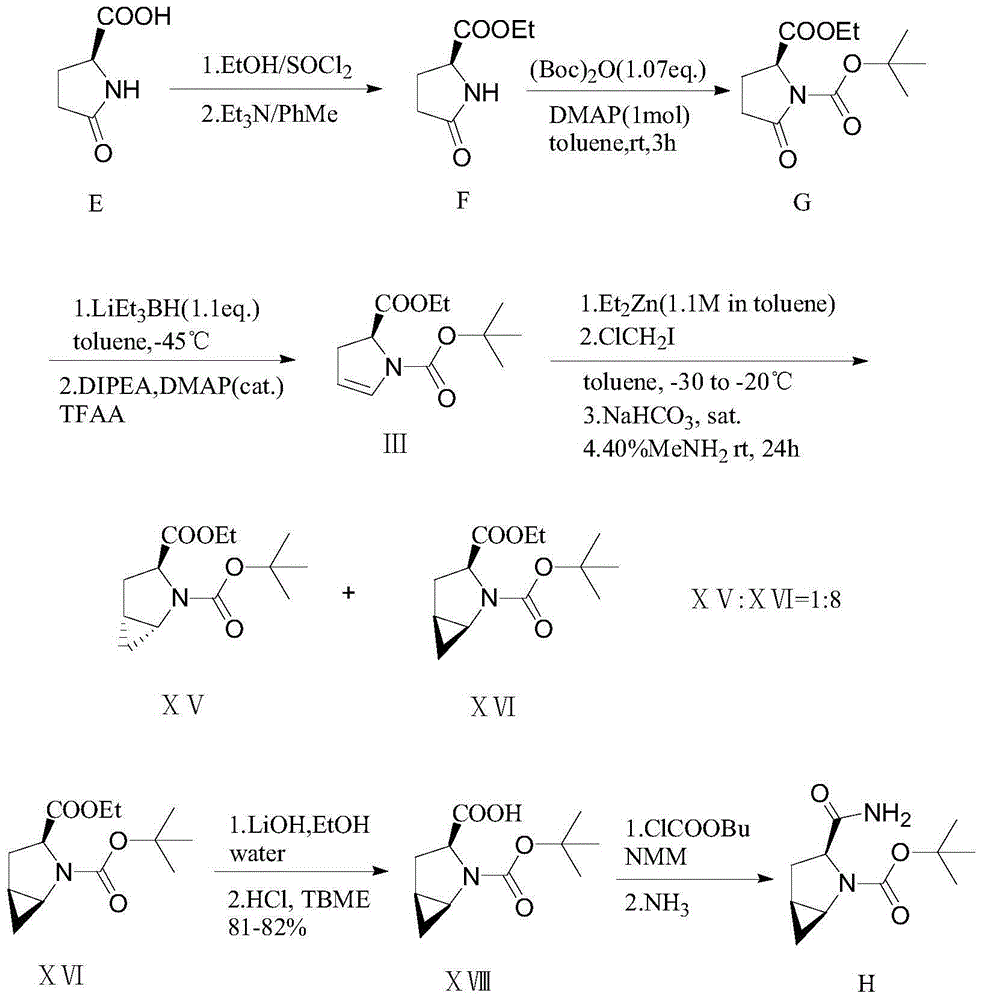
Preparation method of saxagliptin intermediate
The invention provides a preparation method of a saxagliptin intermediate (1S, 3S, 5S)-3-(amino carbonyl)-2-azabicyalo[3.1.0]hexane-2-tert-butyl formate. According to the preparation method, L-pyroglutamic acid is taken as a raw material, and an esterification reaction, Boc protection, reduction, elimination, cyclopropylation, resolution, hydrolyzation, ammonolysis, Boc deprotection and ammonolysis are performed on L-pyroglutamic acid, and finally, the compound (1S, 3S, 5S)-3-(amino carbonyl)-2-azabicyalo[3.1.0]hexane-2-tert-Butyl formate is obtained. The preparation method of the saxagliptin intermediate is cheap and easily available in raw materials, reasonable in process, simple and convenient to operate, high in enantiomer selectivity and high in yield.
