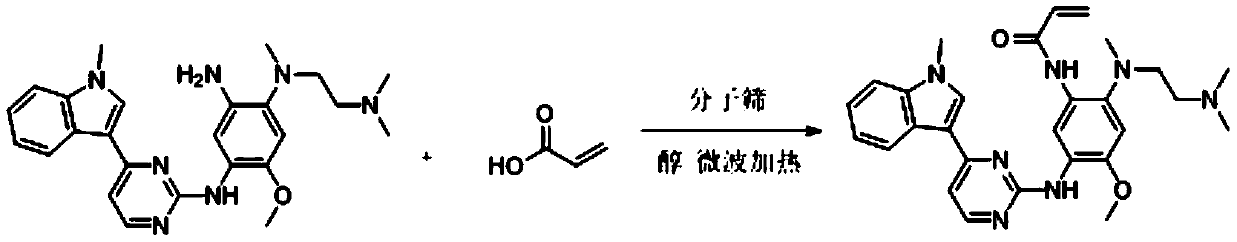
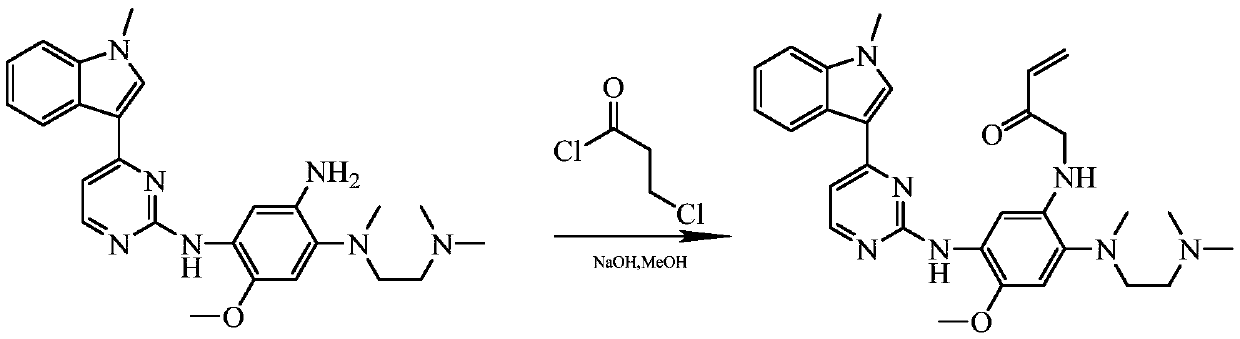
Method for synthesizing osimertinib by molecular sieve catalysis
A technology of molecular sieves and alcohol solvents, which is applied in organic chemistry and other fields, can solve the problems of 3-chloropropionyl chloride's high toxicity and volatility, its inapplicability to large-scale industrial production, and the difficulty of post-processing, so as to reduce highly toxic raw materials Use, easy industrial production, and efficient response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add N-2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indole- 3-yl)-2-pyrimidinyl]amino]aniline (4.45g, 0.010mol), add acrylic acid (0.86g, 0.012mol), HY molecular sieves (0.44g, 0.002mol), isopropanol (32mL, 7.2ml / g), heated to 35°C with microwave, reacted for 4h, dried over anhydrous sodium sulfate, and finally removed the solvent by rotary evaporation to obtain 4.07g of a foamy off-white solid with a yield of 81.6%.
Embodiment 2
[0030] Add N-2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indole- 3-yl)-2-pyrimidinyl]amino]aniline (4.45g, 0.010mol), add acrylic acid (0.86g, 0.012mol), HY molecular sieves (0.66g, 0.003mol), isopropanol (32mL, 7.2ml / g), microwave heated to 35°C, reacted for 4 hours, dried over anhydrous sodium sulfate, and finally removed the solvent by rotary evaporation to obtain 4.88 g of a foamy off-white solid with a yield of 97.9%.
Embodiment 3
[0032] Add N-2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indole- 3-yl)-2-pyrimidinyl]amino]aniline (4.45g, 0.010mol), add acrylic acid (0.86g, 0.012mol), HY molecular sieves (0.66g, 0.003mol), isopropanol (32mL, 7.2ml / g), heated to 30°C with microwave, reacted for 4h, dried over anhydrous sodium sulfate, and finally removed the solvent by rotary evaporation to obtain 4.78g of a foamy off-white solid with a yield of 95.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com