The preparation method of bazedoxifene acetate polymorph a
A technology of bazedoxifene acetate and bazedoxifene, applied in the direction of organic chemistry, to achieve the effects of high product yield, high crystal purity and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
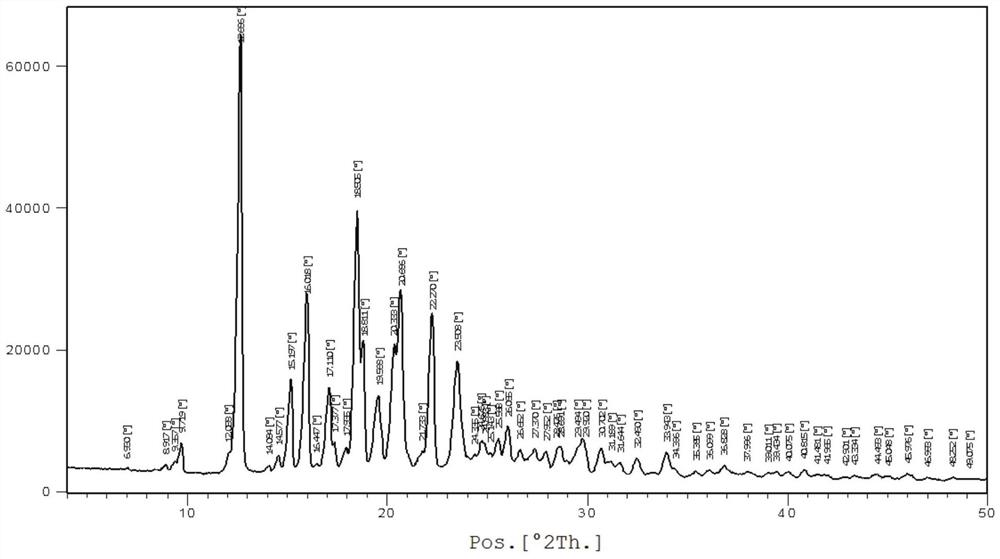
[0028] In a nitrogen atmosphere, add the raw materials bazedoxifene free base (4.7g, 10mmol) and 35mL of methanol into the reaction flask, heat up to 40-45 degrees, stir until dissolved; cool down to 10-20 degrees, dropwise add acetic acid (0.72g , 12mmol) in methanol 10mL solution, keep the temperature and continue to stir for 2-4 hours after dropping; white crystals are precipitated, filtered, washed with cold methanol, and vacuum-dried at 50-60 degrees to obtain white solid bazedoxifene acetate polymorph A 3.9 g, yield 73.6%. For the XRD spectrum of the product bazedoxifene acetate polymorph A, please refer to figure 1 shown.
[0029] Wherein the XRD diffraction spectrum figure 2 The main peaks at θ are: 9.8, 12.7, 15.2, 16.0, 17.1, 17.4, 18.5, 18.8, 19.6, 20.3, 20.7, 22.3, 23.5, 24.9, 25.6, 26.1, 27.4, 28.0, 28.7, 29.9, 30.7; The peaks include: 12.7, 16.0, 18.5, 20.7, 22.3, consistent with the characteristic peaks of polymorphic form A of bazedoxifene acetate. Please ...
Embodiment 2
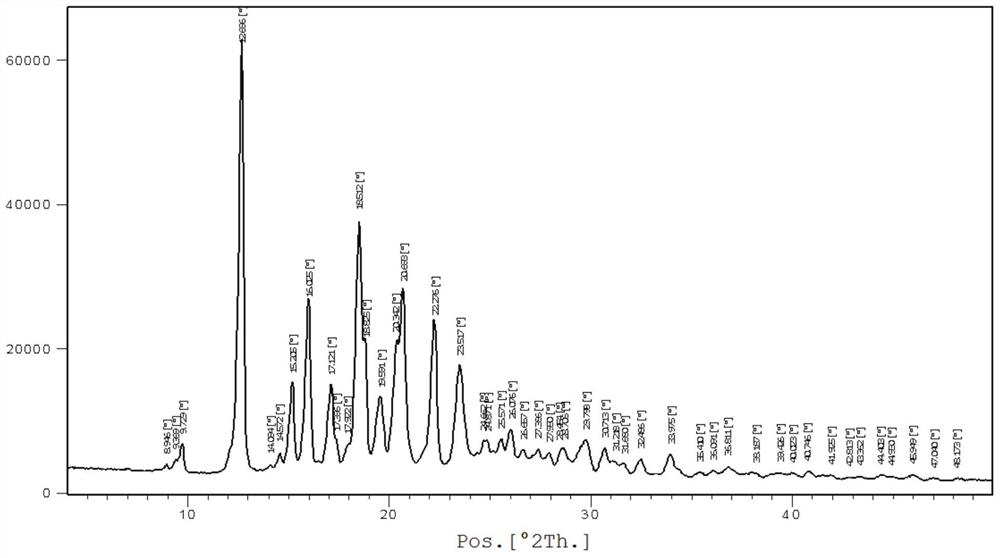
[0034] In a nitrogen atmosphere, add bazedoxifene free base (4.7 g, 10 mmol) and 45 mL of ethanol into the reaction flask, heat up to 35-45 degrees, and stir until dissolved. Cool down to 15-25 degrees, add dropwise acetic acid (0.72g, 12mmol) ethanol 10mL solution, after dropping, keep stirring at this temperature for 2-4 hours, white crystals precipitate, filter, wash with cold ethanol, vacuum at 50-60 degrees After drying, 4.3 g of polymorphic form A of bazedoxifene acetate was obtained as a white solid, with a yield of 81.1%. For the XRD spectrum of the product bazedoxifene acetate polymorph A, please refer to figure 2 shown.
