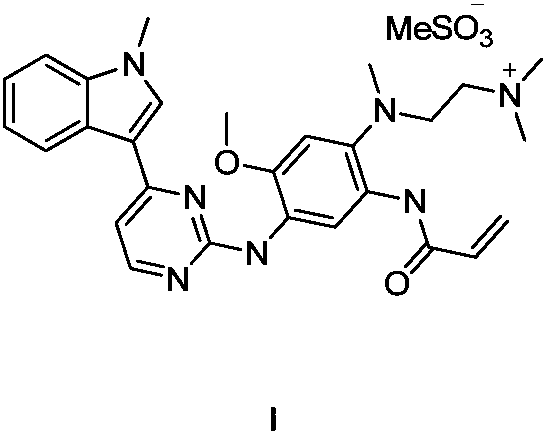
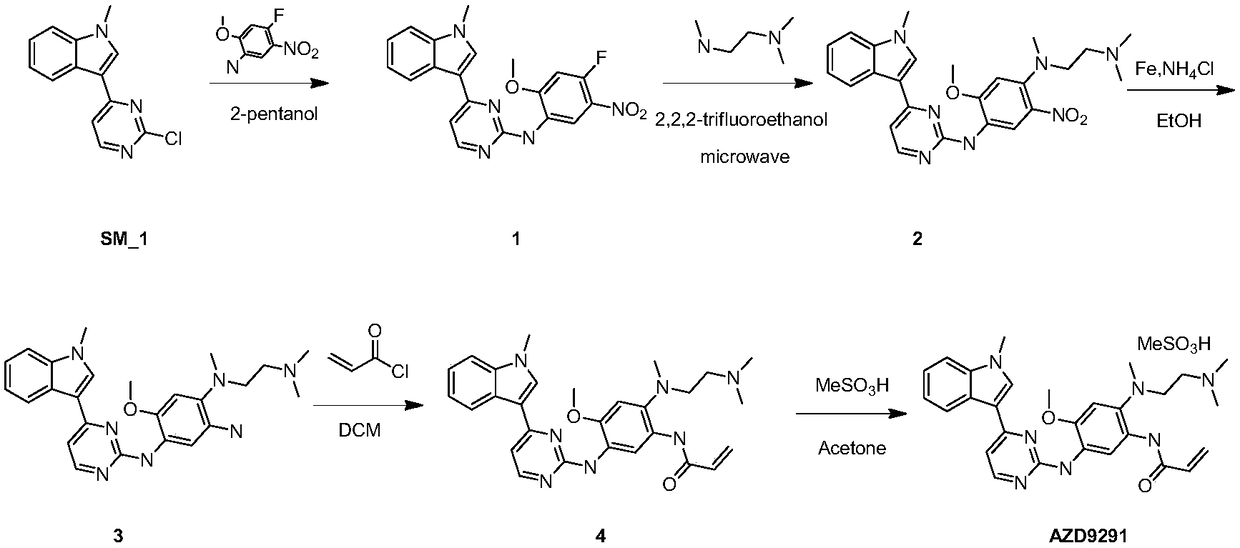
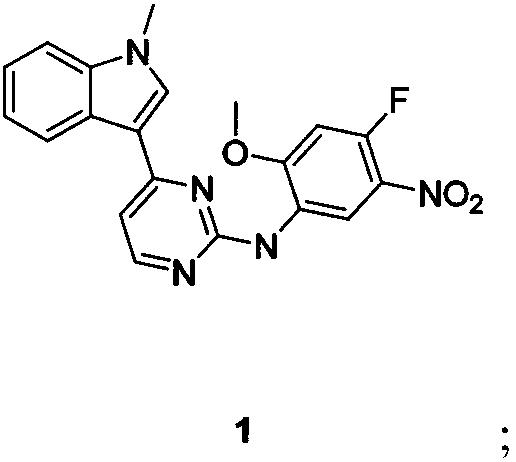
Osimertinib preparation method
A technology for ostinib and compounds, applied in the field of preparation of ostinib, can solve the problems of unrealistic industrial production, poor production practicability, and inability to be used for production, and achieves stable and controllable reaction process, excellent quality and economical efficiency energy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1-methyl-1H-indol-3-yl)-2-pyrimidinamine
[0058] The raw material 3-(2-chloro-4-pyrimidinyl)-1-methyl-1H-indole 2.5kg (10.26mol) and 4-fluoro-2-methoxy-5-nitroaniline 2kg (10.74mol ) was added successively under stirring in a 50L ethanol reactor, after stirring evenly, the oil bath was heated to 120 degrees Celsius, slowly added p-toluenesulfonic acid 2.34kg (12.31mol), and then refluxed overnight to detect that the reaction was complete, stop heating, and cool down to Suction filtration at room temperature, rinse the filter cake with 5L of isopropanol, and dry to obtain 3.7 kg of a yellow solid with a yield of 91.5% (HPLC purity>95%).
Embodiment 2
[0060] N-[(4-Dimethylamino-ethylamino-2-methoxy-5-nitrophenyl)]-4-(1-methyl-1H-indol-3-yl)-2-pyrimidine Amine Synthesis
[0061] N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1-methyl-1H-indol-3-yl)-2-pyrimidinamine 3.7kg (9.41mol) Add 37L of DMF to the reactor, then add 1.43kg (14.03mol) of diisopropylethylamine, stir well, then add N,N,N-trimethylethylenediamine, and heat to 85 degrees Celsius , reacted overnight. After the detection reaction was completed, the heating was stopped, 37 L of water was added, stirred overnight, centrifuged, washed with water, and dried to obtain 3.8 kg of orange-red solid powder with a yield of 84.9% (HPLC purity>99%).
Embodiment 3
[0063] N-[(4-dimethylamino-ethylamino-2-methoxy-5-aminophenyl)]-4-(1-methyl-1H-indol-3-yl)-2-pyrimidinamine synthesis
[0064] The N-[(4-dimethylaminoethylamino-2-methoxy-5-nitrophenyl)]-4-(1-methyl-1H-indol-3-yl)- Add 3.8kg (7.99mol) of 2-pyrimidinamine into an autoclave with 38L of ethanol, then add 380g of palladium carbon into the system, replace with nitrogen three times, then replace with hydrogen twice, set the hydrogen pressure to 2MPa, and heat to 80 degrees React overnight. After the detection reaction is over, cool the autoclave, extract the reaction solution, filter through a diatomite filter cake, rinse the filter cake with ethanol, concentrate to 15kg, cool, stir and crystallize, return to room temperature for 2 hours, filter, and filter the cake Rinse with ethanol and dry to obtain 3.05 kg of silver gray powder with a yield of 85.6% (HPLC purity>99%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com