Owner:SHANGHAI INST OF TECH
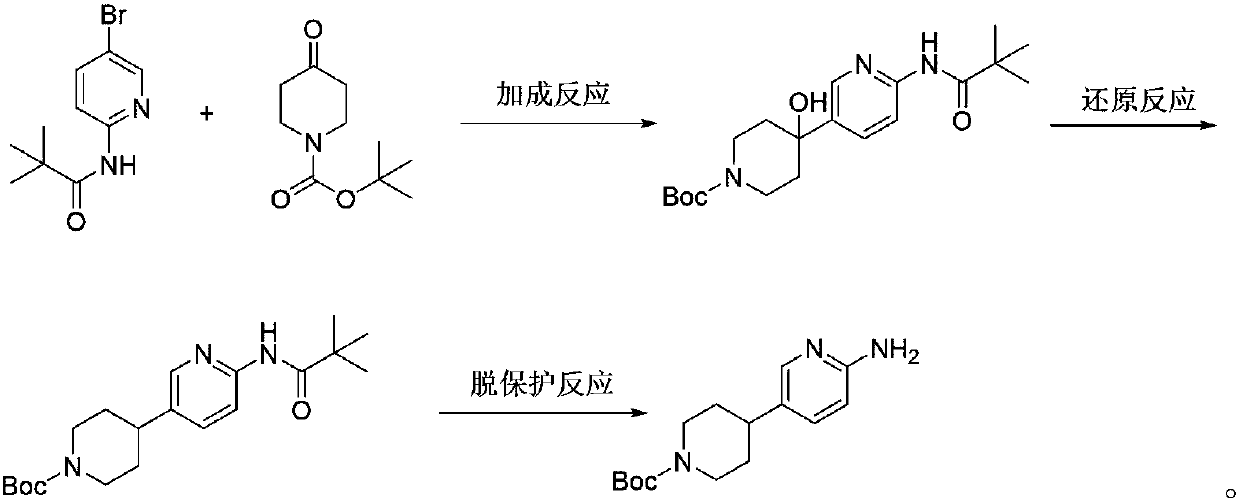
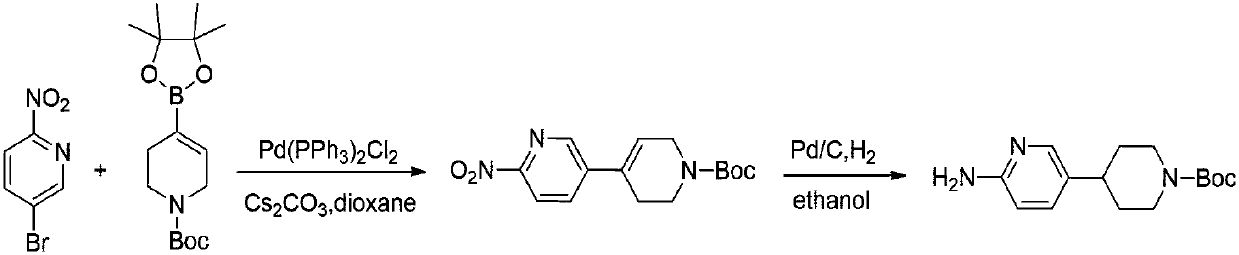
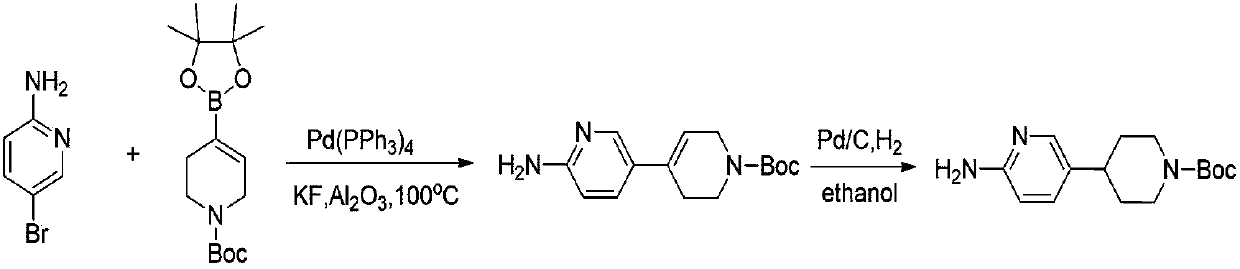
Preparation method of 4-(6-aminopyridine-3-radical) piperidine-1-tert-butyl formate
ActiveCN107827869AReduce manufacturing costReduce pollutionOrganic chemistryTert-Butyloxycarbonyl protecting groupBromine
The invention discloses a preparation method of 4-(6-aminopyridine-3-radical) piperidine-1-tert-butyl formate. The target product 4-(6-aminopyridine-3-radical) piperidine-1-tert-butyl formate is obtained by three steps of reactions by taking N-(5-bromine-piperidine-2-)-2, 2-dimethylacrylamide, N-t-butyloxycarboryl-4-piperidone, raney nickel and the like as raw materials. The preparation method issimple, convenient and stable in process operation, and a product in each step is easy to separate, high in yield and environmentally friendly; the comprehensive yield is 82 percent or above; comparedwith the yield of 42 percent of the existing process, the yield is obviously increased; furthermore, the raw materials are low in cost and readily available, so that the production costs of existingbiological, medical and chemical intermediates are substantially reduced; industrial large-scale production is facilitated.