[0035] Wherein the XRD diffraction spectrum figure 2 The main peaks at θ are: 9.8, 12.7, 15.2, 16.0, 17.1, 17.4, 18.5, 18.8, 19.6, 20.3, 20.7, 22.3, 23.5, 24.9, 25.6, 26.1, 27.4, 28.0, 28.7, 29.8, 30.7; The peaks include: 12.7, 16.0, 18.5, 20.7, 22.3, consistent with the characteristic peaks of polymorphic form A of bazedoxifene acetate. Pleas...
Embodiment 3
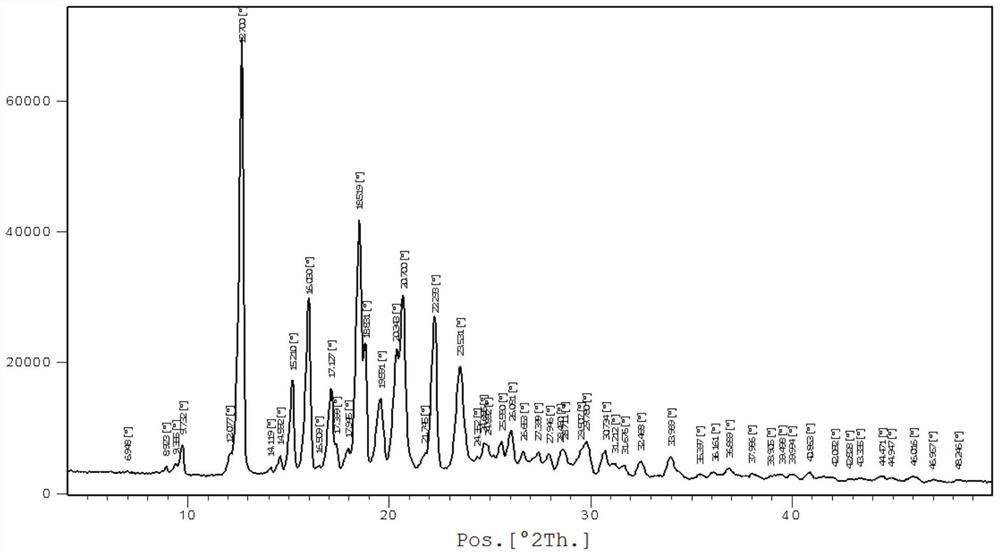
[0040] In a nitrogen atmosphere, the raw materials bazedoxifene free base (4.7 g, 10 mmol) and 50 mL of ethyl acetate were added to the reaction flask, the temperature was raised to 40-45 degrees, and stirred until dissolved. Cool down to 15-25 degrees, add acetic acid (0.72g, 12mmol) in ethyl acetate 10mL solution dropwise, keep stirring at this temperature for 2-4 hours, white crystals precipitate, filter, wash with cold ethyl acetate, 50 Vacuum drying at ~60°C gave 4.5 g of polymorphic form A of bazedoxifene acetate as a white solid, with a yield of 84.9%. For the XRD spectrum of the product bazedoxifene acetate polymorph A, please refer to image 3 shown.
[0041] Wherein the XRD diffraction spectrum figure 2 The main peaks at θ are: 9.8, 12.7, 15.2, 16.0, 17.1, 17.4, 18.5, 18.8, 19.6, 20.3, 20.7, 22.3, 23.5, 24.9, 25.6, 26.1, 27.4, 28.0, 28.7, 29.8, 30.7; The peaks include: 12.7, 16.0, 18.5, 20.7, 22.3, consistent with the characteristic peaks of polymorphic form A of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com