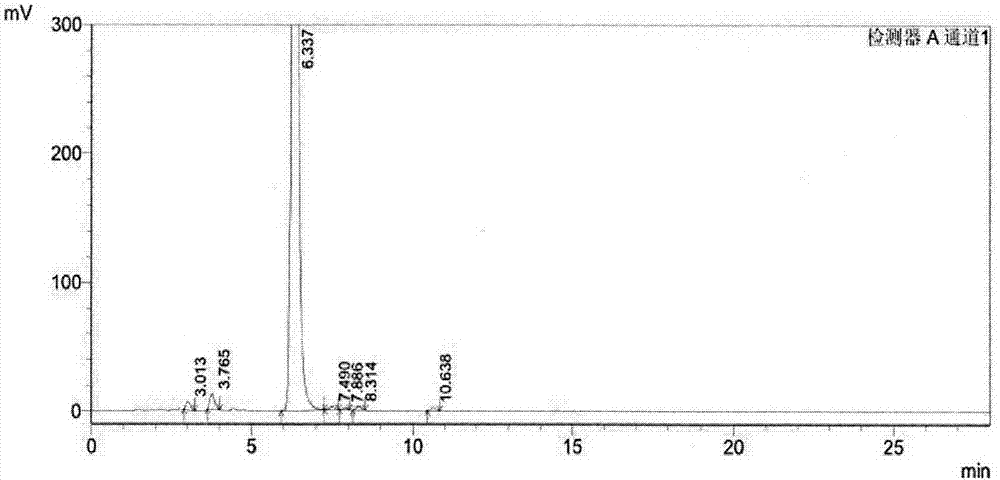
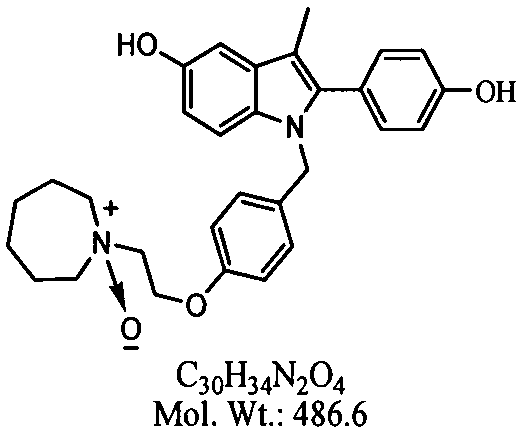
Separation preparation method of bazedoxifene acetate impurity A
A bazedoxifene acetate and impurity technology, applied in the field of reference substance research, can solve the problems of low yield and difficulty in obtaining impurity A with a purity above 96%, and achieve the goal of optimizing chromatographic conditions, shortening separation time, and ensuring stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In this example, 80% bazedoxifene was used as raw material, and 5 μm C 18 The bonded phase filler is a chromatographic column, and a solution containing 0.01M dipotassium hydrogen phosphate (PH8.3)-acetonitrile (40:60, volume ratio) is used as the eluting solvent. The specific process steps are as follows:
[0024] 1. Sample pretreatment
[0025] Add bazedoxifene to the eluting solvent to dissolve, add 3% hydrogen peroxide, let stand for half an hour, dilute the bazedoxifene solution to a concentration of 1 mg / ml with the eluting solvent, and filter through a 0.22 μm microporous membrane.
[0026] 2. Column handling
[0027] The preparative high-performance liquid chromatography column was selected as the stationary phase produced by Japan Sheshido Company as C 18 A preparative column (5 μm, 20×250 mm) was used to equilibrate the chromatographic column with an eluting system solution of about 5 times the column volume.
[0028] 3. Sample loading
[0029] The filtere...
Embodiment 2
[0039] In this example, 90% bazedoxifene was used as raw material, and 10 μmC 18 The bonded phase filler is a chromatographic column, and a solution containing 0.01M dipotassium hydrogen phosphate (PH8.3)-acetonitrile (35:65, volume ratio) is used as the eluting solvent. The specific process steps are as follows:
[0040] 1. Sample pretreatment
[0041] Add bazedoxifene to the elution solvent to dissolve, add 3% hydrogen peroxide, let it stand for 1 hour, dilute it with the elution solvent to a bazedoxifene solution with a concentration of 5 mg / ml, and filter through a 0.22 μm microporous membrane.
[0042] 2. Column handling
[0043] The preparative high-performance liquid chromatography column is selected as a self-packed preparative column, and the particle size of the filler is 10 μm C 8 Bonded phase filler (10μm, 75×250mm), equilibrate the chromatographic column with the elution system solution of about 10 times the column volume.
[0044] 3. Sample loading
[0045] T...
Embodiment 3
[0053] In this example, 85% bazedoxifene was used as raw material, and 10 μm C 18 The bonded phase filler is a chromatographic column, and a solution containing 0.01M dipotassium hydrogen phosphate (PH8.3)-acetonitrile (40:60, volume ratio) is used as the eluting solvent. The specific process steps are as follows:
[0054] 1. Sample pretreatment
[0055] Add bazedoxifene to the elution solvent to dissolve, add 3% hydrogen peroxide, let stand for half an hour, dilute the bazedoxifene solution to a concentration of 5 mg / ml with the elution solvent, and filter through a 0.45 μm microporous membrane.
[0056] 2. Column handling
[0057] The preparative high-performance liquid chromatography column is selected as a self-packed preparative column, and the particle size of the filler is 10 μm C 18 Bonded phase filler (10μm, 75×250mm), equilibrate the chromatographic column with the elution system solution of about 10 times the column volume.
[0058] 3. Sample loading
[0059] Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com