Method for synthesizing natural product (+/-)-folicanthine and intermediate product thereof
A synthetic method and technology of natural products, applied in the field of medicine, can solve problems such as difficulties in industrial production, high reaction temperature, and many reaction steps, and achieve the effects of high yield, low reaction temperature, and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A kind of synthetic method of the intermediate product of natural product (±)-folicanthine, described method is:
[0040] At 25°C, mix 10 mmoles of compound a with 20 mmoles of Cs 2 CO 3 Dissolve in 50 ml of acetonitrile, add 20 mmoles of iodine and stir at 25°C for 30 min; then add saturated Na 2 S 2 o 3 Quench the reaction and continue to add saturated Na 2 S 2 o 3 Shake continuously until the iodine is removed, and extract 3 times with ethyl acetate, each with 100 ml. The combined organic phases were washed with anhydrous Na 2 SO 4 Dry, spin out the solvent, wash and separate on a silica gel column with mobile phase petroleum ether: ethyl acetate = 8:1, the obtained compound b is 8,8'-dimethyl-1,1'-di-p-toluenesulfonate Acyl-2,2′,3,3′,8,8a,8′,8′a-octahydro-1H,1′H-3a,3′a-dipyrrole[2,3-b]indole White solid, 90% yield.
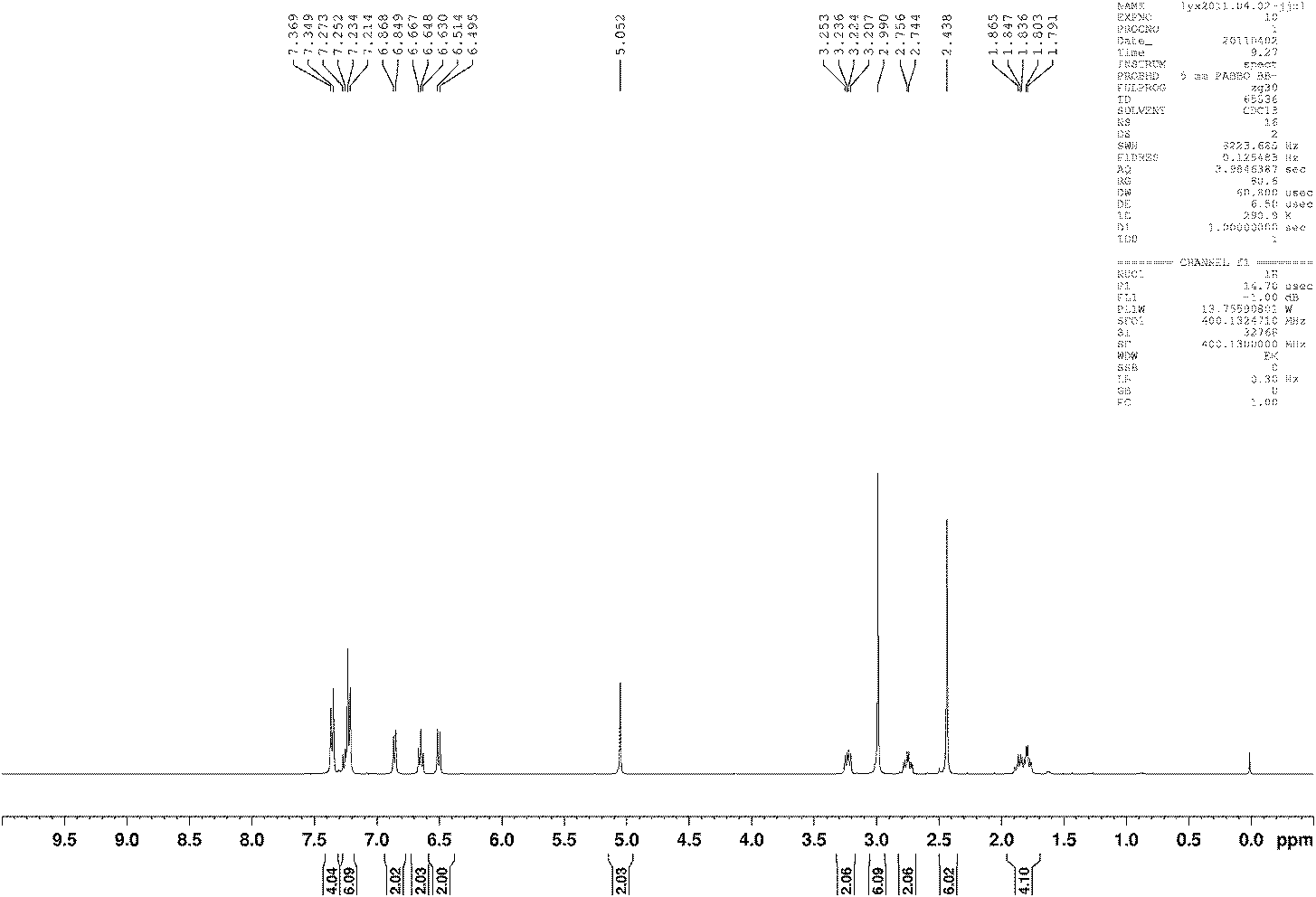
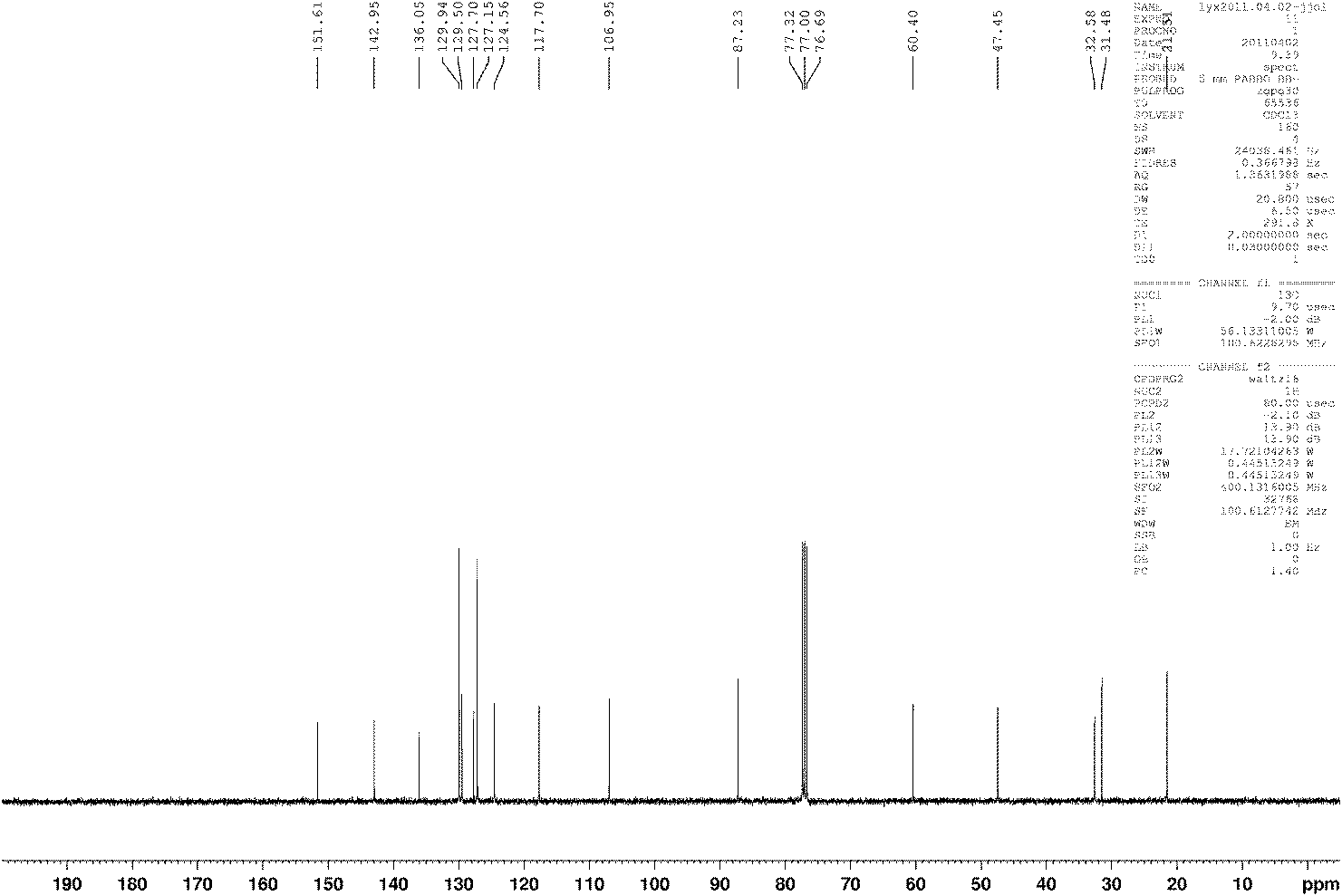
[0041] Characterization data of compound b: white solid Mp: 149-152°C; 1H NMR (400MHz, CDCl 3 , TMS) δ7.36(d, J=8.0Hz, 4H), 7.21-7.27(m, 6H...
Embodiment 2
[0051] A kind of synthetic method of the intermediate product of natural product (±)-folicanthine, described method is:
[0052] At room temperature, 5 mmoles of compound a and 10 mmoles of Cs 2 CO 3 Dissolve in 25 ml of acetonitrile, add 10 mmol of iodine and stir at room temperature for 10 minutes; then add saturated Na 2 S 2 o 3 Quench the reaction and continue to add saturated Na 2 S 2 o 3 Shake continuously until the iodine is removed, and extract 3 times with ethyl acetate, each dosage is 50 ml. The combined organic phases were washed with anhydrous Na 2 SO 4 Dry, spin out the solvent, wash and separate on the silica gel column with mobile phase petroleum ether: ethyl acetate = 8:1, the obtained compound b is 8,8'-dimethyl-1,1'-di-p-toluenesulfonate Acyl-2,2′,3,3′,8,8a,8′,8′a-octahydro-1H,1′H-3a,3′a-dipyrrole[2,3-b]indole White solid, 91% yield.