Owner:SHANGHAI ZAIQI BIO TECH
Thermoplastic acrylic resin coating
The invention discloses a thermoplastic acrylic resin coating. Raw materials of the thermoplastic acrylic resin coating comprise, by weight, 100 parts of a thermoplastic acrylic resin, 50-70 parts of xylene, 50-70 parts of n-butanol, 10-15 parts of a coloring pigment, 3-5 parts of a corrosion-resistant pigment, 0.5-1.5 parts of a wetting dispersant and 0.1-0.5 part of a defoaming agent, wherein the thermoplastic acrylic resin comprises, by weight, 100 parts of a mixture obtained by mixing methyl methacrylate and cyclohexyl methacrylate according to a ratio of 1:3, 15-25 parts of methacrylic acid, 25-30 parts of styrene, 25-30 parts of dibenzoyl peroxide, 3-5 parts of dibenzoyl peroxide, azobisisbutyronitrile or tert-butyl peroxybenzoate, 0.5-1.5 parts of triphenylphosphine and 35-50 parts of a mixed solvent obtained by mixing polyvinyl alcohol and water according to a ratio of 1:35-50. The prepared coating can be applied by diluting with an organic solvent, can also be applied by hot melting, and has good adhesive force to surfaces of cement, asphalt and plastics.
Owner:NANJING HENGAN RESIN CHEM
Corrosion-resistant epoxy resin paint
ActiveCN103013292AImprove consistencyGood batch stabilityPretreated surfacesAnti-corrosive paintsBenzoic acidEpoxy
The invention discloses corrosion-resistant epoxy resin paint. The corrosion-resistant epoxy resin paint comprises the following raw materials in parts by weight: 132-145 parts of E-20 epoxy resin, 21-25 parts of modified phenolic resin, 18-24 parts of 1,2,3-benzotriazole, 2-3 parts of polyacrylic acid, 1-3 parts of ethylene glycol, 2-4 parts of pentaerythritol triacrylate, 0.8-1 part of N-2-(aminoethyl)-3-aminopropyl trimethoxysilane, 1-1.2 parts of poly(ethyl acrylate), 0.2-0.4 part of tert-butyl peroxybenzoate, 0.6-1 part of emulsifier OP-10, 0.1-0.2 part of defoaming agent poly(dimethylsiloxane) and 0.5-0.8 part of dodecyl dimethyl tertiary amine. The corrosion-resistant epoxy resin paint disclosed by the invention adopts an aqueous emulsion finished product prepared from the modified epoxy resin as a main film-forming substance, the whole production process is smooth and easy to control, the consistency of the finished product is greatly improved, and the batch stability is good; and the ethylene glycol and the pentaerythritol triacrylate are selected as solvents, the odor of the product is greatly improved, and the environmental pollution in production and use is reduced.
Owner:TONGLING SANJIA TRANSFORMER
Thermoplastic acrylic resin plastic paint
The invention discloses a thermoplastic acrylic resin plastic paint. Raw materials of the thermoplastic acrylic resin plastic paint comprise, by weight, 100 parts of a thermoplastic acrylic resin, 5-8 parts of a pigment, 50-70 parts of a filler, 1-3 parts of a flatting agent, 1-3 parts of a dispersant and 1.5 parts of a curing agent, wherein the thermoplastic acrylic resin comprises, by weight, 100 parts of a mixture obtained by mixing methyl methacrylate and cyclohexyl methacrylate according to a ratio of 1:3, 15-25 parts of methacrylic acid, 25-30 parts of styrene, 25-30 parts of dibenzoyl peroxide, 3-5 parts of dibenzoyl peroxide, azobisisbutyronitrile or tert-butyl peroxybenzoate, 0.5-1.5 parts of triphenylphosphine and 35-50 parts of a mixed solvent obtained by mixing polyvinyl alcohol and water according to a ratio of 1:35-50. A coating prepared by using the thermoplastic acrylic resin plastic paint can be applied by diluting with an organic solvent, can also be applied by hot melting, and has good adhesive force to surfaces of cement, asphalt and plastics.
