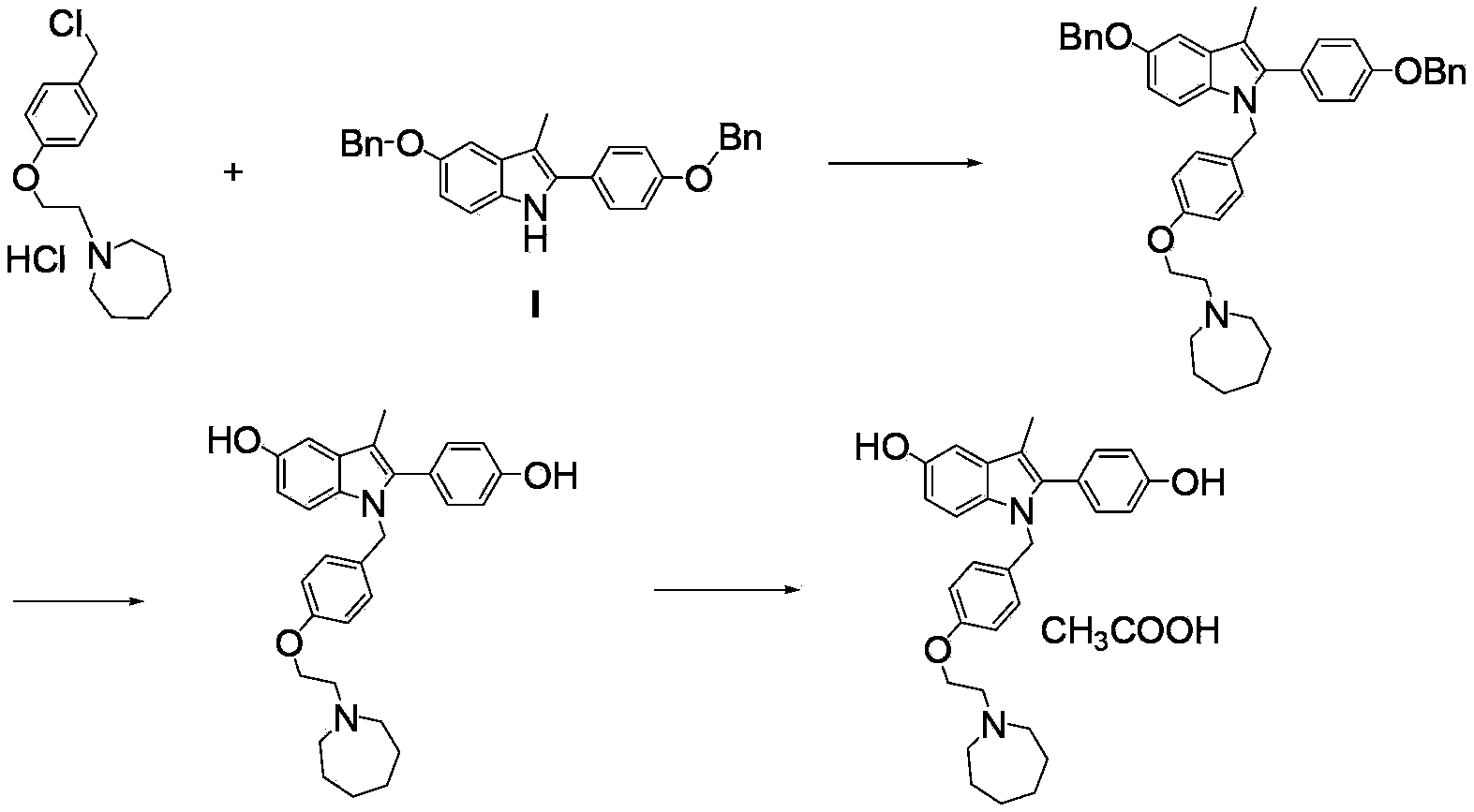
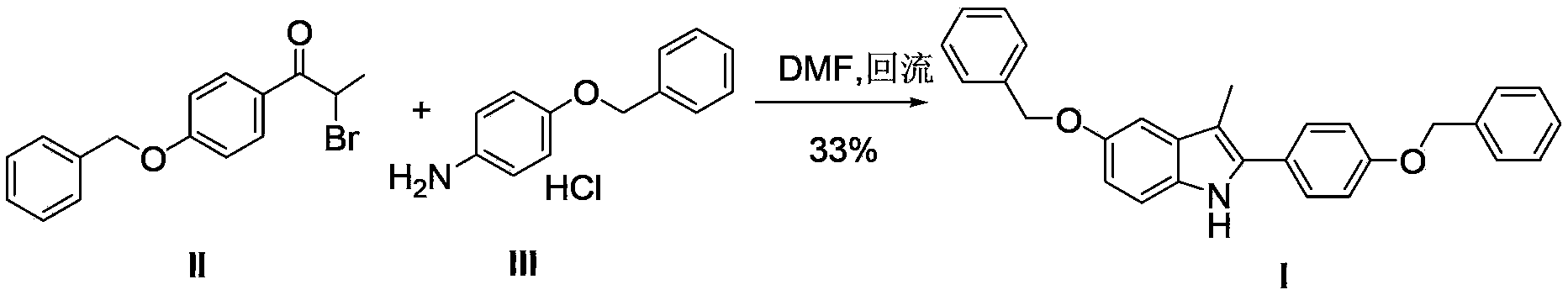
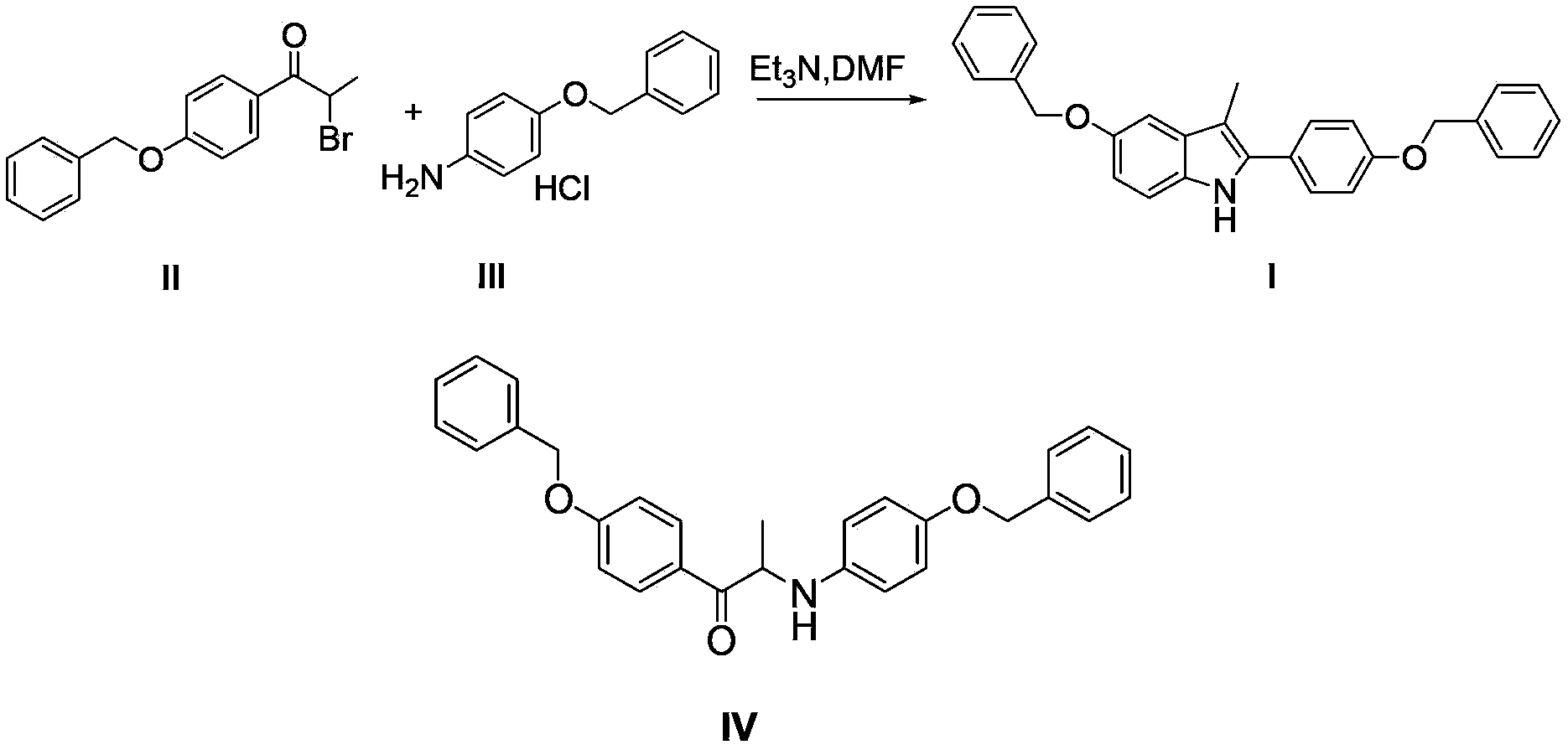
Preparation method for 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
A technology of benzyloxyphenyl and benzyloxy, applied in the field of preparation of 5-benzyloxy-2--3-methyl-1H-indole, can solve the problem of low yield, complicated preparation process and cumbersome operation and other problems, to achieve the effect of high reaction yield, high purity and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole (I)
[0037] Add 2-bromo-(4-benzyloxyphenyl) acetone (12.7g, 0.04mol), p-benzyloxyaniline hydrochloride (11.1g, 0.047mol) to n-butanol (120ml), add triethyl Amine (12.2ml) was heated to 118°C and refluxed. After 3 hours, the reaction of 2-bromo-(4-benzyloxyphenyl)acetone was complete; concentrated hydrochloric