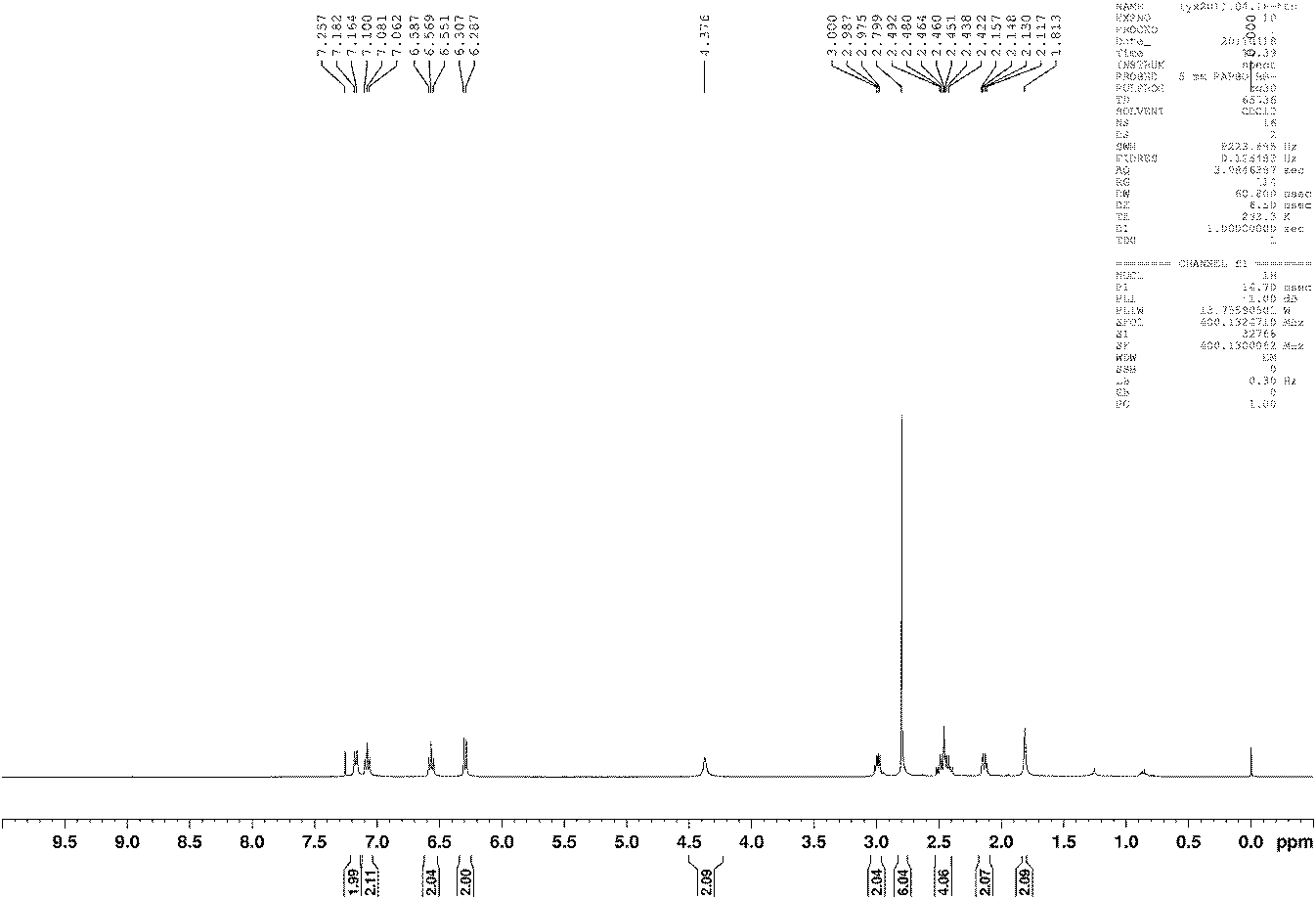
[0053] The synthetic method of preparing natural product (±)-folicanthine with compound b as an intermediate produ...
Embodiment 3
[0059] A kind of synthetic method of the intermediate product of natural product (±)-folicanthine, described method is:
[0060] At room temperature, 2 mmoles of compound a and 4 mmoles of Cs 2 CO 3 Dissolve in 10 ml of acetonitrile, add 4 mmol of iodine and stir at room temperature for 20 minutes; then add saturated Na 2 S 2 o 3 Quench the reaction and continue to add saturated Na 2 S 2 o 3 Shake continuously until the iodine is removed, and extract 3 times with ethyl acetate, each dosage is 20 ml. The combined organic phases were washed with anhydrous Na 2 SO 4 Dry, spin out the solvent, wash and separate on a silica gel column with mobile phase petroleum ether: ethyl acetate = 8:1, the obtained compound b is 8,8'-dimethyl-1,1'-di-p-toluenesulfonate Acyl-2,2′,3,3′,8,8a,8′,8′a-octahydro-1H,1′H-3a,3′a-dipyrrole[2,3-b]indole White solid, 92% yield.
[0061] The synthetic method of preparing natural product (±)-folicanthine with compound b as an intermediate product i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com