Owner:NANJING HENGAN RESIN CHEM
Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate
InactiveCN105111155AStarting materials are cheap and readily availableLow costOrganic chemistryChemical synthesisSynthesis methods
The invention belongs to the technical field of chemical synthesis of N-heterocycle-containing drug intermediates, and particularly relates to a synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate. By using diethyl malonate as a raw material, cyclization reaction, Hofmann reaction, hydrolysis reaction, acylation reaction for recyclization, reduction reaction and the like are performed to conveniently synthesize the target compound product. The method has the advantages of simple synthesis technique, cheap and accessible raw materials, mild reaction conditions, high controllability, low cost and high yield, and is convenient to operate.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate
InactiveCN109503624AReasonable reaction process designMethod route shortOrganic chemistryNonaneSolvent
The invention relates to a method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate, and mainly solves a technical problem of absence of a method suitable for industrialsynthesis at present. The method provided by the invention comprises the following three steps: step one, firstly enabling a compound 1, paraformaldehyde and tetrabutylammonium fluoride added into asolvent N, N-dimethylformamide to be in reaction to obtain a compound 2; step two, enabling the compound 2, ethyl bromoacetate and cesium carbonate to be in reaction in acetone to obtain a compound 3;and step three, enabling the compound 3 and iron and ammonium chloride to be in reaction in ethanol and water to obtain a final compound 4, wherein a reaction formula is as shown in the Specification.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
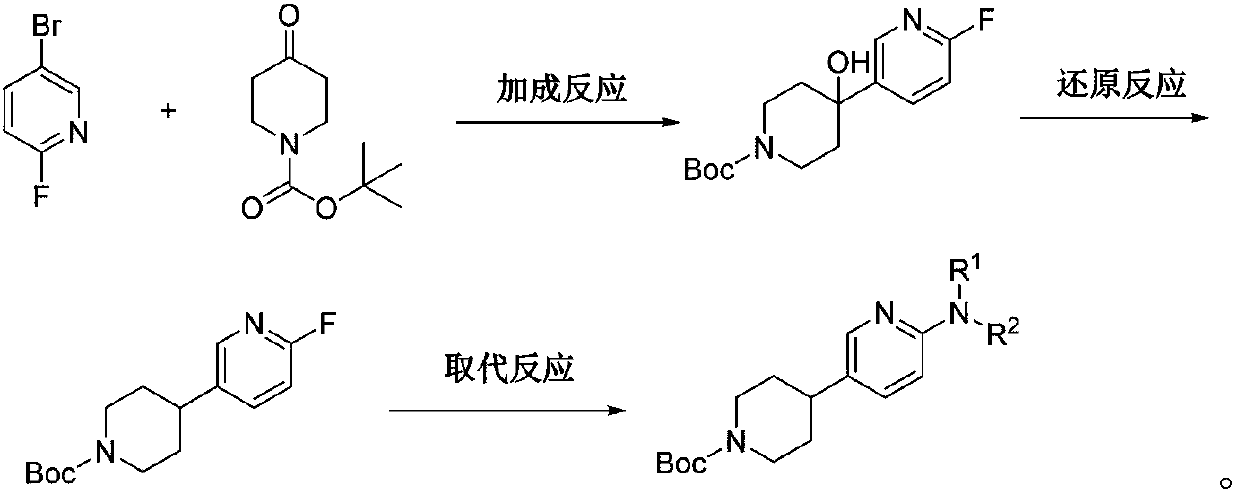
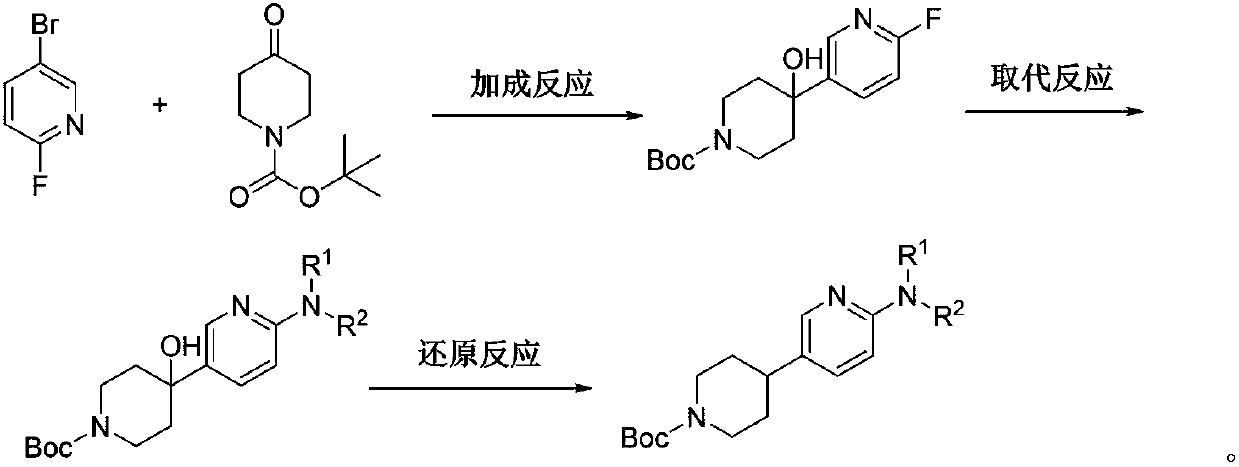
Preparation method of 4-(6-substituted aminopyridine-3-radical) piperidine-1-tert-butyl formate
ActiveCN107759563AEasy to operateShort reaction pathOrganic chemistryTert-Butyloxycarbonyl protecting groupTert-Butyl formate
The invention discloses a preparation method of 4-(6-substituted aminopyridine-3-radical) piperidine-1-tert-butyl formate. The method is characterized in that 2-fluoro-5-bromopyridine, N-t-butyloxycarboryl-4-piperidone, raney nickel, etc. are used as the raw materials and subjected to three-step reaction to prepare the target product that is 4-(6-substituted aminopyridine-3-radical) piperidine-1-tert-butyl formate. The method is simple, convenient and stable in process and operation; the product obtained in each step is easily separated and high in yield; the environment is protected; the rawmaterials are low in price and easy to obtain, so that the production cost of existing biological, medical and chemical intermediates can be greatly decreased; and the method is beneficial for industrial massive production.
