Preparation method of 4-(6-substituted aminopyridine-3-radical) piperidine-1-tert-butyl formate
A technology of tert-butyl formate and aminopyridine, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of many reaction impurities, low synthesis process yield, high price and the like, and achieves the advantages of reducing environmental pollution, optimizing preparation process and reducing production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
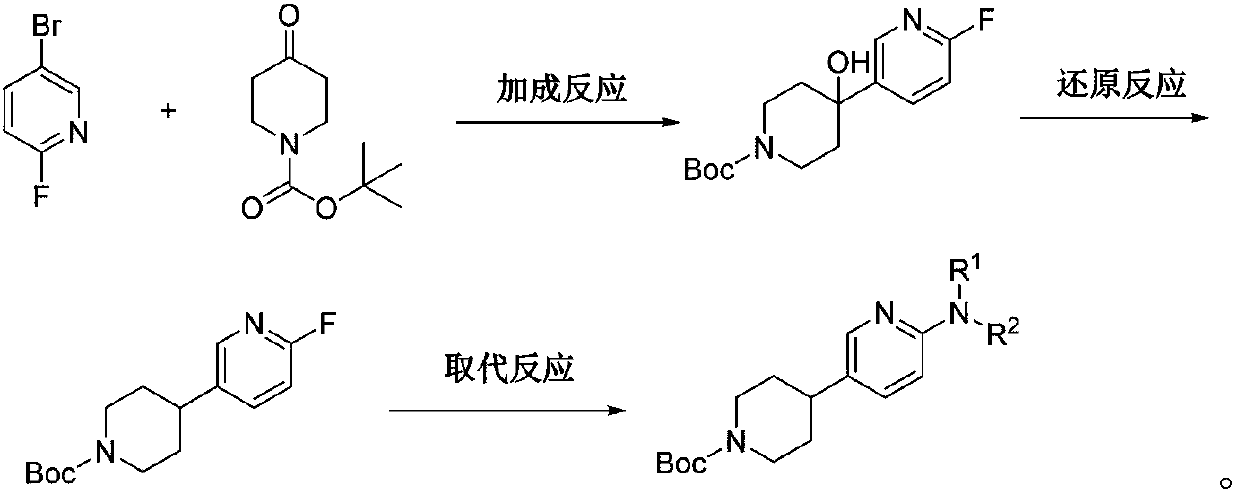
[0037] The first step: the synthesis of 4-(6-fluoro-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine
[0038] Under the protection of nitrogen, in the reaction flask, add 2-fluoro-5-bromopyridine (8.80g, 0.05mol) and tetrahydrofuran (24mL), control the temperature from -10°C to 0°C, and add tetrahydrofuran of isopropylmagnesium bromide dropwise The solution (60mL, 0.06mol) was exchanged at room temperature for 4 hours after dropping, the reaction was completed, and the Grignard reagent was generated. N-tert-butoxycarbonyl-4-piperidone (9.96 g, 0.05 mol) was dissolved in 50 ml of tetrahydrofuran, and the above Grignard reagent was added dropwise, and reacted at 60° C. for 24 hours. TLC monitors the completion of the reaction and adds saturated ammonium chloride to convert Grignard salt into alcohol. After the reaction is completed, extract with ether, dry and spin dry, and purify with ether to obtain 4-(6-fluoro-3-pyridyl)-4-hydroxyl-N- 12.40 g of tert-butoxycarbonylpiperi...
Embodiment 2
[0049] The first step: the synthesis of 4-(6-fluoro-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine
[0050] Under the protection of nitrogen, add 2-fluoro-5-bromopyridine (880g, 5mol) and tetrahydrofuran (2.4L) into the reaction flask, control the temperature from -10°C to 0°C, and add isopropylmagnesium bromide solution in tetrahydrofuran dropwise (10L, 10mol, 1M), exchange at room temperature for 4 hours after dropping, the reaction is complete, and Grignard reagent is generated. N-tert-butoxycarbonyl-4-piperidone (796.8 g, 4 mol) was dissolved in 5 L of tetrahydrofuran, and the above-mentioned Grignard reagent was added dropwise, and reacted at 60°C for 24 hours. TLC monitors the completion of the reaction and adds saturated ammonium chloride to convert Grignard salt into alcohol. After the reaction is completed, extract with ether, dry and spin dry, and purify with ether to obtain 4-(6-fluoro-3-pyridyl)-4-hydroxyl-N- tert-butoxycarbonylpiperidine 1235g, yield 83.4%....
Embodiment 3
[0060] The first step: the synthesis of 4-(6-fluoro-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine
[0061] Under the protection of nitrogen, in the reaction kettle, add 2-fluoro-5-bromopyridine (8.80kg, 50mol) and tetrahydrofuran (24L), control the temperature from -10°C to 0°C, and add the tetrahydrofuran solution of isopropylmagnesium bromide dropwise (75L, 75mol, 1M), exchange at room temperature for 4 hours after dropping, the reaction is complete, and Grignard reagent is generated. Dissolve N-tert-butoxycarbonyl-4-piperidone (8.96 kg, 45 mol) in 50 L of tetrahydrofuran, add the above-mentioned Grignard reagent dropwise, and react at 60° C. for 24 hours. TLC monitors the completion of the reaction and adds saturated ammonium chloride to convert Grignard salt into alcohol. After the reaction is completed, extract with ether, dry and spin dry, and purify with ether to obtain 4-(6-fluoro-3-pyridyl)-4-hydroxyl-N- tert-butoxycarbonylpiperidine 12.29kg, yield 83.04%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com