A kind of method of synthesizing sulfoxide compound
A compound and sulfoxide technology, applied in the field of organic compound synthesis, can solve the problems of harsh reaction conditions and low metal-containing waste, and achieve the effects of high reaction efficiency, high atom utilization rate, and wide application range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
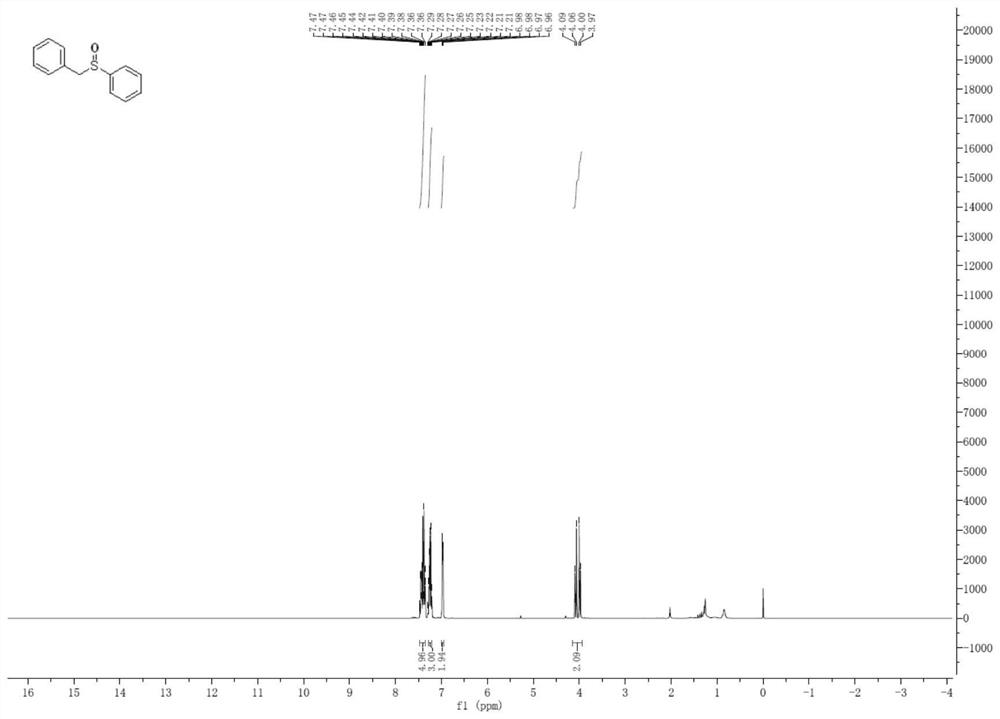
[0043] The preparation of embodiment 1 benzyl phenyl sulfoxide
[0044]
[0045] In a dry Shrek reaction tube, add benzylphenyl sulfide (0.25 mmol) sulfide and PDI (0.5 mol%), PDI is the compound of formula A in methanol (2.0 mL). Next, fill a balloon with oxygen and fix it on top of the Shrek reaction tube. Under normal pressure oxygen atmosphere, irradiated with 15W white CFL to react at room temperature. After the reaction was complete, brine was added to the reaction. The aqueous phase was re-extracted with ethyl acetate. Synthetic organic extracts in Na 2 SO 4 Drying and concentrating in vacuo, the resulting residue was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 10:1), and purified to obtain a white viscous solid (yield: 95%; selectivity: 100%). 1 H NMR (400MHz, CDCl 3 ): δppm 7.47-7.36(m,5H,ArH),7.29-7.21(m,3H,ArH),6.98-6.96(m,2H,ArH),4.00-3.97(m,2H,CH 2 ).