Owner:SHANGHAI ZAIQI BIO TECH
High-rigidity, high-toughness and low-shrinkage-percentage bulk molding compound and preparation method thereof
The invention relates to high-rigidity, high-toughness and low-shrinkage-percentage bulk molding compound and a preparation method thereof. The bulk molding compound is prepared from the following ingredients in weight percentage: 20% to 30% of unsaturated polyester resin, 8% to 12% of polyurethane elastomer resin, 1% to 5% of thermoplastic resin, 8% to 14% of antishrinking agent, 0.2%to 0.4% of tert-butyl peroxybenzoate, 0.2% to 0.4% of tert-butyl cyclohexane peroxide, 0.02%to 0.05% of p-benzoquinone solution, 0.02% to 0.05% of tert-butyl phthalate xylenol solution, 1% to 2% of releasing agent, 15% to 30% of aluminum hydroxide, 1% to 8% of calcium carbonate and 15% to 30% of alkali-free chopped glass fiber. According to the bulk molding compound, the polyurethane elastomer resin and the linear thermoplastic resin are introduced, the polyurethane elastomer resin can be mixed and cross-linked with the unsaturated polyester resin, the linear thermoplastic resin can form a cross network structure with the unsaturated resin, not only are rigidity and toughness of the bulk molding compound obviously improved, but also the shrinkage rate of the bulk molding compound is reduced.
Owner:YUEQING ZHENGYAN ELECTRIC
Alkali-soluble solid dispersant resin and preparation method thereof
The invention provides a preparation method of alkali-soluble solid dispersant resin. The preparation method includes uniformly mixing acrylic acid, styrene, alpha-methyl styrene, maleic anhydride, di-tert-butyl peroxide and / or tert-butyl peroxybenzoate at room temperature to obtain mixture A, adding linear alcohol ethoxylates and solvent diethylene glycol dimethyl ether into a reactor with heating to 140-160 DEG C,adding the mixture A under nitrogen atmosphere, then heating up to 170-185 DEG C for polymerization reaction for 20-60 min, and performing reduced pressure distillation after reaction to obtain the alkali-soluble solid dispersant resin. During mass polymerization of styrene and acrylic acid, the hydrophilic-lipophilic amphiphilic component, namely linear alcohol ethoxylates is added, the terminal hydroxyl group of the linear alcohol ethoxylates reacts with maleic anhydride or acrylic acid in the system to form a hydrophilic-lipophilic side chain, the lipophilic group at the terminal of the side chain changes bonding point distribution of dispersant and pigment, bonding stability of dispersant and pigment is improved beneficially, and dispersing performance on pigment is improved.
Owner:河南省中凌煜新材料科技有限公司
Controllable slow-release phase transfer catalyst microcapsules and preparation method of controllable slow-release phase transfer catalyst microcapsules
InactiveCN106883324AGood dispersionReduce viscosityMicroballoon preparationMicrocapsule preparationBenzoyl peroxidePolyethylene glycol
The invention relates to controllable slow-release phase transfer catalyst microcapsules and a preparation method of the controllable slow-release phase transfer catalyst microcapsules. The method comprises the following steps: sufficiently stirring sodium dodecyl sulfate, polyethylene glycol 6000, a phase transfer catalyst, a strong oxidant, an auxiliary surfactant, ethanol, a pH (Potential of Hydrogen) value regulator and a pyrrole monomer into de-ionized water solution under a room-temperature condition; centrifuging and drying to obtain the phase transfer catalyst microcapsules. Reaction is carried out on the de-ionized water so that an oil-soluble core material is easily subjected to phase transfer catalyst microencapsulation; a preparation process of the controllable slow-release phase transfer catalyst microcapsules is not only limited in the de-ionized water, and the phase transfer catalyst microcapsules with a water-soluble core material can also be prepared in an oily solvent system; a core material phase transfer catalyst is not limited to 2,2'-azobis(2-methylpropionitrile) and can be any one of ammonium persulfate, benzoyl peroxide, tert-butyl hydroperoxide, tert-butyl peroxybenzoate and diisopropyl peroxydicarbonate.
