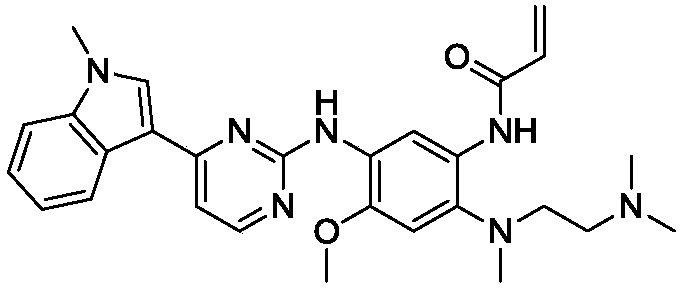
Method for synthesizing osimertinib intermediate through micro-channel reactor
A micro-channel reactor and intermediate technology, applied in the direction of organic chemistry, etc., can solve the problems of low catalyst recovery times, long reaction time, violent explosion, etc., and achieve stable online production and post-processing, yield and purity improvement, Production cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1. The method for synthesizing the intermediate of Osimertinib in a microchannel reactor.
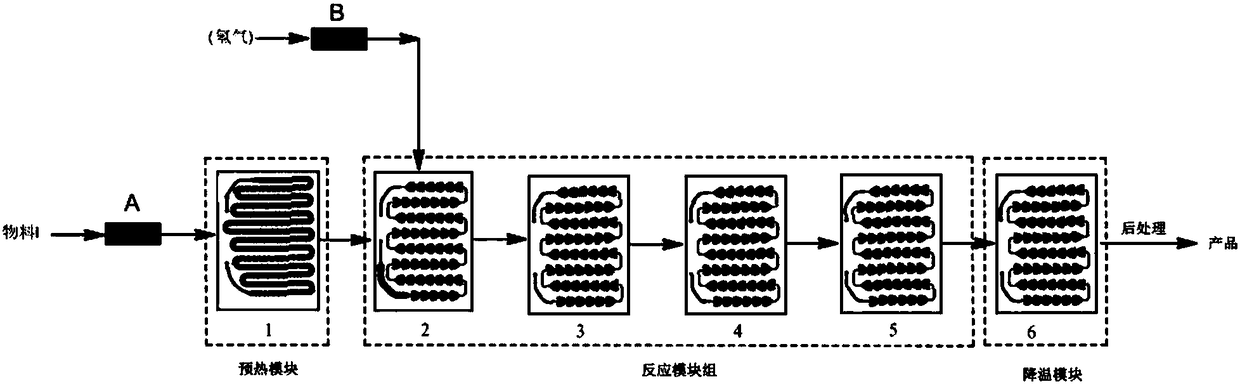
[0039] 1) Weigh 200 g of the raw material hydrogenation precursor nitro, and then add it to a mixture of 4 L of methanol and 100 g of concentrated hydrochloric acid, stir and dissolve, add 10 g of Pd / C catalyst with a mass content of 5% Pd, and fully stir and mix to form Material I, transport the material I to the preheating module 1 of the microchannel reactor for preheating, and then enter the reaction module group of the microchannel reactor after preheating.
[0040] 2) Hydrogen is delivered to the reaction module group of the microchannel reactor and step 1) reacts with the preheated material I in the reaction module group, wherein: adjust the flow rate of the slurry pump so that the flow rate of the material I is 40.0g / min , adjust H 2 The flow rate of the gas flowmeter is 500ml / min, the molar ratio of raw material hydrogenation precursor nitro and hydrogen is 1...
Embodiment 2
[0041] Embodiment 2. The method for synthesizing Osimertinib intermediates in a microchannel reactor.
[0042] 1) Weigh 200 g of the raw material hydrogenation precursor nitro, and then add it into a mixture of 4 L of ethanol and 100 g of concentrated hydrochloric acid, stir and dissolve, add 7 g of Pd / C catalyst with a Pd mass content of 8%, and fully stir and mix to form a material I, the material I is transported to the preheating module 1 of the microchannel reactor for preheating, and then enters the reaction module group of the microchannel reactor after preheating.
[0043] 2) Hydrogen is delivered to the reaction module group of the microchannel reactor and step 1) the material I after preheating is reacted in the reaction module group, wherein: adjust the flow rate of the slurry pump so that the flow rate of the material I is 35.0g / min , adjust H 2 The flow rate of the gas flowmeter is 400ml / min, the molar ratio of raw material hydrogenation precursor nitro and hydro...
Embodiment 3
[0044] Embodiment 3. The method for synthesizing Osimertinib intermediate in microchannel reactor.
[0045] 1) Weigh 300g of the raw material hydrogenation precursor nitro, and then add it to a mixed solution of 5L of ethanol and 150g of concentrated hydrochloric acid, stir and dissolve, add 10g of Pt / C catalyst with a mass content of 10% of Pt, fully stir and mix to form a material I, the material I is transported to the preheating module 1 of the microchannel reactor for preheating, and then enters the reaction module group of the microchannel reactor after preheating.
[0046] 2) Hydrogen is delivered to the reaction module group of the microchannel reactor and step 1) the material I after preheating is reacted in the reaction module group, wherein: adjust the flow rate of the slurry pump so that the flow rate of the material I is 25.0g / min , adjust H 2 The flow rate of the gas flowmeter is 300ml / min, the molar ratio of raw material hydrogenation precursor nitro and hydrog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com