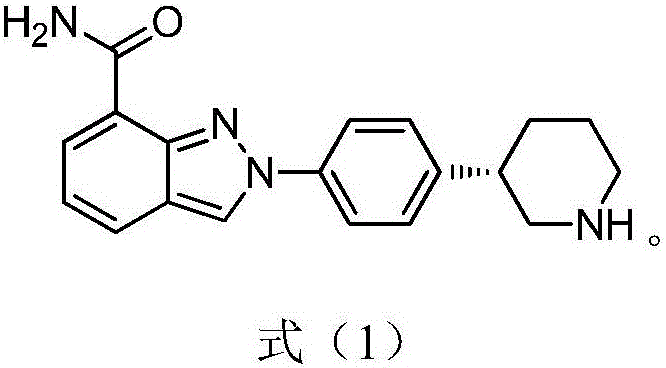
Preparation method of niraparib intermediate of (3S)-3-(4-aminophenyl) piperidine-1-tert-butyl formate
A technology of tert-butyl formate and aminophenyl, which is applied in the field of drug synthesis and can solve problems such as difficult separation, high cost, and inability to split 3-(4-aminophenyl)piperidine-1-carboxylic acid tert-butyl , to achieve the effects of less environmental pollution, easy recycling and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve 2.76g (10mmol) of 3-(4-aminophenyl)piperidine-1-carboxylic acid tert-butyl ester racemate in 85mL of an aqueous ethanol solution (the volume ratio of water to alcohol is 1:16), stir and drop. Add 3.06g of the resolving agent benzenesulfonyl-D-phenylglycine dissolved in 10mL of 50% (v / v) ethanol solution. After the addition is complete, the mixed solution is reacted at 65°C for 2.5 hours, and slowly cooled to 40°C for crystallization 2 After hours, the crystallization was continued at room temperature to obtain (3S)-3-(4-aminophenyl)piperidine-1-carboxylate tert-butyl p-sulfonyl-D-phenylglycinate 2.56 g, with a yield of 90.3%.
[0040] 2.56g (3S)-3-(4-aminophenyl)piperidine-1-carboxylate tert-butyl benzenesulfonyl-D-phenylglycinate was added to 30g ethanol aqueous solution (V water: V ethanol = 1:9 ), heated to 55°C to dissolve, kept for 1.0 hour, stirred and cooled to crystallize to obtain 2.43 g of the product.
[0041] 2.43g (3S)-3-(4-aminophenyl)piperidine-1-car...
Embodiment 2
[0043] Dissolve 2.76g (10mmol) of 3-(4-aminophenyl)piperidine-1-carboxylic acid tert-butyl ester racemate in 90mL aqueous ethanol solution (water to alcohol volume ratio 1:18), and drop with stirring Add 3.00g of the resolving agent benzenesulfonyl-D-phenylglycine dissolved in 10mL of 50% (v / v) ethanol solution. After the addition is complete, the mixed solution is reacted at 60°C for 3 hours, and slowly cooled to 45°C to crystallize 2 After hours, the crystallization was continued at room temperature to obtain (3S)-3-(4-aminophenyl)piperidine-1-carboxylate tert-butyl benzenesulfonyl-D-phenylglycinate 2.60 g, with a yield of 91.5%.
[0044] Add 2.60g (3S)-3-(4-aminophenyl)piperidine-1-carboxylic acid tert-butyl benzenesulfonyl-D-phenylglycinate to 36g ethanol aqueous solution (V water: V ethanol = 1:9 ), heated to 60°C to dissolve, kept for 1.5 hours, stirred and cooled to crystallize to obtain 2.40g of the product.
[0045] Suspend 2.40g (3S)-3-(4-aminophenyl)piperidine-1-carboxy...
Embodiment 3
[0047] Dissolve 2.76g (10mmol) of 3-(4-aminophenyl)piperidine-1-carboxylic acid tert-butyl ester racemate in 78mL of aqueous ethanol solution (volume ratio of water to alcohol is 1:16), stir and drop. Add 3.09g of the resolving agent benzenesulfonyl-D-phenylglycine dissolved in 12mL of 50% (v / v) ethanol solution. After the addition is complete, the mixed solution is reacted at 63°C for 2.5 hours and slowly cooled to 40°C for crystallization 2.0 After hours, the crystallization is continued at room temperature to obtain (3S)-3-(4-aminophenyl)piperidine-1-carboxylate tert-butyl benzenesulfonyl-D-phenylglycinate 2.70g with a yield of 95.1%.
[0048] Add 2.70 g of (3S)-3-(4-aminophenyl)piperidine-1-carboxylate benzenesulfonyl-D-phenylglycinate to 50 g of ethanol aqueous solution (V water: V ethanol = 1:10 ), heated to 58°C for dissolution, kept for 1.0 hour, stirred and cooled to crystallize to obtain 2.54 g of the product.
[0049] (2) Suspend 2.54g (3S)-3-(4-aminophenyl)piperidine-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com