Owner:YANGZHOU POLYTECHNIC INST
High-dielectric-constant polyvinylidene fluoride (PVDF) as well as preparation method and application thereof
ActiveCN109810212AReduce crystallinityExcellent electrical performancePolyvinylidene fluorideOrganic chemicals
The invention relates to the technical field of organic chemical materials and specifically relates to high-dielectric-constant polyvinylidene fluoride (PVDF) and a preparation method thereof. The preparation method comprises the following steps: carrying out a polymerization reaction on vinylidene fluoride, fluoroolefin or a vinylidene fluoride monomer in the presence of an initiator, a chain transfer agent and a pH (Potential of Hydrogen) regulator, wherein the fluoroolefin is one of trifluoroethylene, trifluorochloroethylene, difluorochloroethylene and tetrafluoroethylene; the initiator isorganic peroxide; and the organic peroxide is one of dicumyl peroxide, cumene hydroperoxide, di-tert-butyl peroxide, tert-butyl peroxybenzoate and tert-butyl hydroperoxide. Polyvinylidene fluoride resin prepared by the preparation method involved in the invention has the properties of low crystallization degree, highly-irregular chain segments, high beta-phase type polymers and the like; and a product of the preparation method has excellent electrical performance in electronic devices, energy storage containers and sensors.
Owner:CHENGUANG RES INST OF CHEM IND
Preparation method for 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid
ActiveCN103073480AFast preparationShort synthetic routeOrganic chemistryTert-Butyloxycarbonyl protecting groupCarboxylic acid
The invention relates to a preparation method for a 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid and mainly aims to solve the technical problems that a traditional synthesis process is long in route, high in cost, difficult in reaction control and inconvenient in experimental operation. The 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid is prepared by taking 4-oxohexahydro [c] pyrrole-2 (1H)- formic acid tert-butyl ester as a raw material through a six-step reaction. The reaction formula is shown as follows: , the 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid obtained by the preparation method is a key intermediate for synthetizing a compound with a treatment potential of an active nicotinic acetylcholine receptor of combining and adjusting neurons.
Owner:武汉药明康德新药开发有限公司
Preparation method of 7,9-dioxo-2,6-aza-spiro[3.5]nona-2-tert-butyl formate and intermediate thereof
ActiveCN102936216ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryEthyl chloroformateAfter treatment
The invention relates to the field of medicine intermediate synthesis, and particularly relates to a preparation method of 7,9-dioxo-2,6-aza-spiro[3.5]nona-2-tert-butyl formate (VII) and an intermediate (I) thereof. The method is characterized by comprising the following steps of: enabling the raw material 1-Boc-3-cyanoazetidine to react with ethyl chloroformate and then with methyl iodide; and finally performing reduction reaction to obtain the intermediate (I). The invention also discloses a preparation method for preparing the compound (VII) by taking the 1-Boc-3-cyanoazetidine as the raw material. According to the preparation method provided by the invention, the raw material is cheap and easily available, the reaction conditions are relatively mild, the yield is high, the total yield of the compound VII can reach about 72.0%, and the yield of the compound I is 90%. The raw material is cheap, the after-treatment is simple, and the method is easy to operate and suitable for large-scale preparation.
Owner:SHANDONG DIAI BIOTECH CO LTD
Method for synthesizing Crizotinib intermediate
InactiveCN102898449AEasy to synthesizeReduce manufacturing costGroup 3/13 element organic compoundsPtru catalystBenzoyl peroxide
The invention belongs to the technical field of medicine synthesis, and in particular relates to a method for synthesizing a Crizotinib intermediate. The method comprises steps of: a) reacting a raw material 4-mesylate piperidine-1-formic acid tert-butyl ester (2) with 4-nitro pyrazole to prepare a compound 3; b) reducing nitro by using hydrazine hydrate to obtain an amino compound 4; and c) diazotizing the compound 4 by using tert-butyl nitrite, and reacting the compound 4 with a boric acid ester compound 5 in the presence of a free radical initiator benzoyl peroxide, so as to prepare the Crizotinib intermediate (1). Compared with an existing synthesis method, the method provided by the invention has the following advantages: a diazotization method is used to synthesize the boric acid ester product; and compared with an existing Miyaura boronation method catalyzed by Pd, the method avoids the usage of expensive palladium catalyst and ligand, and has the merits of mild reaction condition, high yield, simple operation, cheap and easily available raw materials and short reaction period, and is quite easy for industrialized mass production.
Owner:TONGJI UNIV
Thermoplastic acrylic resin adhesive
InactiveCN103773289AImprove thermoplasticityImprove adhesionNon-macromolecular adhesive additivesMacromolecular adhesive additivesPolymer scienceAcrylic resin
The invention discloses a thermoplastic acrylic resin. Raw materials of the thermoplastic acrylic resin comprise, by weight, 100 parts of the thermoplastic acrylic resin, 15-20 parts of dibutyl phthalate, 3-5 parts of silica micropowder, 1-1.5 parts of melamine gelatine powder, 0.5 part of a compound emulsifier, 1.2 parts of a neutralizer, 1-3 parts of a catalyst and 25-30 parts of an organic solvent, wherein the thermoplastic acrylic resin comprises, by weight, 100 parts of a mixture obtained by mixing methyl methacrylate and cyclohexyl methacrylate according to a ratio of 1:3, 15-25 parts of methacrylic acid, 25-30 parts of styrene, 25-30 parts of dibenzoyl peroxide, 3-5 parts of dibenzoyl peroxide, azobisisbutyronitrile or tert-butyl peroxybenzoate, 0.5-1.5 parts of triphenylphosphine and 35-50 parts of a mixed solvent obtained by mixing polyvinyl alcohol and water according to a ratio of 1:35-50. A coating prepared by using the thermoplastic acrylic resin adhesive can be applied by diluting with an organic solvent, and can also be applied by hot melting.