acid (1.0ml) was added, and the temperature was raised to 118°C to continue the reaction for 7 hours. The reaction liquid was cooled, filtered with suction, and dried to obtain 15.2 g of white solid, HPLC purity: 98%, yield 90.5%.
[0038] 1 H-NMR (400MHz, d 6-DMSO)δ(ppm):7.798(br s,1H,-NH-),7.102-7.557(m,14H,Ar-H),7.255(d,1H,J=2.4Hz,Ar-H),7.174 (d,1H,J=4.0Hz,Ar-H),6.978(dd,1H,J=4.0Hz,2.4Hz,Ar-H),5.192(s,2H,-OCH 2 -),5.153(s,2H,-OCH 2 -),2.436(s,3H,-CH 3 )MS (m / z): 420 (M+1)
Embodiment 2
[0040] Synthesis of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole (I)
[0041] Add 2-bromo-(4-benzyloxyphenyl) acetone (12.7g, 0.04mol), p-benzyloxyaniline hydrochloride (11.1g, 0.047mol) to isobutanol (120ml), add triethyl Amine (12.2ml), heated to 107°C and refluxed, 2-bromo-(4-benzyloxyphenyl)acetone reacted completely after 3 hours; added p-toluenesulfonic acid monohydrate (1.7g), heated to 107°C The reaction was continued for 14 hours. The reaction solution was cooled, filtered with suction, and dried to obtain 14.4 g of a tan solid, with an HPLC purity of 97% and a yield of 86%.
Embodiment 3
[0043] Synthesis of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole (I)
[0044] Add 2-bromo-(4-benzyloxyphenyl) acetone (12.7g, 0.04mol), p-benzyloxyaniline hydrochloride (11.1g, 0.047mol) to isopropanol (120ml), add pyridine ( 7.1ml), the temperature was raised to 84°C, and after 4 hours, the reaction of 2-bromo-(4-benzyloxyphenyl)acetone was complete; acetic acid (0.7ml) was added to continue the reaction for 15 hours. The reaction solution was cooled, filtered with suction, and dried to obtain 14.6 g of white solid, HPLC purity: 96%, yield 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com