Embodiment 2
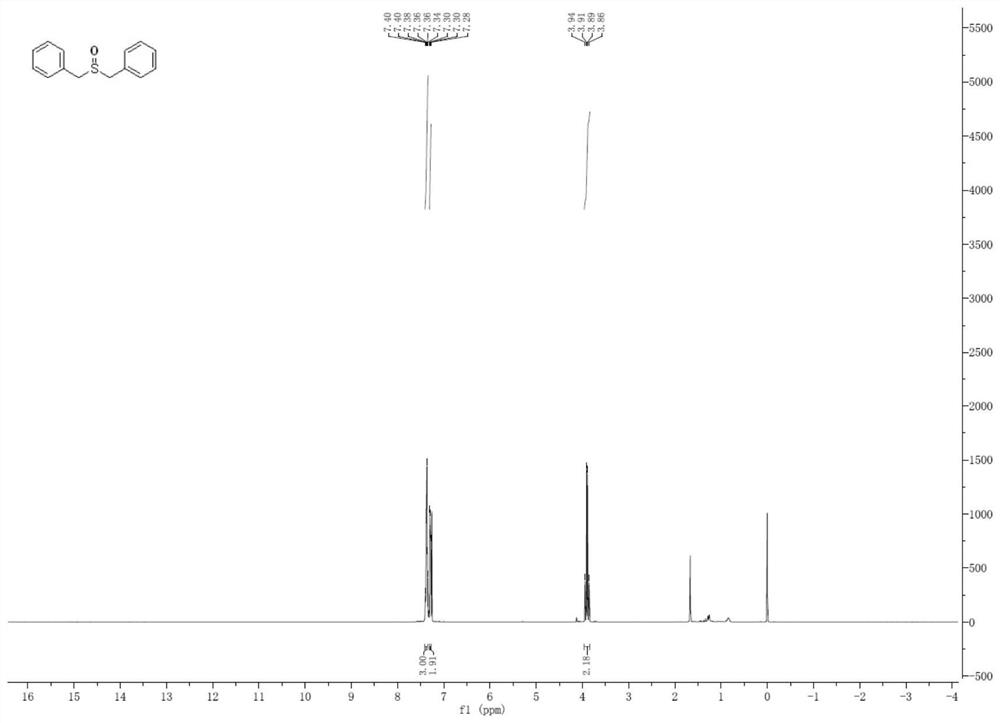
[0046] The preparation of embodiment 2 benzhydryl sulfoxide
[0047]
[0048] In a dry Shrek reaction tube, add benzhydryl sulfide (0.25 mmol) sulfide and PDI (0.5 mol%), PDI is the compound of formula B in methanol (2.0 mL). Next, fill a balloon with oxygen and fix it on top of the Shrek reaction tube. Under normal pressure oxygen atmosphere, irradiated with 15W white CFL to react at room temperature. After the reaction was complete, brine was added to the reaction. The aqueous phase was re-extracted with ethyl acetate. Synthetic organic extracts in Na 2 SO 4 Drying and concentration in vacuo, the resulting residue was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 10:1), and purified to obtain a white viscous solid (yield: 90%; selectivity: 100%). 1 H NMR (400MHz, CDCl 3 ): δppm 7.40-7.34(m,3H,ArH),7.30-7.28(m,2H,ArH),3.94-3.86(m,2H,CH 2 ).
Embodiment 3
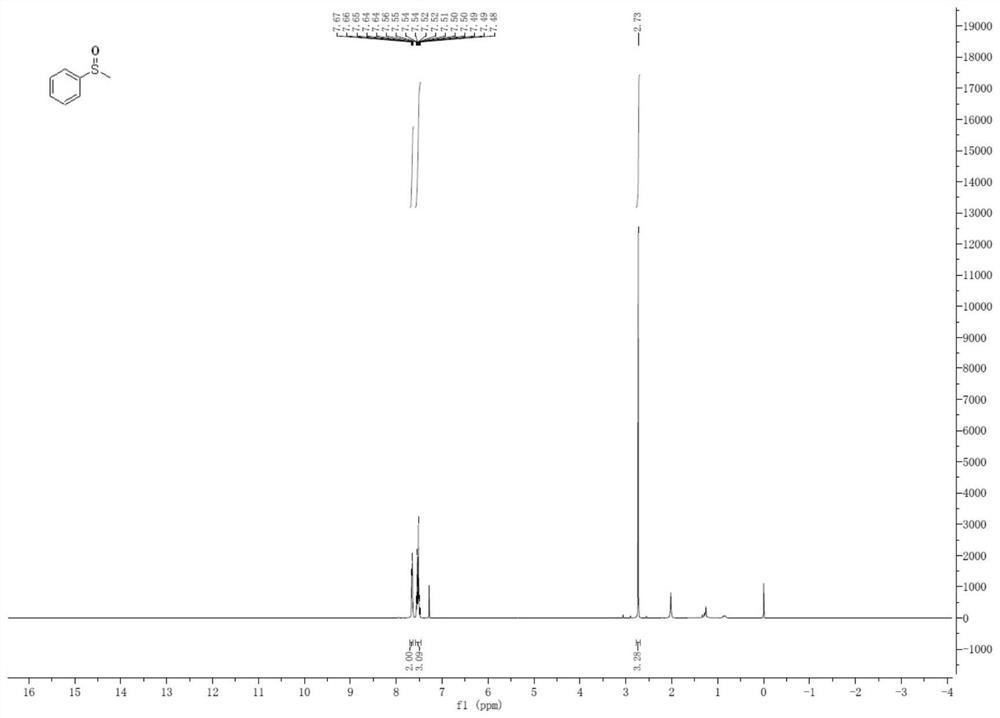
[0049] The preparation of embodiment 3 methyl phenyl sulfoxide
[0050]
[0051] In a dry Shrek reaction tube, methyl phenyl sulfide (0.25 mmol) sulfide and PDI (0.5 mol%), the compound of formula A, were added in methanol (2.0 mL). Next, fill a balloon with oxygen and fix it on top of the Shrek reaction tube. Under normal pressure oxygen atmosphere, irradiated with 15W white CFL to react at room temperature. After the reaction was complete, brine was added to the reaction. The aqueous phase was re-extracted with ethyl acetate. Synthetic organic extracts in Na 2 SO 4 Drying and concentration in vacuo, the resulting residue was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 10:1), and purified to obtain a white viscous solid (yield: 94%; selectivity: 100%). 1 H NMR (400MHz, CDCl 3 ): δppm 7.67-7.64(m,2H,ArH),7.56-7.48(m,3H,ArH),2.73(s,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com