Owner:NANJING HENGAN RESIN CHEM
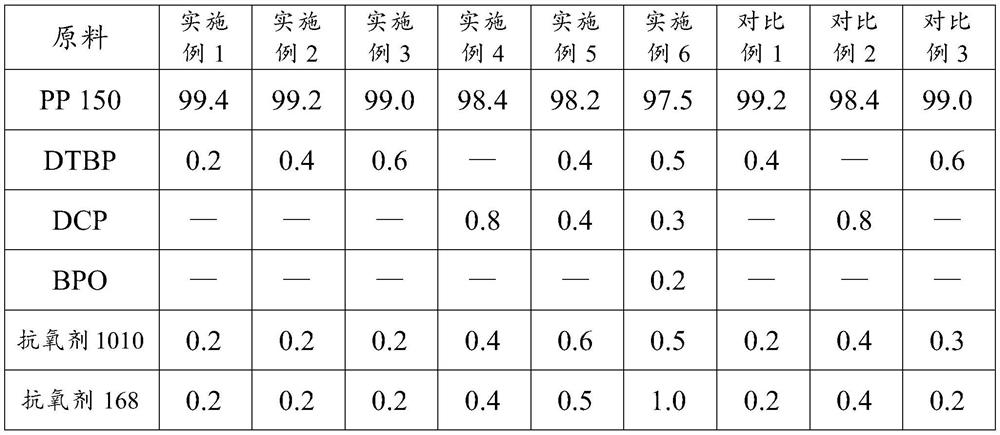
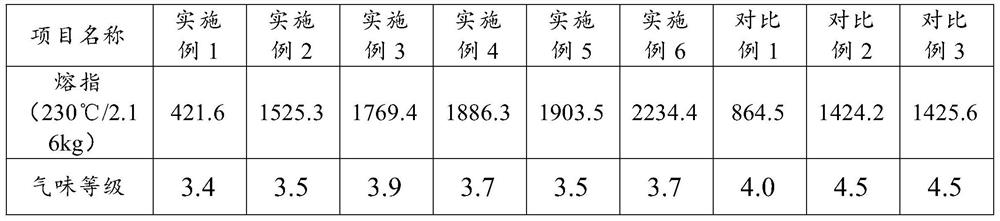
Low-odor polypropylene material with ultrahigh melt index and preparation method thereof
The invention relates to low-odor polypropylene with an ultrahigh melt index and a preparation method thereof. The low-odor polypropylene is prepared from the following raw materials in percentage bymass: 97%-99.5% of polypropylene, 0.05%-1.5% of a peroxide initiator and 0.1%-2% of an antioxidant, wherein the peroxide initiator is one or a mixture of more selected from the group consisting of benzoyl peroxide, tert-butyl peroxybenzoate, di-tert-butyl peroxide and dicumyl peroxide. The preparation method is simple in process; the peroxide initiator and the antioxidant are added in sections, the peroxide initiator is added in the third section of a twin-screw extruder, and the antioxidant is added in the seventh area by using a side feeding method, so the consumption of peroxide and the smell of the ultrahigh-melt-index polypropylene material are reduced, and the effect of the antioxidant is exerted to the maximum extent; and the prepared polypropylene material has the characteristics of low cost, high and stable melt index (1500+ / -100g / min) and low odor.
Owner:中广核俊尔(上海)新材料有限公司
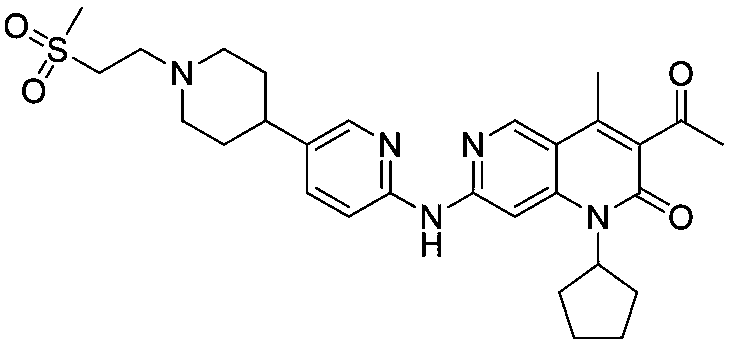
Preparation method of ribociclib and product and use thereof
InactiveCN109400612AAchieve primary separationMild responseOrganic chemistryCarboxylic acidRibociclib
A preparation method of ribociclib comprises the following steps: 1) utilizing 4-(6-aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester and 2-chloro-4-cyclopentyl-N,N-dimethyl-7H-pyrrole[2,3-d]pyrimidine-6-formamide as raw materials and conducting reaction in a protective atmosphere with palladium acetate / BINAP as a catalyst, cesium carbonate as an acid absorber and 4-methyl-2-pentanone as a solvent to obtain 4-(6-(7-cyclopentyl-6-(dimethylaminoformyl)-7H-pyrrolo[2,3-d]pyrimidine-2-yl)aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester); 2) dissolving the4-(6-(7-cyclopentyl-6-(dimethylaminoformyl)-7H-pyrrolo[2,3-d]pyrimidine-2-yl)aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester) obtained in step 1) in an organic solvent, adding acid dropwise at the room temperature to remove tert-butyl formate, conducting liquid separation, adding a water-soluble organic solvent to an aqueous layer, separating solids and filtering to obtain ribociclib acid salt; 3) adding water to the ribociclib acid salt for dissolving, adding an adsorbent, filtering and adding alkali to the filtrate to obtain the ribociclib.
Owner:CHONGQING SANSHENG IND CO LTD
Flame-retardant thermal insulation paint used for curtain walls and preparation method of flame-retardant thermal insulation paint
InactiveCN104987795AImprove flame retardant performanceGood heat insulationFireproof paintsWeather resistanceAcrylic resin
The invention discloses flame-retardant thermal insulation paint used for curtain walls and a preparation method of the flame-retardant thermal insulation paint. The flame-retardant thermal insulation paint is composed of, by weight, 100 parts to 115 parts of modified acrylic resin, 1 part to 1.6 parts of flatting agents, 3 parts to 4 parts of (1S,3S,5S)-3-(amino-carbonyl)-2-azabicyalo[3.1.0]hexane-2-tert-butyl formate, 0.8 part to 1.6 parts of guanidine carbonate, 1 part to 1.5 parts of zinc molybdate and 10 parts to 15 parts of bi(3,4-dimethyl benzylidene)sorbitol. The flame-retardant thermal insulation paint has the advantages that the flame-retardant effect and the thermal insulating effect of the paint are good; environmental protection is achieved, pollution is little, the machining process is simple, and operation is convenient; glass beads can not be separated out when the flame-retardant thermal insulation paint is used for a long time; the weather resistance of the paint is high, and paint components can not be separated out.
Owner:王舒怡
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/686d6434-d716-4732-89d1-53ce0fc7652e/BDA0002287786710000021.png)
![Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/686d6434-d716-4732-89d1-53ce0fc7652e/BDA0002287786710000031.png)
![Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/686d6434-d716-4732-89d1-53ce0fc7652e/BDA0002287786710000041.png)


![Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98933b6c-efbc-47d1-a3d7-5eaa2ddeed5c/HDA0000793427800000011.PNG)
![Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98933b6c-efbc-47d1-a3d7-5eaa2ddeed5c/HDA0000793427800000012.PNG)
![Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98933b6c-efbc-47d1-a3d7-5eaa2ddeed5c/HDA0000793427800000021.PNG)
![Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0d300b0-9773-430c-84c7-853737052f0a/DEST_PATH_IMAGE001.png)
![Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0d300b0-9773-430c-84c7-853737052f0a/DEST_PATH_IMAGE003.png)




![Preparation method for 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid Preparation method for 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8d7d3a8d-d396-44fc-96b2-5f5bf3e85271/11857DEST_PATH_IMAGE003.PNG)
![Preparation method for 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid Preparation method for 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8d7d3a8d-d396-44fc-96b2-5f5bf3e85271/2013100477459100002DEST_PATH_IMAGE001.PNG)
![Preparation method for 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid Preparation method for 2-(t-butyloxycarbonyl) octahydrocyclopenta [c] pyrrole-5-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8d7d3a8d-d396-44fc-96b2-5f5bf3e85271/2013100477459100002DEST_PATH_IMAGE003.PNG)
![Preparation method of 7,9-dioxo-2,6-aza-spiro[3.5]nona-2-tert-butyl formate and intermediate thereof Preparation method of 7,9-dioxo-2,6-aza-spiro[3.5]nona-2-tert-butyl formate and intermediate thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2510c4b-f30a-427b-9432-acffab2349d7/BDA00002526654700011.PNG)
![Preparation method of 7,9-dioxo-2,6-aza-spiro[3.5]nona-2-tert-butyl formate and intermediate thereof Preparation method of 7,9-dioxo-2,6-aza-spiro[3.5]nona-2-tert-butyl formate and intermediate thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2510c4b-f30a-427b-9432-acffab2349d7/BDA00002526654700021.PNG)
![Preparation method of 7,9-dioxo-2,6-aza-spiro[3.5]nona-2-tert-butyl formate and intermediate thereof Preparation method of 7,9-dioxo-2,6-aza-spiro[3.5]nona-2-tert-butyl formate and intermediate thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2510c4b-f30a-427b-9432-acffab2349d7/BDA00002526654700022.PNG)